Summary
The cytoplasmic Elmo1:Dock180 complex acts as a guanine nucleotide exchange factor (GEF) for the small GTPase Rac and functions downstream of the phagocytic receptor BAI1 during apoptotic cell clearance, and in the entry of Salmonella and Shigella into cells [1–7]. We discovered an unexpected binding between Elmo1 and Mediator complex subunit Med31. The Mediator complex is a regulatory hub for nearly all gene transcription via RNA polymerase II (Pol II), bridging the general transcription machinery with gene-specific regulatory proteins [8–14]. Med31 is the smallest and the most evolutionarily conserved Mediator subunit [15, 16] and knockout of Med31 results in embryonic lethality in mice [17]; however, Med31 function in specific biological contexts is not understood. We observed that in primary macrophages, during Salmonella infection, Elmo1 and Med31 specifically affected expression of cytokine genes Il10 and Il33 among the >25 genes monitored. While endogenous Med31 is predominantly nuclear localized, Elmo1 increased the cytoplasmic localization of Med31. We discover ubiquitination as a novel post-translational modification of Med31, with the cytoplasmic mono-ubiquitinated form of Med31 being enhanced by Elmo1. These data identify Elmo1 as a novel regulator of Med31, revealing a previously unrecognized link between cytoplasmic signaling proteins and the Mediator complex.
Results and Discussion
Elmo1 binds the Mediator complex subunit Med31
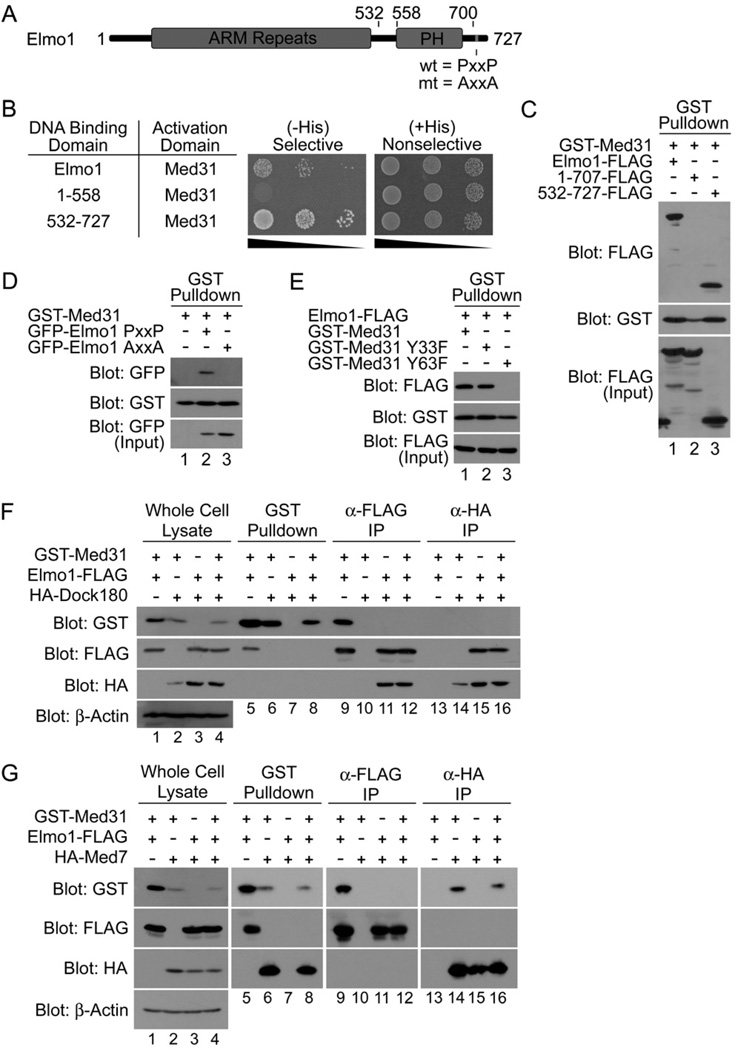
In a yeast two-hybrid screen where full-length murine Elmo1 (amino acids 1–727) (Fig. 1A) was used as bait, we unexpectedly identified Med31 as a binding partner from a 7-day mouse embryo library (Fig. 1B). The Elmo1:Med31 interaction was confirmed in mammalian cells, where Med31 coprecipitated full-length Elmo1 from lysates (Fig. 1C) under a variety of salt and detergent concentrations (Fig. S1), suggesting a stable interaction. Elmo1 binding to Med31 also occurred at endogenous levels of Med31 (Fig. S2). Further mapping of the interaction in the yeast two-hybrid system and in mammalian cells revealed that Med31 interacts with the C-terminal portion (amino acids 532–727) of Elmo1, but not the N-terminal regions (Fig. 1B and 1C). Deletion of the C-terminal 20 amino acids of Elmo1 (1–707), which contains a PxxP motif, failed to bind Med31 (Fig. 1C). Proline to alanine mutations within the PxxP motif of full-length Elmo1 (Elmo1 AxxA) abrogated the Elmo1-Med31 binding (Fig. 1D), suggesting a critical role for this PxxP motif. These observations suggested a novel and unexpected interaction between a predominantly nuclear protein Med31 and a cytoplasmic signaling protein Elmo1.
