Abstract
Introduction
Age-related decreases in tongue muscle mass and strength have been reported. It may be possible to prevent age-related tongue muscle changes using neuromuscular electrical stimulation (NMES). Our hypothesis was that alterations in muscle contractile properties and myosin heavy chain composition would be found following NMES.
Methods
Fifty-four young, middle-aged and old Fischer 344/Brown Norway rats were included. Twenty-four rats underwent bilateral electrical stimulation of the hypoglossal nerves for 8 weeks and were compared with control or sham rats. Muscle contractile properties and myosin heavy chain (MHC) in the genioglossus (GG), styloglossus (SG) and hyoglossus (HG) muscles were examined.
Results
In comparison with unstimulated control rats, we found reduced muscle fatigue, increased contraction and half decay times and increased twitch and tetanic tension. Increased Type I MHC was found, except for GG in old and middle-aged rats.
Discussion
Transitions in tongue muscle contractile properties and phenotype were found following NMES.
Keywords: muscle contraction, tongue, electrical stimulation, aging, swallowing
Introduction
It is well known that strength, endurance and sensorimotor function are affected by the aging process and may be due to reductions in muscle mass and cross sectional area, reduction in number or size of muscle fibers, transformation or selective loss of specific muscle fiber types9,18,22,26,48 and reduction in muscle strength. 46 In addition to age-related muscular changes in the extremities, decrements in critical cranial functions have also been reported with increasing age, including problems with swallowing. 12,15,24,37,62 Older individuals swallow more slowly in a manner that may compromise airway penetration and increase the risk of aspiration.23 Tongue muscle atrophy and/or weakness may contribute to degradation in deglutitive function. 5,13,28,33,36,38,47,49,61
Skeletal muscles, including muscles of the tongue, are capable of considerable plasticity, including alterations in strength and phenotype. 44,50,60 Recent studies in humans and rats suggest that targeted tongue exercise49,53,55,61,71 or neuromuscular electrical stimulation (NMES) 60,74 are associated with alterations in tongue strength, physiology, phenotype and neuromuscular junction morphology. However, the manner in which these alterations may contribute to physiological changes in tongue muscle contraction or how they may be applicable to muscle plasticity for aging lingual muscles has not been well-studied.
We tested the hypothesis that alterations in muscle contractile and biochemical properties of lingual muscles would be found after 8 weeks of bilateral hyoglossal nerve stimulation in rats. We compared physiologic and biochemical measures of 3 extrinsic tongue muscles (genioglossus, GG; hyoglossus, HG; and styloglossus, SG) in young adult, middle-aged, and old rats under control, stimulated, and sham (unstimulated) conditions.
Methods
This study was performed in accordance with the PHS policy on care and use of laboratory animals, the NIH guide for care and use of laboratory animals, and the animal welfare act. The animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin-Madison School of Medicine and Public Health.
Animal Subjects and Experimental Design Overview
Data from 54 male Fischer 344/Brown Norway rats are reported. This strain has a median lifespan of approximately 33 months for males. 34 As shown in Table 1, 26 rats were 9 months of age (Young Adult), 13 were 24 months old (Middle-Aged group), and 15 were 32 months old (Old group). The rats were assigned randomly into either an experimental group (Stimulation Group; n = 24) that underwent implantation of an electrode assembly and received chronic bilateral electrical stimulation of the hypoglossal nerves for 8 weeks, or a Control Group (n = 20) that did not receive electrical stimulation, but were housed in our animal care facility for 8 weeks. In addition, 10 young adult rats were assigned to a Sham Group that received electrode implantation but did not receive any nerve stimulation across 8 weeks of observation.
Table 1.
Sample sizes for each age group and stimulation treatment condition. Rats in the Stimulation Group received bilateral hypoglossal nerve stimulation 5 days per week for 8 weeks. Control Group rats did not receive stimulation. Likewise, Sham Group rats did not receive stimulation but were implanted with the stimulation electrode assemblies bilaterally.
| Stimulation (n) | Control (n) | Sham (n) | |
|---|---|---|---|
| Young Adult | 8 | 8 | 10 |
| Middle Aged | 7 | 6 | 0 |
| Old | 9 | 6 | 0 |
Following the 8-week study period, tongue muscle contractile properties were recorded in vivo for all 54 rats. Animals were then anesthetized and euthanized, and the genioglossus (GG), hyoglossus (HG) and styloglossus (SG) muscles were harvested, frozen, and stored at minus 80° C for later analysis. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed, and gels were silver-stained and analyzed to determine the myosin heavy chain composition of the muscles of interest.
Implantation of hypoglossal stimulation electrodes and chronic electrical stimulation
Rats randomly assigned to the Stimulation Group or Sham Group (18 Young Adult rats, 7 Middle Aged rats, and 9 Old rats) were anesthetized with isoflurane. Following induction, each animal was clipped, prepped, and placed in sternal recumbancy on a water-filled heat blanket to regulate body temperature that was maintained at 37°C throughout surgery. Each rat was implanted with a prefabricated electrode assembly for chronic stimulation of the hypoglossal nerves modified from that developed by Zealear. 17 The assembly consisted of a plastic Dacron skin plug for making connections to an external stimulator, 6 coiled Teflon-coated multistranded stainless steel lead wires, and 2 nerve cuffs made of silastic tubing. The pair of nerve cuffs were equipped with two electrodes for hypoglossal nerve stimulation, and the remaining 2 leads served as electrodes for recording electromyographic (EMG) responses from the tongue base that were evoked by hypoglossal nerve stimulation on each side. Tongue recordings made later in awake animals were referenced to an electrode placed on the tail. The tongue EMG electrodes were included in the assembly to insure that hypoglossal nerve damage did not occur from the cuffs over the course of the experiment. Three to 4 animals were implanted at a time. The animals had a recovery period of 4 weeks to allow skin incision healing and implant fibrosis.
A Grass S88 stimulator was employed for nerve stimulation in the Stimulation Group animals only and was administered 5 days per week for 8 weeks. The protocol consisted of 5 sets of 12 repetitions, where each repetition included 1.0 sec of stimulation followed by a 1.0 sec rest period. There was a 2 min rest interval between sets. Pulses were 0.2 ms in width and were delivered at 40 Hz. A stimulation frequency of 40 Hz was used, because it has been shown to maximally activate slowly contracting, fast-fatiguing and fast-fatigue-resistant motor units3, has been used successfully in previous studies of muscle fatigue in rat hindlimb, 7 and represents an approximate midpoint between low (10 Hz) and high (100 Hz) frequencies used previously in the literature. 4,8,10,42,60 We used supramaximal current intensity, defined as 1.5 times the current level needed to generate maximum tongue tension, which varied for each rat (usually between 300 – 500 μA). A 1.0 sec rest interval between stimulus trains allowed sufficient time for recovery of blood flow and perfusion of tongue muscles. Sham Group rats did not receive any hypoglossal nerve stimulation.
