Abstract
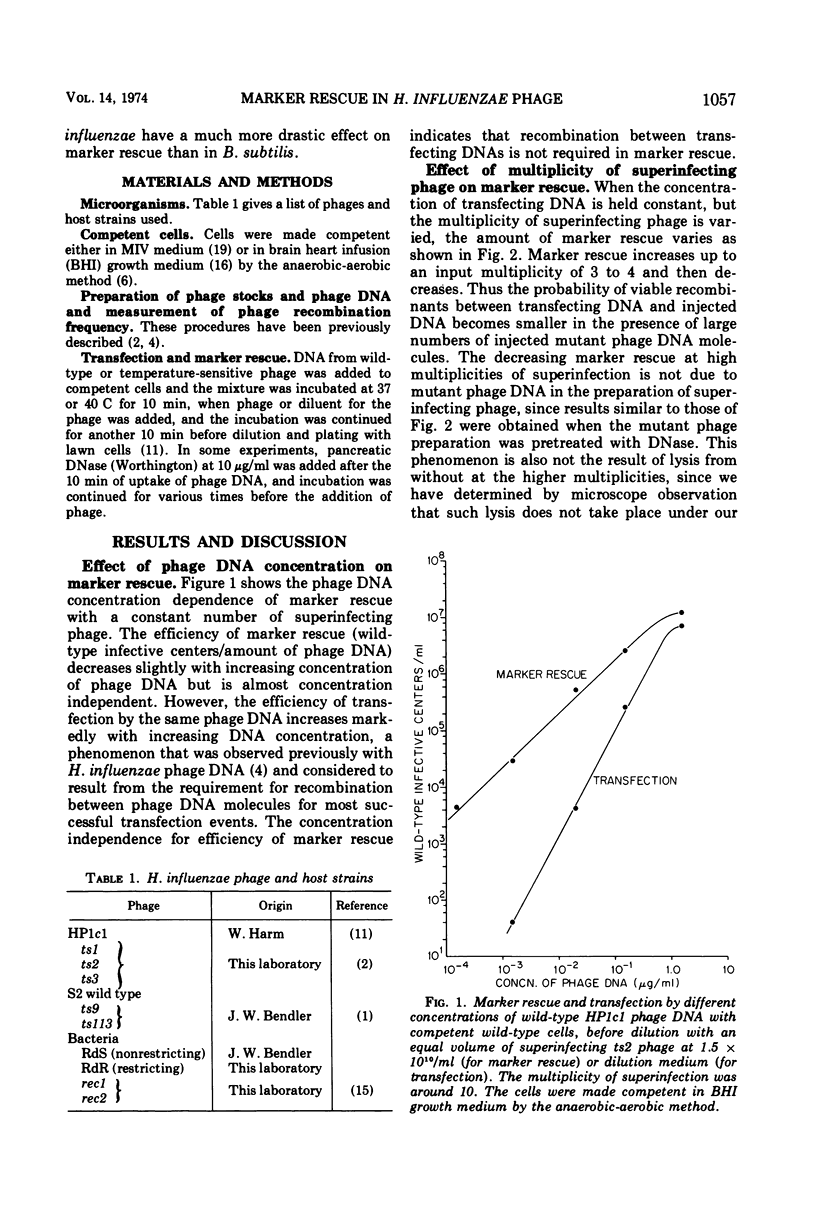
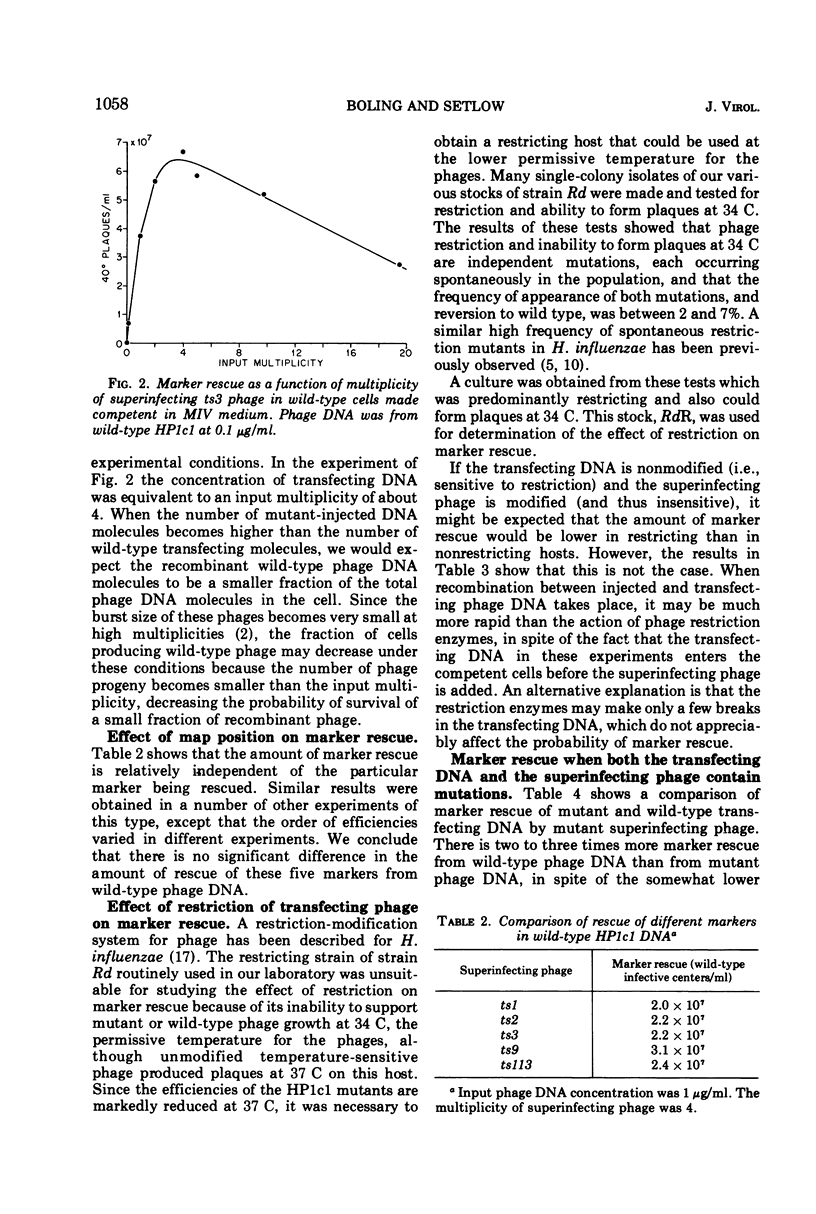
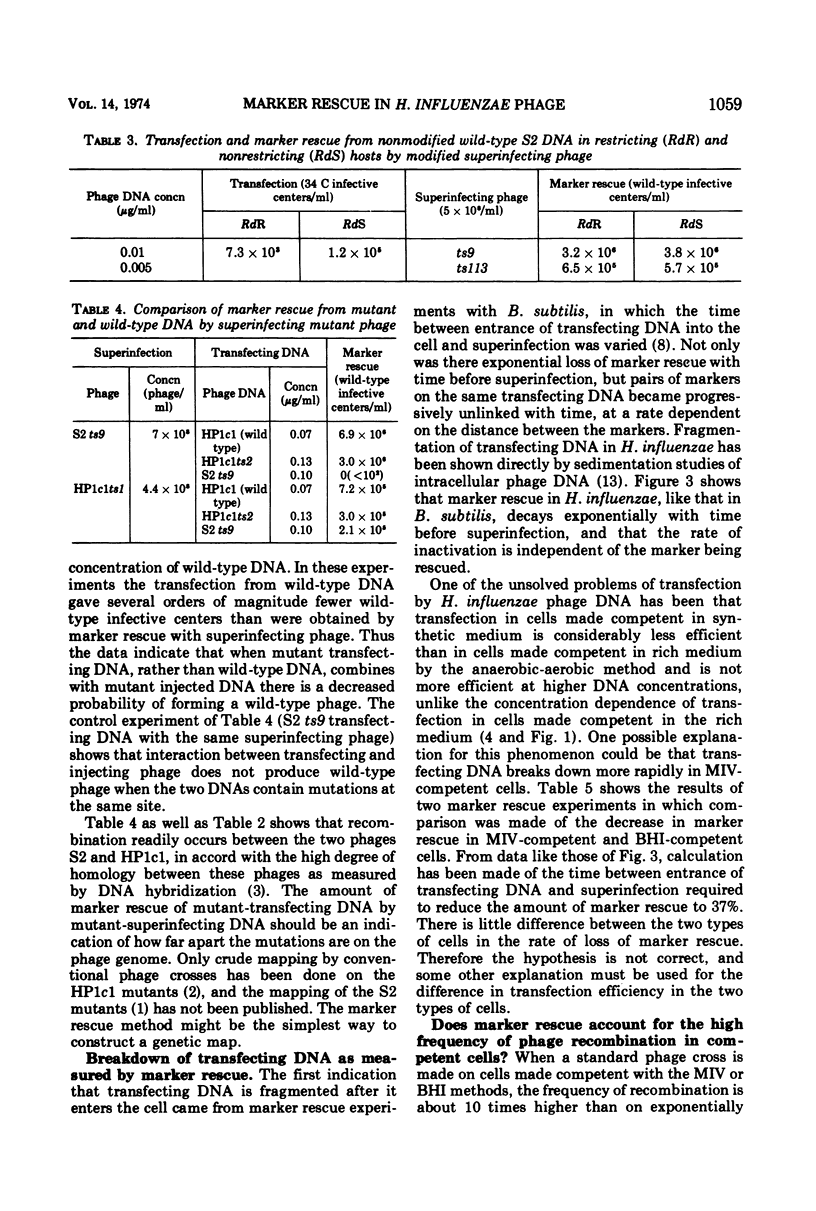
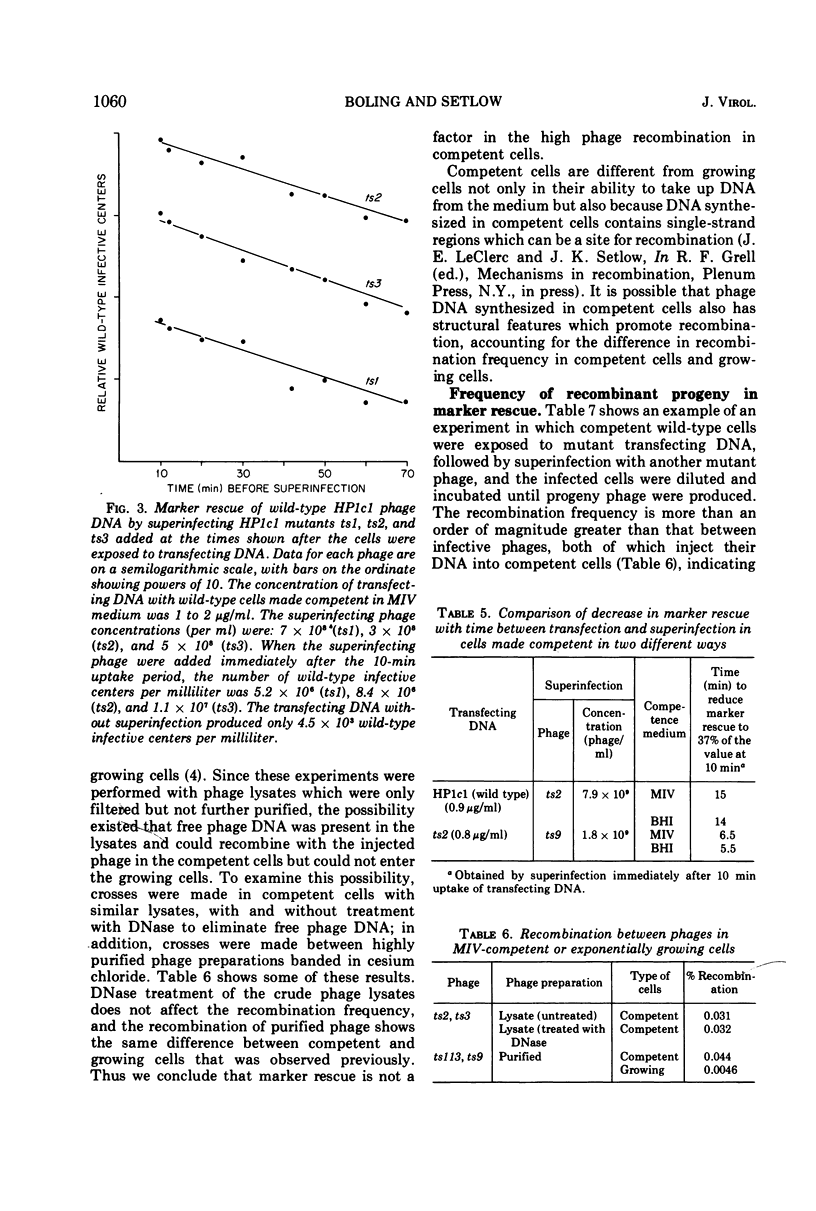
Rescue of wild-type markers from transfecting phage DNA in cómpetent Haemophilus influenzae cells by superinfection with temperature-sensitive phage (marker rescue) is approximately linearly dependent upon the concentration of transfecting DNA. The amount of marker rescue with a constant amount of transfecting DNA increases with increasing multiplicities of superinfecting phage up to about 4, and then decreases at higher multiplicities. Host restriction of transfecting DNA does not affect marker rescue. The frequency of wild-type recombinants from marker rescue is much greater than that from multiple infection with whole phages, and is comparable to that obtained with two mutant-transfecting DNAs. The amount of marker rescue decreases exponentially with time between entrance of the transfecting DNA and superinfection, and the rate of decrease is independent of map position of the rescued marker. Marker rescue is drastically reduced in the recombination-defective strains, rec1 and rec2.