Figure 1. The engulfment protein Elmo1 interacts with the Mediator complex subunit Med31.
A. Schematic representation of Elmo1 protein. C-terminal PxxP (wild type) and AxxA (mutant) motifs are indicated. PH – pleckstrin homology. B. In a yeast two-hybrid assay, Med31 interacts with full length Elmo1 and the C-terminal PxxP-containing fragment of Elmo1 (532–727), but not with a PxxP-deletion mutant of Elmo1 (1–558), as determined by growth on selective (His-Leu-Trp-) and nonselective (His+Leu-Trp-) media at ten-fold serial dilutions. C. The C-terminal PxxP motif of Elmo1 is required for the interaction with Med31. The interaction in mammalian cells between GST-Med31 and Elmo1-FLAG, or the Elmo1 fragments, were analyzed by transiently expressing the indicated proteins in 293T cells, and analysis by precipitations and immunoblotting as shown. D. Mutation of the Elmo1 PxxP motif to AxxA abolishes the interaction with Med31. E. Med31 residue Y63 is required for binding to Elmo1. F. Med31:Elmo1 and Elmo1:Dock180 complexes are distinct. Epitope-tagged Med31, Elmo1 and Dock180 were transiently expressed as indicated, precipitated via epitope-tag, and immunoblotted as shown. G. Elmo1 is not part of the Med31:Med7 subcomplex. Indicated proteins were expressed and their association was determined by precipitation with the indicated tags and immunoblotting.
Elmo1 binds the same region of Med31 involved in binding to Med7
The structure of yeast Med31, co-crystallized with the N-terminal region of another Mediator subunit Med7 (Med7N), revealed that the hydroxyl groups of two evolutionarily conserved tyrosine residues in Med31 (Y33 and Y63) were in close proximity to proline rich regions of Med7N [16] (Fig. S3). To test whether Med31 may use these tyrosines to bind the PxxP motif of Elmo1, we introduced phenylalanine substitutions (Y33F or Y63F) into murine Med31. The Y33F, but not the Y63F mutant, retained the ability to coprecipitate Elmo1 (Fig. 1E). These data identified Y63 in Med31 and the PxxP motif within Elmo1 as key components of the interaction, and suggested that other proline-rich containing proteins can replace Med7N in binding to Med31.
Elmo1:Med31 interaction is distinct from the Elmo1:Dock180 complex
To date, all of the functions associated with Elmo1 in mammalian and C. elegans models are mediated through its interaction with the large protein Dock180, with the Elmo1:Dock180 complex functioning as a bipartite GEF for the GTPase Rac [18]. We asked whether Med31 can be part of the Elmo1:Dock180 complex. While Elmo1 could readily associate with either Med31 or Dock180 (Fig. 1F Lanes 5,9,11 and 15), Med31 did not bind Dock180 nor was it part of the Elmo1:Dock180 complex (Fig. 1F Lanes 6,8,10,12,14 and 16). When all three proteins were coexpressed, Elmo1 appeared to favor Dock180 binding over Med31 (Fig. 1F Lanes 8, 12 & 16). These data suggest that the Elmo1:Med31 complex is distinct from the Elmo1:Dock180 complex.
As a corollary, we asked whether Elmo1 might associate with the Med31:Med7 subcomplex. While Med31 could associate independently with both Elmo1 (Fig. 1G Lane 5 & 9) and Med7 (Fig. 1G Lane 6 & 14), Elmo1 was not found in the same complex with Med7 when coexpressed with or without Med31 (Fig. 1G). Although qualitative, when all three proteins were overexpressed, Med31 appeared to favor binding Med7 over Elmo1 (Fig. 1G Lanes 8, 12 & 16). These data suggest that the Elmo1:Med31 complex is distinct from the Med31:Med7 complex, and by extrapolation, the larger Mediator complex.