Muscle Contractile Property Recordings
Recording of tongue muscle contraction properties was performed 1–7 days following the 8 weeks of electrical stimulation. This 7-day time period was required to allow completion of experiments for all rats. We do not expect that this 1–7 day delay introduced detraining artifacts, but detraining studies in the rat tongue as a function of age have not been performed and we cannot completely rule out this possibility. Although not completely relevant to the current work, a recent study involving treadmill running in normal Wistar rats found that a 1-week detraining period did not alter functional capacity on an exercise test. 73
On the day of the experiment, body weights were recorded, and animals were anesthetized via intraperiotoneal injection of sodium pentobarbital (70 mg/kg). Each animal was placed in the dorsal recumbent position under an operating microscope (Zeiss). For the Control Group rats, the hypoglossal nerves were exposed bilaterally using a ventral approach to allow access for the nerve cuff stimulation electrodes. This surgical approach is well documented in the literature. 30,52,67 Nerve exposure was not necessary for the Stimulation Group and Sham Group rats, because these animals were previously implanted with stimulation electrode assemblies. The core temperature of each animal was monitored at all times and was maintained at 37 – 39° C. A small suture was placed into the tip of the tongue for connection to a force transducer (Kent Scientific, Torrington, CT).
Whole Nerve Stimulation
The tongue was protruded manually from the mouth during the experiment. Optimal direction and line tension on the suture were determined for each animal to yield maximum peak muscle twitch forces. Whole hypoglossal nerves were then stimulated bilaterally via the electrode cuffs surrounding the nerve, and retrusive tongue muscle contractile properties were recorded. These isolated hypoglossal nerve stimulation pulses (1-Hz rectangular-wave pulses, pulse width 0.1 ms) were delivered at supramaximal levels. Supramaximal stimulation levels (1.5 times maximum stimulation level; generally between 300 and 500 μA; A-M Systems, Carlsborg, WA) controlled for small differences in stimulation electrode placement. These stimulation parameters have been reported previously. 19 Three 10-sec trials with a 1-min rest period between trials were recorded.
The following measurements were made: (1) twitch contraction time (CT), the interval (ms) between the onset of stimulation and the point of 50% maximal twitch tension; (2) half-decay time (HDT), the interval (ms) between the onset of stimulation and the point of 50% decay from peak twitch tension; (3) maximum twitch tension, the peak tension [g] generated following a single electrical stimulus; (4) tetanic tension, the maximal tension (g) of each stimulated fused wave; and (5) fatigue index, determined from repetitive stimulation. That is, tongue muscles were stimulated repeatedly at 100 Hz for 2 minutes. The fatigue index was calculated by constructing a ratio of the average tetanic tension (g) at the end of 2 minutes of stimulation relative to the initial tetanic tension (g) and multiplying by 100 to express the value as the percentage of initial tension. A high fatigue index indicated a resistance to fatigue. 25,35
Medial Branch Stimulation
Following retrusive tongue whole-hypoglossal nerve stimulation, a 2–5 mm section of the lateral branch of the hypoglossal nerve was removed bilaterally. The animal was repositioned to optimize direction and line tension for forthcoming elicited protrusive tongue actions. Following a 45-min stabilization period, whole hypoglossal nerves were then stimulated again. Due to the lateral hypoglossal branch section, only the medial branch was effectively stimulated, and protrusive muscle contractile properties were measured. The same measurements were made for protrusive muscle contractions as described above for retrusive actions.
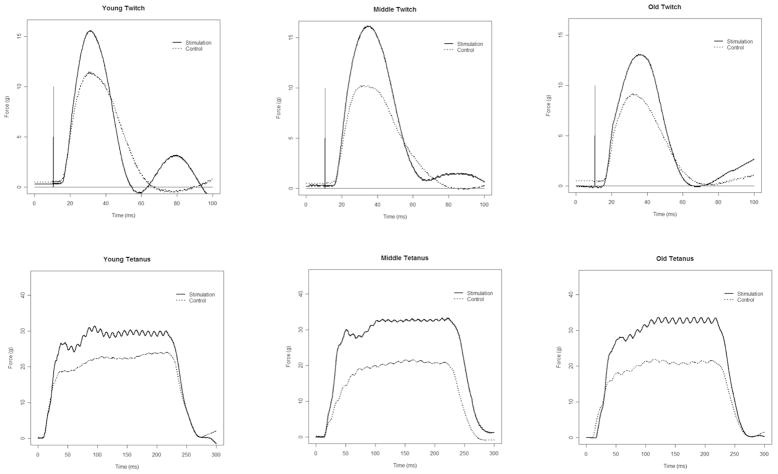
The stimulation signal and tongue force signal were acquired digitally on a dedicated laboratory computer equipped with an A/D converter (Data Translation, Marlboro, MA) using data acquisition software written and customized for our use (Acquire Ver. 1.3.0). Representative twitch and tetanic tension signals from a Young, Middle-aged and Old animal in the Stimulation and Control Groups are shown in Figure 1.
Figure 1.
Representative twitch and tetanic tension signals during medial nerve stimulation from Young Adult, Middle-aged and Old animals in the Stimulation Group and Control (no stimulation) Group. Significantly greater twitch and tetanic tension were found in the Stimulation Group in comparison with the Control Group (p<.05)
Following data collection, the transducer was disconnected from the tongue, and the anesthetized animal was euthanized by an overdose of Beuthanasia via intracardiac injection. The genioglossus (GG), hyoglossus (HG), and styloglossus (SG) muscles on both sides were then quickly extracted and suspended in oxygenated Ringers solution. Muscles were wrapped in foil, labeled, frozen in liquid nitrogen, and then stored in a minus 80° C freezer.
Myosin Heavy Chain
Using a cryostat, 60 μm sections were made of the frozen GG, HG and SG muscles from the left or right side of the tongue in each animal, as determined by a previously assigned random schedule. SDS-PAGE was performed with a 0.75 mm thick 6% acrylamide/30% glycerol separating gel (18 × 16 cm) and a 4% acrylamide/30% glycerol stacking gel using small bundles of muscle fibers extracted from a muscle section at the midpoint of the muscle under a stereomicroscope using a stainless steel needle.