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendler J. W., Goodgal S. H. Prophage S2 mutants in Haemophilus influenzae: a technique for their production and isolation. Science. 1968 Oct 25;162(3852):464–465. doi: 10.1126/science.162.3852.464. [DOI] [PubMed] [Google Scholar]
- Boling M. E., Allison D. P., Setlow J. K. Bacteriophage of Haemophilus influenzae. 3. Morphology, DNA homology, and immunity properties of HPlcl, S2, and the defective bacteriophage from strain Rd. J Virol. 1973 Apr;11(4):585–591. doi: 10.1128/jvi.11.4.585-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K., Allison D. P. Bacteriophage of Haemophilus influenzae. I. Differences between infection by whole phage, extracted phage DNA and prophage DNA extracted from lysogenic cells. J Mol Biol. 1972 Feb 14;63(3):335–348. doi: 10.1016/0022-2836(72)90431-7. [DOI] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. Dependence of Vegetative Recombination Among Haemophilus influenzae Bacteriophage on the Host Cell. J Virol. 1969 Sep;4(3):240–243. doi: 10.1128/jvi.4.3.240-243.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Glover S. W., Piekarowicz A. Host specificity of DNA in Haemophilus influenzae: restriction and modification in strain Rd. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1610–1617. doi: 10.1016/0006-291x(72)90793-0. [DOI] [PubMed] [Google Scholar]
- Gromkova R., Bendler J., Goodgal S. Restriction and modification of bacteriophage S2 in Haemophilus influenzae. J Bacteriol. 1973 Jun;114(3):1151–1157. doi: 10.1128/jb.114.3.1151-1157.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARM W., RUPERT C. S. INFECTION OF TRANSFORMABLE CELLS OF HAEMOPHILUS INFLUENZAE BY BACTERIOPHAGE AND BACTERIOPHAGE DNA. Z Vererbungsl. 1963 Dec 30;94:336–348. doi: 10.1007/BF00897593. [DOI] [PubMed] [Google Scholar]
- Klotz G., Spatz H. C. A biological assay for intracellular SPP1 DNA. Mol Gen Genet. 1971;110(4):367–373. doi: 10.1007/BF00438279. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Allison D. P. Intracellular events during infection by Haemophilus influenzae phage and transfection by its DNA. J Mol Biol. 1973 Apr 25;75(4):581–599. doi: 10.1016/0022-2836(73)90293-3. [DOI] [PubMed] [Google Scholar]
- Rottländer E., Trautner T. A. Genetic and transfection studies with B, subtilis phage SP 50. I. Phage mutants with restricted growth on B. subtilis strain 168. Mol Gen Genet. 1970;108(1):47–60. doi: 10.1007/BF00343184. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Randolph M. L., Boling M. E., Mattingly A., Price G., Gordon M. P. Repair of DNA in Haemophilus influenzae. II. Excision, repair of single-strand breaks, defects in transformation, and host cell modification in UV-sensitive mutants. Cold Spring Harb Symp Quant Biol. 1968;33:209–218. doi: 10.1101/sqb.1968.033.01.024. [DOI] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. The role of recombination in transfection of B. subtilis. Mol Gen Genet. 1971;113(2):174–190. doi: 10.1007/BF00333191. [DOI] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