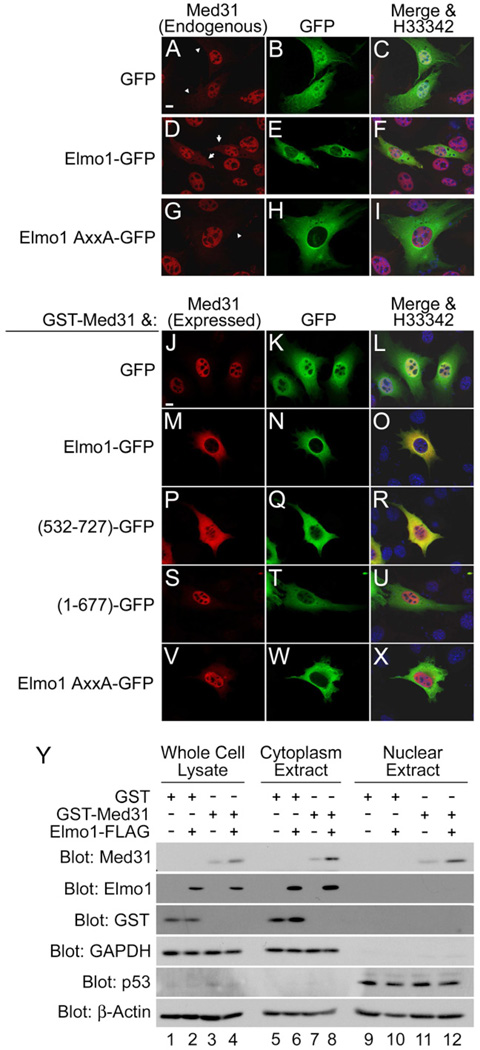
Elmo1 promotes relocalization of Med31 from the nucleus to the cytoplasm
Med31 is thought to be a predominantly nuclear protein. Consistent with this, we observed by fluorescence microscopy that endogenous Med31 was largely nuclear localized (Fig. 2A-C) though faint cytoplasmic localization was reproducibly detected. Additionally, overexpressed Med31 is also primarily nuclear localized (Fig. 2J-L). Surprisingly, expression of Elmo1 in cells resulted in increased cytoplasmic localization of endogenous Med31 (Fig. 2D-F) in 85% of Elmo1-expressing cells. In contrast, expression of the Elmo1 AxxA mutant that does not bind Med31 did not affect the subcellular localization of endogenous Med31 (Fig. 2G-I). Elmo1 also resulted in increased cytoplasmic localization of overexpressed Med31 (Fig. 2M-O). The C-terminal portion of Elmo1 (532–727) that is sufficient to bind Med31 also resulted in cytoplasmic translocation of Med31 (Fig. 2P-R). Med31 remained largely nuclear when coexpressed with mutants of Elmo1 unable to bind Med31, either the PxxP deletion mutant (Fig. 2S-U) or the Elmo1 AxxA mutant (Fig. 2V-X).
Figure 2. Elmo1 interacts with Med31 in the cytoplasm.
(A-C) Endogenous Med31 in NIH/3T3 cells is primarily nuclear localized, although cytoplasmic localization is also noticed. (D-F) expression of Elmo1-GFP resulted in increased levels of cytoplasmic Med31 in 85% of transfected cell compared to only 15% of cells expressing (G-I) Elmo1 AxxA-GFP. Transfected cells with (arrow) and without (arrowhead) increased cytoplasmic localization of endogenous Med31. (J-X) Med31 shows increased cytoplasmic localization when coexpressed with Elmo1 proteins or fragments that interact with Med31. Cells expressing GST-Med31 with GFP-tagged versions of the indicated Elmo1 proteins were visualized by immunofluorescence. Scale bar is 10µm. Y. Immunoblotting of whole cell lysates, cytoplasmic extracts, and nuclear extracts from 293T cells expressing GST-Med31, Elmo1-FLAG, or both. GAPDH was used as a cytoplasmic marker, p53 as a nuclear marker, and β-actin as a loading control.
Biochemical fractionation studies also revealed that Elmo1 was largely cytoplasmic, with minimal detection in nuclear extracts (Fig. 2Y Lanes 6,8,10 and 12). However, Med31 was detectable in both nuclear and cytoplasmic fractions (Fig. 2Y Lanes 7, 8, 11 and 12), consistent with the immunofluorescence imaging (Fig. 2J-O). The level of Med31 in the cytoplasmic fraction increased in the presence of Elmo1. These data suggest that the Elmo1:Med31 interaction occurs predominantly in the cytoplasm. To our knowledge, this is the first demonstration of a cytoplasmic binding partner of Med31.
Mice and humans express three Elmo homologs, Elmo1, Elmo2, and Elmo3. Elmo1 is ubiquitously expressed, with enriched expression in cells of the monocytes/macrophage lineage, while Elmo2 and Elmo3 have more restricted expression patterns [1]. Elmo1 and Elmo2 readily associated with Med31, while Elmo3 did not bind Med31 (Fig. S4A). Consistent with this result, coexpression of Med31 with Elmo1 or Elmo2 resulted in increased cytoplasmic localization of Med31 (Fig. S4E-J), while coexpression with Elmo3 did not (Fig. S4K-M). Collectively, these data reveal that Elmo1 and Elmo2 proteins promote the cytoplasmic localization of Med31, dependent upon the C-terminal PxxP motif-mediated binding to Med31.