The gel was then stained using a silver staining kit to visualize protein bands. Individual silver-stained gels were digitally imaged, and the density of each band was determined using computer-assisted image analysis and densitometry by one of the investigators (AJS) who was blind to animal age and treatment group (UN-Scan-IT gel Version 6.1, Silk Scientific, Inc, Utah, USA). Specifically, the percentage of each MHC isoform in each column of the gel was calculated using the density of a particular MHC band over the total density for all MHC bands in that column. In this manner, relative MHC composition for each muscle was calculated.29,43,45,51,57,64,75
To assess measurement reliability, density measurements from GG, HG and SG gels from approximately half of the rats across age and stimulation treatment groups were measured again by an investigator (HW) blind to age, stimulation group and original measurement values. Intraclass correlation coefficients and 95% confidence intervals between the original and repeated measurements were 0.98 (.97 – .99) for the GG and SG muscles, and 0.93 (.90 – .95) for the HG muscles.
Data Analysis
Examination of age effects, stimulation effects, and interactions were performed using Analysis of Variance (ANOVA) or t-tests when only 2 groups were compared. For the myosin heavy chain variables expressed as percentages, analyses were repeated based on ranks, and similar results were found relative to ANOVA findings. Thus, only ANOVA results will be presented. Pair-wise comparisons were made between groups using the Fisher protected least significant difference tests (LSD). SAS statistical software was used for all analyses (SAS Institute Inc., Cary, NC). The critical value for obtaining statistical significance was set at α=.05. Effect sizes were also calculated for comparisons that were found to be significantly different. The Cohen d was calculated as the difference between means standardized by the pooled variance. The Cohen guidelines for interpretation of effect sizes suggest that an effect size greater than 0.8 can be considered “large” and thus indicates a large relative difference between means independent of the sample size for each comparison, while an effect size between 0.5 and 0.8 is considered “medium.” 14
For some variables, missing data resulted in slightly smaller sample sizes and are reflected in the degrees of freedom for each analysis. Error degrees of freedom of 38 represented a full dataset. Loss of data occurred for a few muscle contraction variables due to unanticipated expiration of 2 rats during muscle contractile property recording and a computer error (n=1). Use of muscle tissue in other experiments limited availability for the MHC analysis in a few cases (GG n=4; SG n=3; HG n=3). Otherwise, all available data were included in the analysis.
Results
Twitch and tetanic tension measures were compared in young adult Control Group and Sham Group to determine if presence of the stimulation cuff alone, without NMES treatment, may have affected tongue muscle contractile properties. T-tests revealed that there was not a significant difference in twitch tension between the young adult Control Group and Sham Group with whole nerve stimulation (Control mean [SE] = 28.9 g [2.5]; Sham mean [SE] = 25.8 g [1.5]; t16=−1.10, p=.28). Similarly, there was not a significant difference in tetanic tension (Control mean [SE] =91.1 g [7.0]; Sham mean [SE] = 80.5 g [4.9]; t16=1.27, p=.22, respectively). When only the medial branch was stimulated effectively, tetanic tension was also not significantly different in the young adult Control and Sham Groups (Control mean [SE] = 21.1 g [2.9]; Sham mean [SE] = 20.5 g [1.7]; t15=.18, p=.86). However, twitch tension was significantly reduced in the Sham Group relative to the Control Group with isolated medial nerve stimulation (Control mean [SE]= 9.2 g [1.1]; Sham mean [SE] = 6.1 g [0.7]; t15=2.36, p=.03; d = 1.15). Thus, presence of the stimulation cuff around the hypoglossal nerve was associated with reduced twitch tension for evoked protrusive tongue actions. This finding is taken into account when the results for twitch tension with medial branch stimulation are interpreted.
Whole Nerve Stimulation
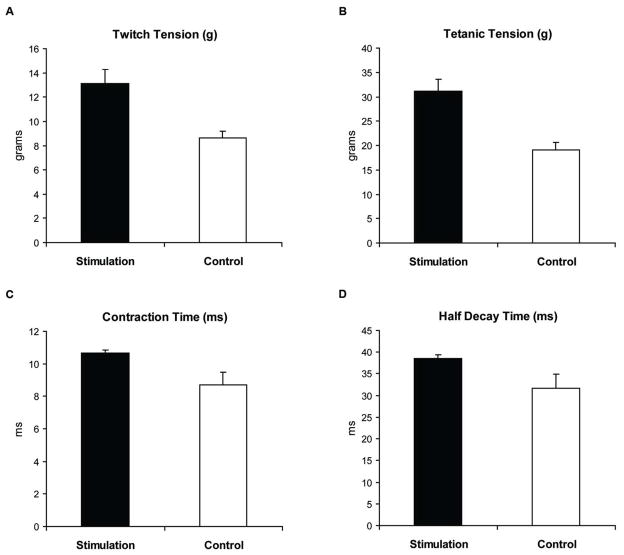
Descriptive data for each measure by age group and treatment group are shown in Table 2. No significant interaction effects (age group by stimulation treatment) were observed. No main effects for age group were found for any of the dependent variables. A significant main effect for stimulation treatment was obtained for contraction time, half decay time and fatigue ratio. Specifically, as shown in Figures 2A and 2B, contraction time and half decay time were significantly longer in the Stimulation Group versus the Control Group (F[1,37]=8.6, p=.006, d=.90; F[1,37]=6.8, p=.01, d=.81; respectively). Stimulation Group rats also demonstrated significantly less fatigue than rats in the Control Group (Figure 2C, F[1,36]=15.5, p=.0004, d=1.16). No other significant differences were found relative to stimulation treatment.
Table 2. Whole Nerve Stimulation.
Means and standard errors (in parentheses) of muscle contractile properties within each age group and stimulation treatment group during retrusive actions of the tongue elicited by bilateral stimulation of the whole hypoglossal nerve.
| Maximum Twitch Tension (g) | Contraction Time (ms) | Half Decay Time (ms) | Maximum Tetanic Tension (g) | Fatigue Index (%) | ||
|---|---|---|---|---|---|---|
| Young Adult | Stimulation | 27.9 (1.6) | 10.4 (0.3) | 37.1 (1.3) | 78.6 (5.0) | 87.1 (0.8) |
| Control | 28.9 (2.5) | 7.3 (1.4) | 28.1 (5.4) | 91.1 (7.0) | 73.7 (4.4) | |
| Middle Aged | Stimulation | 32.5 (1.9) | 10.5 (0.3) | 39.3 (1.4) | 89.2 (6.9) | 87.4 (1.8) |
| Control | 25.8 (2.6) | 6.9 (1.9) | 25.9 (6.9) | 77.5 (5.5) | 80.9 (2.7) | |
| Old | Simulation | 31.0 (2.0) | 9.9 (0.2) | 36.6 (0.8) | 86.8 (4.7) | 81.7 (2.4) |
| Control | 33.4 (1.5) | 9.9 (0.3) | 36.1 (1.2) | 83.0 (2.9) | 72.6 (4.0) |
Figure 2. A, B, C. Whole Nerve Stimulation.