Med31 and Elmo1 are required for specific gene expression of Il33 and Il10 during Salmonella infection
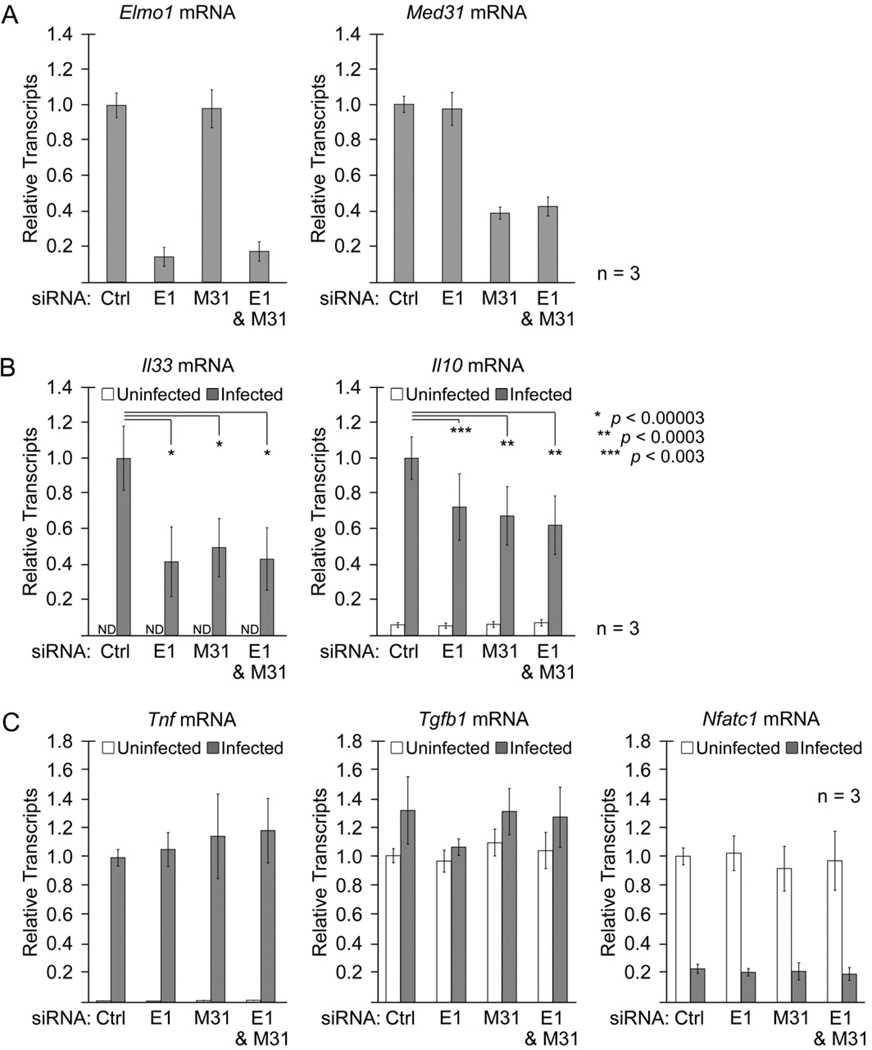
Previously in a Drosophila cell culture model, dMed31 was shown to be required for expression of lipopolysaccharide (LPS)- and heat shock-activated endogenous genes, but dispensable for transcription of synthetic promoters [19]. Recent studies have also revealed that BAI1, the membrane receptor upstream of Elmo1 can engage the LPS of Gram-negative bacteria [6], and that the BAI1:Elmo1 module can mediate uptake of these bacteria into macrophages. Therefore, we asked whether murine Med31 and Elmo1 play a role in LPS-activated gene expression in primary bone marrow-derived macrophages (BMDM). We performed siRNA-mediated knockdown of Elmo1, Med31, or both Elmo1 and Med31 in the BMDM. Forty-eight hours after siRNA transfection, Elmo1 transcript levels were reduced by ~ 85%, while Med31, transcript levels were reduced by ~ 60% (Fig. 3A). siRNA knockdown of Elmo1 did not result in the compensatory upregulation of Elmo2 (Table S1) observed in BMDM isolated from Elmo1–deficient mice [20], nor were Elmo3 transcript levels affected (Table S1). The siRNA-transfected BMDMs were then infected with the Gram-negative enteric pathogen Salmonella enterica serovar Typhimurium (with a ΔinvG bacterial strain that depends on Bai1-Elmo1-dependent cellular entry). After 6hr, we profiled the BMDM for 25 different gene transcripts, including genes encoding for cytokines, transcription factors, and cell cycle regulators via quantitative RT-PCR (qRT-PCR) (Table S2). We categorized the genes into three groups based on change in expression after Salmonella infection: genes whose expression was upregulated >2-fold from uninfected BMDMs, those whose expression was lowered >2-fold from uninfected BMDMs, and those genes that had no change in expression after infection.
Figure 3. siRNA knockdown of Med31 and Elmo1 decreases transcription of specific genes in BMDM after Salmonella infection.
A. qRT-PCR analysis of knockdown efficiency in BMDMs transfected with siRNA targeting Elmo1 (E1) and/or Med31 (M31). Total siRNA transfected in each sample was kept constant by addition of control (Ctrl) siRNA. B. Analysis of Il33 and Il10 transcript levels from siRNA-transfected BMDMs, either 6hr post Salmonella (ΔinvG) infection (grey bars) or uninfected (white bars), demonstrating a statistically significant decrease in transcript levels with knockdown of Elmo1 or Med31. C. siRNA knockdown of Elmo1 or Med31 in BMDM cells in response to Salmonella (ΔinvG) infection does not affect other transcripts with three examples shown here: a gene that was upregulated post infection (Tnf), one transcript unchanged with infection (Tgfb1), and one transcript downregulated after infection (Nfatc1). All transcript levels were normalized to the reference gene Hprt1. Data are represented as mean +/− standard deviation. Data for other transcripts analyzed is shown in Supplemental Table S2.
Among the 12 genes upregulated during Salmonella infection (Table S2), two genes encoding cytokines interleukin 33 (IL-33) and interleukin 10 (IL-10) were particularly sensitive to loss of Elmo1 and Med31. Basally, Il33 transcript levels were not detectable in uninfected BMDMs, but Il33 expression was increased following Salmonella infection (Fig. 3B). Knockdown of Med31 reduced the upregulation of Il33 by >50%; similarly, knockdown of Elmo1 also decreased Il33 expression >50% after Salmonella infection (Fig. 3B). BMDMs express low levels of Il10 transcripts in the uninfected state, but infection results in a >15-fold increase in Il10 expression (Fig. 3B). Knockdown of either Med31 or Elmo1 inhibited the upregulation of Il10 (Fig. 3B). Interestingly, concurrent knockdown of both Med31 and Elmo1 did not further decrease the transcript levels of Il33 or Il10 (Fig. 3B). One likely explanation is that Elmo1 and Med31 might function in the same pathway downstream of Salmonella infection. This requirement of Elmo1 and Med31 for upregulation of Il33 and Il10 cytokine genes after Salmonella infection appeared specific, as their knockdown did not affect upregulation of the gene encoding tumor necrosis factor (Tnf) (Fig. 3C).
Importantly, knockdown of Elmo1 and Med31 did not affect the basal transcript level of any of the genes analyzed; similarly, the transcript levels of genes that were downregulated (4 genes) or unchanged (9 genes) in response to Salmonella infection (Table S2) were also unaffected by loss of Elmo1 or Med31. All cytokine genes measured were upregulated following infection, with the exception of the gene encoding for transforming growth factor β1 (Tgfb1) whose expression was unchanged (Fig. 3C). Three of the four genes that were downregulated after infection encode for transcription factors, including the nuclear factor of activated T-cells (NFAT)c1 gene (Nfatc1) (Fig. 3C). Knockdown of Elmo1Med31, or both, had no effect on Tgfb1 or Nfatc1 gene expression, suggesting that siRNA knockdown of Elmo1 and Med31 does not impact transcription globally. Interestingly, all three genes unaffected by the siRNA knockdown of Elmo1 and Med31 (TnfTgfb1, and Nfatc1) have high levels of basal expression in the uninfected state; since Pol II-dependent transcription requires the Mediator complex, these data suggest Med31 is dispensable for expression of some genes, and that separate regulatory mechanisms likely exist for basal and activated gene transcription.