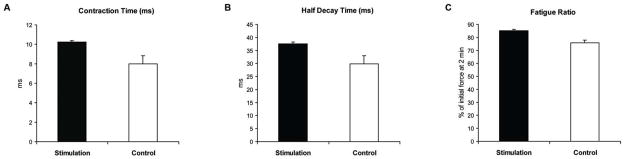
Following 8 weeks of bilateral hypoglossal nerve stimulation (Stimulation Group) compared to the no-stimulation Control Group, (A) tongue muscle contraction times were significantly longer, (b) half decay times were longer, and (c) fatigue index was greater, indicating less muscle fatigue. The fatigue index was calculated by constructing a ratio of the average tetanic tension (g) at the end of 2 minutes of stimulation relative to the initial tetanic tension (g), and multiplying by 100 to express the value as the percentage of initial tension.
Medial Branch Stimulation
Descriptive data comparisons for each measure by age group and treatment group are shown in Table 3. No significant interaction effects (age group by stimulation treatment) were observed. No main effects for age group were found for any of the dependent variables. However, significant stimulation treatment main effects were observed for twitch tension and tetanic tension, with greater mean values for the Stimulation Group in comparison with the Control Group (Figures 3A and 3B; F[1,37] = 10.1, p=.003, d=1.0; F[1,37] = 16.6, p=.0002, d=1.27; respectively). In addition, significant stimulation treatment main effects were observed for contraction time and half-decay time, with longer time intervals found in the Stimulation rats than in Controls (Figure 3C and 3D; F[1,37] = 6.2, p=.02; d=.77; F[1,37] = 4.5, p=.04, d=.68; respectively). No stimulation treatment differences were found for fatigue ratio
Table 3. Medial Branch Stimulation.
Means and standard errors (in parentheses) of muscle contractile properties within each age group and stimulation treatment group during protrusive actions of the tongue elicited by bilateral stimulation of the medial branch of the hypoglossal nerve (following section of the lateral branch).
| Maximum Twitch Tension (g) | Contraction Time (ms) | Half Decay Time (ms) | Maximum Tetanic Tension (g) | Fatigue Index (%) | ||
|---|---|---|---|---|---|---|
| Young Adult | Stimulation | 11.6 (2.3) | 10.7 (0.2) | 38.3 (1.2) | 26.4 (3.3) | 77.7 (3.2) |
| Control | 9.2 (1.1) | 7.9 (1.4) | 28.8 (5.4) | 21.1 (2.9) | 65.3 (5.2) | |
| Middle Aged | Stimulation | 14.3 (1.8) | 10.9 (0.3) | 38.9 (1.7) | 33.2 (3.6) | 76.3 (3.9) |
| Control | 7.9 (1.1) | 7.6 (1.9) | 28.0 (7.4) | 18.2 (2.9) | 72.3 (4.9) | |
| Old | Simulation | 13.3 (2.1) | 10.4 (0.4) | 38.4 (1.4) | 33.4 (4.7) | 80.1 (3.9) |
| Control | 8.7 (0.9) | 10.8 (0.2) | 39.3 (0.9) | 17.5 (1.6) | 79.5 (4.8) |
Figure 3. A, B, C, D. Medial Branch Stimulation.
Following 8 weeks of bilateral hypoglossal nerve stimulation (Stimulation Group) compared to the no-stimulation Control Group, (A) twitch tension was greater, (b) tetanic tension was greater, (c) contraction times were longer, and (D) half decay times were longer in the Stimulation Group.
Tongue Muscle Myosin Heavy Chain Composition
Overview of Findings
Representative silver-stained SDS-PAGE gels for a Young Adult, Middle-Aged and Old animal in the Stimulation and Control Groups are found in Figure 4. The percent of each MHC isoform (Types IIb, IIx, IIa, I) found in the GG, SG and HG muscles is shown in Tables 4, 5 and 6. On the average, Type IIx MHC occupied the greatest proportion of each muscle, regardless of age group or stimulation condition. Conversely, Type I MHC occupied the smallest proportion of each muscle.
Figure 4.
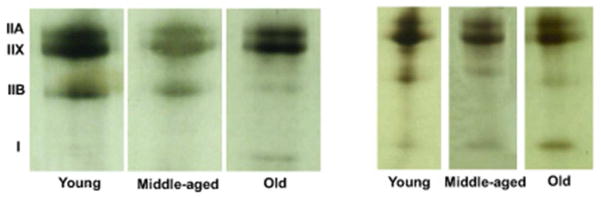
Representative silver-stained SDS-PAGE for the GG muscle in a young, middle-aged and old animal in the Stimulation and Control (no stimulation) Groups. As suggested in this gel, a higher proportion of Type IIb MHC and a smaller proportion of Type I MHC were found in Young versus the Old animals. In addition, an increased proportion of Type I MHC was found with stimulation in the Young animals.
Table 4.
Average percent and standard errors (in parentheses) of myosin heavy chain (MHC) isoforms in the Genioglossus (GG) muscle for each age group and stimulation treatment group.
| GG Muscle | |||||
|---|---|---|---|---|---|
| IIb | IIx | IIa | I | ||
| Young Adult | Stimulation | 12.6 (1.5) | 48.1 (1.3) | 36.5 (2.0) | 2.9 (.6) |
| Control | 17.3 (2.0) | 47.1 (2.0) | 34.5 (.9) | 1.1 (.5) | |
| Middle Aged | Stimulation | 11.9 (1.2) | 46.9 (1.1) | 38.1 (.9) | 3.0 (.7) |
| Control | 10.5 (1.9) | 49.2 (1.4) | 38.0 (1.5) | 2.3 (.5) | |
| Old | Simulation | 11.4 (1.1) | 48.1 (.9) | 37.4 (1.0) | 3.2 (.5) |
| Control | 9.8 (1.8) | 45.0 (.8) | 40.1 (2.2) | 5.1 (.6) | |
Table 5.
Average percent and standard errors (in parentheses) of myosin heavy chain (MHC) isoforms in the Hyoglossus (HG) muscle for each age group and stimulation treatment group.
| HG Muscle | |||||
|---|---|---|---|---|---|
| IIb | IIx | IIa | I | ||
| Young Adult | Stimulation | 25.2 (4.3) | 42.2 (2.7) | 29.4 (2.3) | 3.3 (0.5) |
| Control | 35.7 (2.0) | 37.5 (1.3) | 26.3 (1.2) | 0.56 (0.4) | |
| Middle Aged | Stimulation | 26.9 (2.6) | 43.4 (2.7) | 27.5 (1.4) | 2.2 (0.8) |
| Control | 31.7 (1.3) | 40.3 (2.4) | 26.9 (1.7) | 1.1 (0.5) | |
| Old | Simulation | 28.8 (3.2) | 41.3 (1.7) | 25.6 (1.8) | 4.3 (0.7) |
| Control | 35.2 (4.7) | 38.1 (1.3) | 24.4 (3.4) | 2.4 (0.3) | |
Table 6.
Average percent and standard errors (in parentheses) of myosin heavy chain (MHC) isoforms in the Styloglossus (SG) muscle for each age group and stimulation treatment group.
| SG Muscle | |||||
|---|---|---|---|---|---|
| IIb | IIx | IIa | I | ||
| Young Adult | Stimulation | 19.6 (3.1) | 45.4 (2.1) | 31.9 (2.4) | 3.1 (0.7) |
| Control | 25.9 (1.9) | 40.3 (1.2) | 33.3 (1.1) | 0.50 (0.3) | |
| Middle Aged | Stimulation | 16.8 (3.1) | 44.9 (1.6) | 36.4 (2.8) | 2.0 (0.6) |
| Control | 23.6 (2.9) | 42.6 (1.9) | 32.5 (1.8) | 1.4 (0.7) | |
| Old | Simulation | 19.6 (2.5) | 45.8 (1.7) | 31.9 (1.9) | 2.7 (0.7) |
| Control | 22.2 (4.4) | 43.2 (.8) | 32.9 (3.8) | 1.8 (0.6) | |
Genioglossus Muscle
On the average, the GG muscle (Table 4) contained between 1.1 and 5.1% Type I MHC and greater than 94% Type II MHC (a, b, x combined). A significant main effect for age group in the absence of a significant stimulation treatment by age group interaction was found for GG Type IIb MHC (F[2,34] = 4.6, p=.02). Post hoc LSDs revealed a greater percentage of Type IIb MHC in Young GG versus Middle-Aged and Old (p=.03, d=.74; and p=.009, d=.86; respectively). A significant stimulation treatment by age group interaction effect was noted for GG Type I MHC (F[2,34] = 4.9, p=.01). Post hoc LSDs revealed a significantly greater percentage of GG Type I MHC in the Young Stimulation group versus the Young Control Group (p=.04, d=1.11), but a significantly greater percentage of GG Type I MHC in the Old Control Group versus Old Stimulation (p=.03, d=1.23). Significant differences in GG Type I MHC were not found as a function of stimulation treatment in the Middle-Aged group. In addition, Old Control GG muscles contained a greater percentage of GG Type I MHC than Young Control GG (p=.0001, d=3.03) and Middle-Aged Control GG (p=.008, d=1.06). No other statistically significant age group, stimulation treatment, or interaction effects were found.
Hyoglossus Muscle
On the average, the HG muscle (Table 5) contained between 0.6 and 4.3% Type I MHC and greater than 95.7% Type II MHC (a, b, x combined). A significant main effect for age group and stimulation treatment in the absence of a significant stimulation treatment by age group interaction effect was found for HG Type 1 MHC (Age Group: F[2,34] = 4.6, p=.02; Stimulation Treatment: F[1,34] = 15.5, p=.0004). Post hoc LSDs comparing age groups revealed a greater percentage of Type 1 MHC in Old HG versus Young and Middle-Aged (p=.02, d=.86; and p=.01, d=1.08; respectively). The Stimulation Group (Mean [SE]= 3.4%[0.4]) had a larger percentage of HG Type I MHC than the Control Group (Mean [SE] = 1.2% [0.3]). No other statistically significant age group, stimulation treatment, or interaction effects were found.
Styloglossus Muscle
Type I MHC represented 0.5 to 3.1% of the SG muscle on average, and Type II MHC (a, b, x combined) represented over 96.9% of the muscle (Table 6). A significant main effect for stimulation treatment in the absence of a significant stimulation treatment by age group interaction effect was found for SG Type I MHC (F[1,33] = 6.4, p=.02, d=.96), with a greater percentage of SG Type I MHC in the Stimulation Group versus the Control Group (Control mean [SE] = 1.1% [0.3]; Stimulation mean [SE] = 2.7% [0.4]). No other statistically significant age group, stimulation treatment, or interaction effects were found.
Discussion
The hypothesis of this study was that alterations in extrinsic tongue muscle contractile properties and biochemical structure would be found following 8 weeks of bilateral hypoglossal nerve stimulation. Our results supported this hypothesis. Animals in the stimulation treatment condition showed both muscle contractile and biochemical alterations when compared with control animals. Specifically, stimulated muscles exhibited less fatigue, increased contraction and half decay times, increased twitch and tetanic tension, and, except for the Old and Middle-Aged GG muscle, a significant increase in Type I MHC when compared with control animals. Thus, transitions in tongue muscle phenotype were found following 8 weeks of stimulation treatment.
Alterations in tongue muscle contraction with NMES treatment
Muscle contractile properties elicited with stimulation of the whole hypoglossal nerve resulted in a significant increase in contraction times, half decay times, and reduced levels of fatigue in animals that underwent 8 weeks of NMES treatment. However, alterations in twitch and tetanic tension with whole nerve stimulation were not altered statistically as a function of age group or NMES treatment. Results for twitch and tetanic tensions may be due to activation of both medial and lateral branches of the hypoglossal associated with whole nerve stimulation, leading to co-contraction of protruser and retrusor muscles and resulting in a net retrusive action, as observed in other studies. 19 Thus, co-contraction of muscles with opposing actions may have limited the potential magnitude of observable retrusive forces. To specifically examine retrusive tongue forces, an experimental paradigm that isolated retrusive actions via section of the medial branch of the hypoglossal nerve was required but was not performed in this study. Accordingly, increased retrusive tongue forces may have changed as a function of chronic hypoglossal nerve stimulation, but they could not be measured with our paradigm and should be examined in future research.