Elmo1 promotes the ubiquitination of Med31
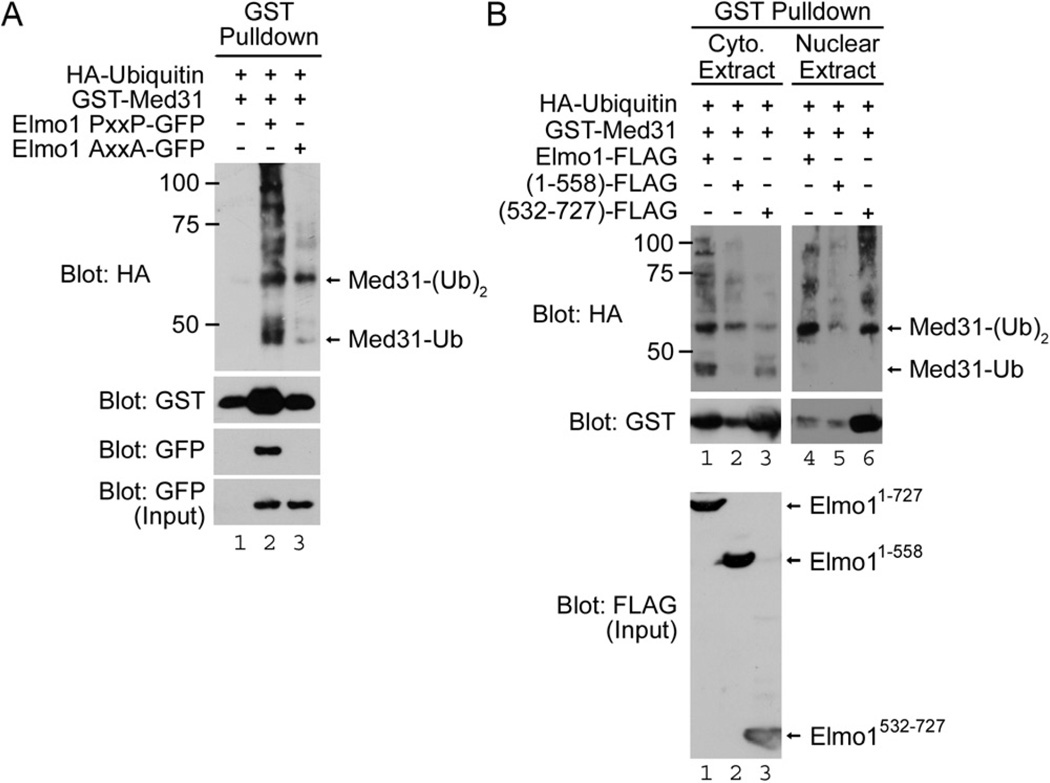
Elmo1 binding has been linked to modification of the ubiquitination state of Dock180, wherein coexpression of Elmo1 with Dock180 decreased the poly-ubiquitination state of Dock180, and in turn, improved the stability/half life of Dock180 [21]. We asked whether the Med31 ubiquitination status might be influenced by Elmo1. Surprisingly, the overall ubiquitination status of Med31 was increased when coexpressed with Elmo1 (Fig. 4A). Based on the predicted molecular weight of proteins migrating on SDS-PAGE, we observed two prominent bands that corresponded to mono- and di-ubiquitinated Med31 (Med31-Ub and Med31-(Ub)2, respectively), in addition to higher polyubiquitinated forms (Fig. 4A). While polyubiquitination (>4 ubiquitin moieties) is often linked to proteosomal degradation of the ubiquitin-modified protein [22], mono- and di-ubiquitination have been linked to signaling (e.g. in gene transcription via NF-κB) [23–25].
Figure 4. Elmo1 promotes the ubiquitination of Med31.
A. Elmo1 promotes the mono- and di-ubiquitination of Med31. Immunoblotting of GST precipitates from cell lysates expressing HA-Ubiquitin, GST-Med31, and Elmo1-GFP (wild type, PxxP) or Elmo1 AxxA-GFP (mutant). Molecular weight makers are indicated. B. Wild type Elmo1 promotes the cytoplasmic mono-ubiquitination of Med31. Immunoblotting of GST precipitations from cytoplasmic and nuclear extracts of cells expressing HA-Ubiquitin, GST-Med31, and Elmo1-FLAG, or Elmo1 truncation mutants 1–558 and 532–727. Mono-ubiquitinated Med31-Ub was primarily found within the cytoplasmic extract, while Med31-(Ub)2 was observed within both cytoplasmic and nuclear extracts. Coexpression of Med31 with either full length (1–727) or PxxP-containing fragment (532–727) of Elmo1 enhanced the levels of both cytoplasmic Med31-Ub and nuclear Med31-(Ub)2 compared to coexpression with a PxxP-deletion mutant of Elmo1 (1–558).
While increased levels of di-ubiquitinated Med31-(Ub)2 were observed with coexpression of both Elmo1 and Elmo1 AxxA (Fig. 4A), mono-ubiquitinated Med31 was present at higher levels when coexpressed with wild type Elmo1. As further support for the Elmo1:Med31 binding regulating Med31 ubiquitination status, the Elmo1 AxxA mutant did not increase the mono-ubiquitinated form (Fig. 4A). When we examined the subcellular localization of ubiquitinated forms of Med31, the Med31-Ub was found primarily in the cytoplasm (Fig. 4B), while Med31-(Ub)2 was readily detectable in both cytoplasmic and nuclear fractions (Fig. 4B). Coexpression with either full length or the C-terminal portion of Elmo1 resulted in increased cytoplasmic Med31-Ub, while an N-terminal mutant of Elmo1 that lacks the PxxP motif did not (Fig. 4B). Collectively, these results reveal that Elmo proteins that interact with Med31 could promote the mono-ubiquitination of Med31 in the cytoplasmic fractions.