Conversely, when only the medial branch of the hypoglossal nerve was stimulated in our post-treatment muscle recordings, we found statistically significant increases in twitch and tetanic tension and contraction and decay times following NMES versus control animals. No significant differences were noted for fatigue. Thus, for protrusive tongue actions mediated by the medial branch innervation, we found that force properties and temporal properties were affected by NMES. The significant increases in twitch tension following NMES versus control must be interpreted with some caution, because we also found significant differences between the Young Control Group and the Sham Group. In this comparison, the Sham Group had lower average twitch tensions than non-implanted controls. Thus, the presence of the stimulation cuff around the nerve appeared to affect function of the nerve and subsequently reduced generation of tongue twitch tension in one experimental condition. However, despite the reduced twitch tension due to presence of the cuff, following 8 weeks of NMES, twitch tension during protrusive actions was significantly greater than in non-treated controls, suggesting that NMES treatment was successful in potentially overcoming the putative negative effects of the stimulation cuff around a nerve.
Taken together, the different profiles of change characteristic of chronic stimulation for retrusive and protrusive actions suggest that adaptation of the tongue as a whole with stimulation treatment may provide increased tension characteristics, increased contraction and decay times and reduced fatigue. These changes may ultimately provide greater strength and endurance during tongue movements. In this experiment, the artificial dichotomy of separate retrusive and protrusive actions may not be relevant to the performance of natural movements for swallowing and other critical cranial functions. However, it is the capacity for change in all of the parameters noted that may enhance function of individual muscles of the tongue to manifest improved function while acting in concert in the performance of natural, goal-directed movements.
Alterations in myosin heavy chain properties with chronic stimulation treatment
Our results showed that MHC properties of the genioglossus, styloglossus and hyoglossus were altered by stimulation treatment in similar ways. Namely, except for the Old and Middle-Aged GG muscle, there was an increase in the content of Type I MHC following stimulation treatment. Lack of a signfiicant increase in GG Type I MHC within the Old and Middle-Aged groups suggests a somewhat reduced capacity for muscle plasticity in older protrusor muscles that may not have been differentially addressed by our stimulation paradigm. Aspects of our stimulation protocol that may have limited our findings are discussed further below. However, findings of increased Type I MHC with stimulation are consistent with phenotype transitions in fast-twitch limb muscles stimulated at low frequencies and with a recent study in rabbit tongue in which chronic low frequency stimulation resulted in increased Type I MHC and decreased Type II MHC in the normally fast-contracting GG muscle.42,60 In contrast, studies of denervated slow-twitch muscles such as soleus which were stimulated chronically at high frequencies have resulted in greater Type II MHC and reduced Type I MHC. 4,8,10,42 A potential explanation for the directionality of these fast-to-slow or slow-to-fast phenotype transitions is that the experimentally-applied electrical stimulation frequencies may have mimicked nerve impulses that occur naturally in a different type of muscle fiber, and phenotype was altered to correspond with the change in neural input. 39,42 This explanation has been reinforced by crossed reinnervation studies in muscles. 1,39,42 That is, fast and slow muscles cross reinnervated by each others’ nerves appear to switch their properties in accordance with their new neural input. In aged fast-twitch muscles, “nearest neighbor” 42 fast-to-slow phenotype transitions within the Type II muscle fiber category have been reported: Type IIb MHC appeared to transition to Type IIx MHC, and/or Type IIx MHC transitioned to Type IIa MHC. 16,45 Similar transformations from fast to slow MHC have been observed in fast-twitch muscles with a disturbed nerve supply. 2,39 Thus, findings of a fast-to-slow progession in denervated muscle and with aging in combination with findings of larger and fewer motor units with aging 21,40 have led to hypotheses that denervation-reinnervation is a major cause of musculoskeletal changes seen with sarcopenia.18,20
Prior studies have found that the electrical stimulation frequency and duration, or type of exercise employed (endurance vs. resistance) can induce variability in degree of phenotype transformation. 6,27,42,44 Alterations in MHC expression have been found to be dose-dependent with greater durations of stimulation or exercise training associated with larger changes along the fast-to-slow MHC continuum. 31,32 In a prior report, the highest durations of training were required to affect the fast-to-slow MHC change in the predominantly fast-contracting EDL muscle in comparison with other more slowly contracting limb muscles. 32 While we developed our stimulation protocol to model clinically relevant strength training paradigms in which 3 sets of 10–12 near-maximal contractions are produced, larger changes in muscle contractile properties or MHC profiles may have been obtained if we had stimulated at different frequencies for greater than 8 weeks, or if individual stimulation sessions were longer than 10 minutes per day. Studies of the effects of chronic stimulation on muscle have involved animal models with almost constant stimulation for 10 to 24 hours per day over the course of days or weeks. 11,31,42 In rabbit tongue, 7 days of continuous 24 hour per day stimulation were performed and resulted in larger phenotypic changes than those found in rat in our study. 60 However, these durations of stimulation would be difficult to translate to clinical populations. The dose-response aspect of stimulation of tongue muscles and phenotype shift should be investigated further in future studies with the goal of defining optimal stimulation parameters for obtaining optimal physiological outcomes. We are currently performing studies toward this goal in our laboratory.
Our goal in including middle-aged and old rats was to determine if age-related changes in muscles of the tongue could be reversed or prevented. Use of animals at an early stage of aging (24 months old) allowed examination of the intervention’s ability to delay or prevent age-related changes in tongue muscle, while examination of an older group of animals (32 months old) pertained to the reversibility of neuromuscular decline. However, with regard to a delay or reversal of age-related changes, we did not find that tongue muscle characteristics became more young-like with stimulation treatment. That is, we did not observe a conversion or replacement of MHC isoforms to more rapidly contracting isoforms consistent with young muscles. Instead, with stimulation treatment, we found an even larger proportion of Type I MHC in middle aged and old muscles than observed with aging alone. The shift toward Type I MHC may be a characteristic of muscle plasticity with aging as a positive adaptive mechanism to promote endurance, because Type I muscle fibers are more fatigue-resistant than Type II muscle. Exercise or stimulation may function to improve the efficiency of this transformation toward the goal of improved function within the context of overall aging. Thus, perhaps muscle phenotype is modified with aging to maximize endurance capabilities at expense of speed, and the stimulation treatment accelerated this process to levels not observed with natural aging alone. Accordingly, the use of chronic neuromuscular stimulation in this study may have served both to prevent and also treat age-related changes in tongue muscles.
While statistically significant age group effects were found in the MHC composition of the muscles studied, we did not find statistically significant age group effects in muscle contractile properties as we have reported in previous studies. 52,67,68 For example, we previously reported significantly reduced tetanic tension in old rats when compared with young during elicited tongue protrusion. 68 Although tetanic tension for elicited protrusive actions was somewhat smaller in old animals relative to young and middle aged rats in this study (see Table 2), these differences were not statistically significant. Because we used the same strain of genetically identical rats, the same equipment, similar sample sizes and the same procedures in all studies, this lack of an age group effect is difficult to explain but may be due to individual variation in animal batches or animal handling during training by different personnel.
Clinical Implications
Neuromuscular electrical stimulation (NMES) is a clinical modality used currently in physical therapy that has been applied to the treatment of swallowing disorders. 41,58 Clinically, NMES for the treatment of dysphagia generally involves surface stimulation of muscles through the skin using a variety of electrode arrays placed on the neck. The results of clinical investigations of the effectiveness of NMES in improving swallowing outcomes have been mixed. While some studies have reported beneficial effects, 41,63,66,70,72 other studies that used randomized treatment allocation or cross-over designs have not found benefits from NMES use in swallowing treatment over traditional treatment methods. 56,65 One source of variability in the application of this treatment method and in the research that has been forthcoming may be that the neuromuscular targets of stimulation applied to the neck are indirect and potentially imprecise. Relatively diffuse and superficial surface stimulation may have unpredictable effects. For example, recent work has shown that stimulation through commercially prescribed electrode arrays may pull the larynx down during the swallow in a non-optimal manner. 54,59 Our study does not address these issues, because direct nerve stimulation was used, and thus precise neuromuscular targets were specified and optimized. We found differences in muscle contractile properties and biochemistry that may be beneficial in promoting endurance during swallowing including reduced fatigue and increased contractile tension. Thus, perhaps as NMES for dysphagia treatment evolves to the point in which particular muscles can be targeted and customized for individual patients, we will have a clearer understanding of the potential benefits of this treatment approach. The data reported here suggest that changes in tongue muscle phenotype and physiology may be possible following intensive and targeted stimulation of the muscles of the tongue.
Acknowledgments
The authors are grateful for the assistance of Laura Mann Dhanansayan in the completion of this work. This study was supported by grants from the National Institute of Deafness and Other Communication Disorders (R01DC005935 and R01DC008149). Dr. Michelle Ciucci provided extremely helpful suggestions on an earlier version of this paper. Excellent comments and suggestions were received from two anonymous reviewers that greatly improved the clarity of this paper.
List of Acronyms and Abbreviations
- A/D
Analog to Digital
- C
Celsius
- cm
centimeter
- EMG
Electromyographic or Electromyography
- g
gram
- GG
Genioglossus muscle
- HG
Hyoglossus muscle
- Hz
Hertz
- IACUC
Institutional Animal Care and Use Committee
- IP
intraperitoneal
- kg
kilogram
- μA
microamp
- μm
micron
- min
minutes
- mg
milligram
- MHC
Myosin Heavy Chain
- mm
millimeter
- ms
millisecond
- NIH
National Institutes of Health
- NMES
Neuromuscular Electrical Stimulation
- PHS
Public Health Service
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SE
Standard Error
- sec
second
- SG
Styloglossus muscle
References
- 1.Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutmann E, Melichna J, Syrový I. Contraction properties and ATPase activity in fast and slow muscle of the rat during denervation. Experimental Neurology. 1972;36:488–497. doi: 10.1016/0014-4886(72)90008-8. [DOI] [PubMed] [Google Scholar]
- 3.Burke RE, Levine DN, Tsaris P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lomo T, Westgaard RH, Dahl HA. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proceedings of the Royal Society of London Series B, Containing Papers of a Biological Character Royal Society. 1974;187:99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- 5.Baum BJ, Bodner L. Aging and oral motor function: evidence for altered performance among older persons. J Dent Res. 1983;62:2–6. doi: 10.1177/00220345830620010401. [DOI] [PubMed] [Google Scholar]
- 6.Edstrom L, Grimby L. Effect of exercise on the motor unit. Muscle Nerve. 1986;9:104–126. doi: 10.1002/mus.880090203. [DOI] [PubMed] [Google Scholar]
- 7.Enoka RM, Rankin LL, Joyner MJ, Stuart DG. Fatigue-related changes in neuromuscular excitability of rat hindlimb. Muscle & Nerve. 1988;11:1123–1132. doi: 10.1002/mus.880111104. [DOI] [PubMed] [Google Scholar]
- 8.Gorza L, Gundersen K, Lømo T, Schiaffino S, Westgaard RH. Slow-to-fast transformation of denervated soleus muscles by chronic high-frequency stimulation in the rat. J Physiol. 1988;402:627–649. doi: 10.1113/jphysiol.1988.sp017226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lexell J, Taylor CC, Sjostrom M. What is the cause of ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15 to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 10.Westgaard RH, Lømo T. Control of contractile properties within adaptive ranges by patterns of impulse activity in the rat. J Neurosci. 1988;8:4415–4426. doi: 10.1523/JNEUROSCI.08-12-04415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Termin A, Staron RS, Pette D. Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles. Eur J Biochem. 1989;186:749–754. doi: 10.1111/j.1432-1033.1989.tb15269.x. [DOI] [PubMed] [Google Scholar]
- 12.Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: radiologic findings in 56 cases. AJR Am J Roentgenol. 1991;156:1181–1184. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama M. Histological study on aging changes in the human tongue. J Otolaryngol Japan. 1991;94:541–555. doi: 10.3950/jibiinkoka.94.541. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 15.Robbins JA, Hamilton JW, Lot GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 16.Larsson L, Biral D, Campione M, Schiaffino S. An age-related type IIB to IIX myosin heavy chain switching in rat skeletal muscle. Acta Physiol Scand. 1993;147:227–234. doi: 10.1111/j.1748-1716.1993.tb09493.x. [DOI] [PubMed] [Google Scholar]
- 17.Widick MH, Tanabe T, Fortune S, Zealear DL. Awake evoked electromyography recording from the chronically implanted rat. Laryngoscope. 1994;104:420–425. doi: 10.1288/00005537-199404000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Carlson BM. Factors influencing the repair and adaptation of muscles in aged individuals: Satellite cells and innervation. J Gerontol. 1995;50:96–100. doi: 10.1093/gerona/50a.special_issue.96. [DOI] [PubMed] [Google Scholar]
- 19.Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol. 1995;74:547–555. doi: 10.1152/jn.1995.74.2.547. [DOI] [PubMed] [Google Scholar]
- 20.Larsson L. Motor units: remodeling in aged animals. J Gerontol. 1995;50A:91–95. doi: 10.1093/gerona/50a.special_issue.91. [DOI] [PubMed] [Google Scholar]