Since ubiquitination occurs at lysine residues, we aligned the Med31 protein sequences from yeast to human (Fig. S3) and mutated the two conserved lysine residues within the central consensus sequence to arginine residues (K44R and K53R) and tested whether they would inhibit ubiquitination of Med31. Unfortunately, mutation of these two conserved lysine residues did not disrupt the ubiquitination of Med31 (data not shown), suggesting that ubiquitination may occur at a non-conserved lysine residue in murine Med31, or perhaps compensatory ubiquitination may occur at more than one site on Med31.
Collectively, the data presented in this report makes several interesting observations. First, this work identifies cytoplasmic Elmo1 as a previously unrecognized partner of the Med31 subunit of the Mediator complex. Second, Elmo1 alters the cytoplasmic versus nuclear distribution of Med31. Third, we identify a previously unknown post-translational modification of Med31 and the mono-ubiquitinated form is modified by Elmo1. Fourth, in a physiological context where Elmo1 has previously been shown to play a role, Med31 and Elmo1 are required for the activated expression of the same subset of genes in primary macrophages; in particular, during the response of immune cells to Salmonella infection, both Elmo1 and Med31 influence the same set of genes, suggesting a possible conserved role for Med31 in LPS-activated gene expression. Interestingly, among the >25 genes analyzed, Elmo1 and Med31 were required for transcription of the cytokine genes Il33 and Il10, suggesting a specificity in the expression of genes controlled by these two molecules.
The Mediator complex has been reported to include over 30 subunits, and many subunits are believed to influence a distinct subset of genes through their myriad protein-protein interactions with non-Mediator complex proteins [11, 14], but the role of cytoplasmic proteins in regulating specific Mediator subunits has been unclear. Given the striking cytoplasmic relocalization of Med31 when coexpressed with Elmo1, our initial hypothesis was that Elmo1 might affect Med31-dependent gene transcription by sequestering Med31 in the cytoplasm. Our siRNA studies in macrophages are inconsistent with a simple sequestration model. Although our mutational studies were unsuccessful in identifying the specific lysine residues ubiquitinated on Med31, future studies targeting multiple lysines might provide insights on how ubiquitination may influence Med31 interactions with other components of the Mediator complex. Since Elmo1 and Med31 (as well as the Mediator complex) are highly evolutionarily conserved, this work suggests new considerations on the regulation of specific genes during biological processes, including pathogen-induced gene transcription.
Experimental Procedures
Preparation of bone marrow-derived macrophages and Salmonella infection
Bone marrow-derived macrophages were generated from C57BL/6 donor mice as previously described [26] and maintained in differentiation media consisting of Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum, 10% L929-conditioned media and antibiotics. Using Amaxa mouse macrophage nucleofector kit (Lonza; Basel, Switzerland) BMDMs were transfected with an siGENOME SMARTpool containing four siRNAs targeting mouse Elmo1 (M-041254-00: Dharmacon; Lafayette, CO) and/or Med31 (M-047061-01) then incubated for 48hr to recover; total siRNA was kept constant with siGENOME non-targeting siRNA pool #2 (D-001206-14). siRNA-transfected BMDM were plated at 1.0×105 cells/well in 96 well plates and infected with Salmonella enterica serovar Typhimurium (ΔinvG) as previously described [6]. BMDM mRNA was isolated 6hr post infection using RNeasy kit (QIAGEN; Valencia, CA). cDNA was generated from isolated mRNA using random primers and the SuperScript III First-Strand Synthesis kit (Life Technologies; Carlsbad, CA). Transcript levels were measured by qRT-PCR using TaqMan gene expression assays (see Supplemental Methods) and One-Step Plus RT-PCR instrument (Life Technologies). Results were normalized to transcripts of the reference gene Hprt1.
Please see Supplementary data for the detailed materials and methods.
Supplementary Material
Highlights.
Elmo1 interacts with the transcriptional Mediator complex subunit Med31
The binding of Elmo1 to Med31 promotes translocation Med31 to the cytoplasm
Elmo1 and Med31 are needed for the upregulation of cytokine genes Il10 and Il33
Elmo1 influences the ubiquitination state of Med31
Acknowledgments
We thank the members of the Ravichandran laboratory for helpful discussions and Jason Kinchen for careful reading of this manuscript. This work was supported by a grant from the NIH to Kodi S. Ravichandran (GM-64998).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 2.Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–582. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 3.Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, Chen Q, Klingele D, Hengartner MO, Ravichandran KS. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem. 2004;279:6087–6097. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- 4.Lu M, Ravichandran KS. Dock180-ELMO cooperation in Rac activation. Methods Enzymol. 2006;406:388–402. doi: 10.1016/S0076-6879(06)06028-9. [DOI] [PubMed] [Google Scholar]
- 5.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Owen KA, Ly KT, Park D, Black SG, Wilson JM, Sifri CD, Ravichandran KS, Ernst PB, Casanova JE. Brain angiogenesis inhibitor 1 (BAI1) is a pattern recognition receptor that mediates macrophage binding and engulfment of Gram-negative bacteria. Proc Natl Acad Sci U S A. 2011;108:2136–2141. doi: 10.1073/pnas.1014775108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handa Y, Suzuki M, Ohya K, Iwai H, Ishijima N, Koleske AJ, Fukui Y, Sasakawa C. Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery. Nat Cell Biol. 2007;9:121–128. doi: 10.1038/ncb1526. [DOI] [PubMed] [Google Scholar]
- 8.Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends Biochem Sci. 2005;30:235–239. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Malik S, Roeder RG. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet. 2010;11:761–772. doi: 10.1038/nrg2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taatjes DJ. The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem Sci. 2010;35:315–322. doi: 10.1016/j.tibs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conaway RC, Conaway JW. Function and regulation of the Mediator complex. Curr Opin Genet Dev. 2011;21:225–230. doi: 10.1016/j.gde.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imasaki T, Calero G, Cai G, Tsai KL, Yamada K, Cardelli F, Erdjument-Bromage H, Tempst P, Berger I, Kornberg GL, et al. Architecture of the Mediator head module. Nature. 2011;475:240–243. doi: 10.1038/nature10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soutourina J, Wydau S, Ambroise Y, Boschiero C, Werner M. Direct interaction of RNA polymerase II and mediator required for transcription in vivo. Science. 2011;331:1451–1454. doi: 10.1126/science.1200188. [DOI] [PubMed] [Google Scholar]
- 14.Hentges KE. Mediator complex proteins are required for diverse developmental processes. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Bourbon HM, Aguilera A, Ansari AZ, Asturias FJ, Berk AJ, Bjorklund S, Blackwell TK, Borggrefe T, Carey M, Carlson M, et al. A unified nomenclature for protein subunits of mediator complexes linking transcriptional regulators to RNA polymerase II. Mol Cell. 2004;14:553–557. doi: 10.1016/j.molcel.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Koschubs T, Seizl M, Lariviere L, Kurth F, Baumli S, Martin DE, Cramer P. Identification, structure, and functional requirement of the Mediator submodule Med7N/31. The EMBO journal. 2009;28:69–80. doi: 10.1038/emboj.2008.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risley MD, Clowes C, Yu M, Mitchell K, Hentges KE. The Mediator complex protein Med31 is required for embryonic growth and cell proliferation during mammalian development. Dev Biol. 2010;342:146–156. doi: 10.1016/j.ydbio.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Lu M, Kinchen JM, Rossman KL, Grimsley C, deBakker C, Brugnera E, Tosello-Trampont AC, Haney LB, Klingele D, Sondek J, et al. PH domain of ELMO functions in trans to regulate Rac activation via Dock180. Nat Struct Mol Biol. 2004;11:756–762. doi: 10.1038/nsmb800. [DOI] [PubMed] [Google Scholar]
- 19.Kim TW, Kwon YJ, Kim JM, Song YH, Kim SN, Kim YJ. MED16 and MED23 of Mediator are coactivators of lipopolysaccharide- and heat-shock-induced transcriptional activators. Proc Natl Acad Sci U S A. 2004;101:12153–12158. doi: 10.1073/pnas.0401985101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott MR, Zheng S, Park D, Woodson RI, Reardon MA, Juncadella IJ, Kinchen JM, Zhang J, Lysiak JJ, Ravichandran KS. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature. 2010;467:333–337. doi: 10.1038/nature09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makino Y, Tsuda M, Ichihara S, Watanabe T, Sakai M, Sawa H, Nagashima K, Hatakeyama S, Tanaka S. Elmo1 inhibits ubiquitylation of Dock180. J Cell Sci. 2006;119:923–932. doi: 10.1242/jcs.02797. [DOI] [PubMed] [Google Scholar]
- 22.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. The EMBO journal. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harhaj EW, Dixit VM. Deubiquitinases in the regulation of NF-kappaB signaling. Cell Res. 2011;21:22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Chen ZJ. Expanding role of ubiquitination in NF-kappaB signaling. Cell Res. 2011;21:6–21. doi: 10.1038/cr.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strieter ER, Korasick DA. DA Unraveling the complexity of ubiquitin signaling. ACS chemical biology. 7:52–63. doi: 10.1021/cb2004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Chapter 14. In: Coligan John E, et al., editors. Current protocols in immunology. 2008. Unit 14 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.