- 21.Larsson L, Ansved T. Effects of ageing on the motor unit. Progress in Neurobiology. 1995;45:397–458. doi: 10.1016/0301-0082(95)98601-z. [DOI] [PubMed] [Google Scholar]
- 22.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 23.Robbins J, Levine R, Wood J, Roecker ED, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol. 1995;50:M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 24.Shaw DW, Cook IJ, Gabb M, Holloway RH, Simula ME, Panagopoulos V, Dent J. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol. 1995;268:G389–G396. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- 25.van Lunteren E, Vafaie H, Salomone RJ. Comparative effects of aging on pharyngeal and diaphragm muscles. Respir Physiol. 1995;99:113–125. doi: 10.1016/0034-5687(94)00077-d. [DOI] [PubMed] [Google Scholar]
- 26.Brown M, Hasser EM. Differential effects of reduced muscle use (hindlimb unweighting) on skeletal muscle with aging. Aging (Milano. 1996;8:99–105. doi: 10.1007/BF03339562. [DOI] [PubMed] [Google Scholar]
- 27.Hamalainen N, Pette D. Slow-to-fast transitions in myosin expression of rat soleus muscle by phasic high-frequency stimulation. FEBS Lett. 1996;399:220–222. doi: 10.1016/s0014-5793(96)01325-7. [DOI] [PubMed] [Google Scholar]
- 28.Lazarus CL, Logemann JA, Pauloski BR, Colangelo LA, Kahrilas PJ, Mittal BB, Pierce M. Swallowing disorders in head and neck cancer patients treated with radiotherapy and adjuvant chemotherapy. Laryngoscope. 1996;106:1157–1166. doi: 10.1097/00005537-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Humphries BJ, Newton RU, Abernethy PJ, Blake KD. Reliability of an electrophoretic and image processing analysis of human skeletal muscle taken from m vastus lateralis. European Journal of Applied Physiology and Occupational Physiology. 1997;75:532–536. doi: 10.1007/s004210050200. [DOI] [PubMed] [Google Scholar]
- 30.Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol (Lond) 1998;507:265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutherland H, Jarvis JC, Kwende MM, Gilroy SJ, Salmons S. The dose-related response of rabbit fast muscle to long-term low-frequency stimulation. Muscle & Nerve. 1998;21:1632–1646. doi: 10.1002/(sici)1097-4598(199812)21:12<1632::aid-mus3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 32.Demirel H, Power SK, Naito H, Hughes M, Coombes JS. Exercise induced alterations in skeletal muscle myosin heavy chain phenotype: dose-response relationship. J Appl Physiol. 1999;86:1002–1008. doi: 10.1152/jappl.1999.86.3.1002. [DOI] [PubMed] [Google Scholar]
- 33.Mortimore IL, Fiddes P, Stephens S, Douglas NJ. Tongue protrusion force and fatiguability in male and female subjects. Eur Respir J. 1999;14:191–195. doi: 10.1034/j.1399-3003.1999.14a32.x. [DOI] [PubMed] [Google Scholar]
- 34.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol [A] Biol Sci Med Sci [A] Biol S. 1999;54A:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 35.Fuller DD, Fregosi RF. Fatiguing contractions of tongue protrudor and retractor muscles: influence of systemic hypoxia. Journal of Applied Physiology. 2000;88:2123–2130. doi: 10.1152/jappl.2000.88.6.2123. [DOI] [PubMed] [Google Scholar]
- 36.Lazarus CL, Logemann JA, Pauloski BR, Rademaker AW, Larson CR, Mittal BB, Pierce M. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. J Speech Lang Hear Res. 2000;43:1011–1023. doi: 10.1044/jslhr.4304.1011. [DOI] [PubMed] [Google Scholar]
- 37.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Hear Res. 2000;43:1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 38.Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol [A] Biol Sci Med Sci. 2000;55:M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 39.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microscopy Research and Technique. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Takeda N, Thomas GR, Ludlow CL. Aging effects on motor units in the human thyroarytenoid muscle. Laryngoscope. 2000;110:1018–1025. doi: 10.1097/00005537-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 41.Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respir Care. 2001;46:466–474. [PubMed] [Google Scholar]
- 42.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochemistry and Cell Biology. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- 43.D’Antona G, Megighian A, Bortolotto S, Pellegrino MA, Marchese-Ragona R, Staffieri A, Bottinelli R, Reggiani C. Contractile properties and myosin heavy chain isoform composition in single fibre of human laryngeal muscles. Journal of Muscle Research and Cell Motility. 2002;23:187–195. doi: 10.1023/a:1020963021105. [DOI] [PubMed] [Google Scholar]
- 44.Lowe DA, Alway SE. Animal models for inducing muscle hypertrophy: Are they relevant for clinical applications in humans? J Orthop Sports Phys Ther. 2002;32:36–43. doi: 10.2519/jospt.2002.32.2.36. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki T, Connor NP, Lee K, Bless DM, Ford CN, Inagi K. Age-related alterations in myosin heavy chain isoforms in rat laryngeal muscles. Ann Otol Rhinol Laryngol. 2002;111:962–967. doi: 10.1177/000348940211101102. [DOI] [PubMed] [Google Scholar]
- 46.Vandervoort AA. Aging of the human neuromuscular system. Muscle & Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 47.Clark HM, Henson PA, Barber WD, Stierwalt JAG, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. American Journal of Speech Language Pathology. 2003;12:40–50. doi: 10.1044/1058-0360(2003/051). [DOI] [PubMed] [Google Scholar]
- 48.Doherty TJ. Aging and Sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 49.Lazarus C, Logemann JA, Huang CF, Rademaker AW. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55:199–205. doi: 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- 50.Ingalls CP. Nature vs. nurture: can exercise really alter fiber type composition in human skeletal muscle? Journal of Applied Physiology. 2004;97:1591–1592. doi: 10.1152/classicessays.00010.2004. [DOI] [PubMed] [Google Scholar]
- 51.Kingham PJ, Birchall MA, Burt R, Jones A, Terenghi G. Reinnervation of laryngeal muscles: a study of changes in myosin heavy chain expression. Muscle & Nerve. 2005;32:761–766. doi: 10.1002/mus.20409. [DOI] [PubMed] [Google Scholar]
- 52.Ota F, Connor NP, Konopacki RA. Alterations in contractile properties of tongue muscles in old rats. Ann Otol Rhinol Laryngol. 2005;114:799–803. doi: 10.1177/000348940511401010. [DOI] [PubMed] [Google Scholar]
- 53.Robbins JA, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind J. The Effects of Lingual Exercise on Swallowing in Older Adults. J Am Geriatr Soc. 2005;53:1493–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 54.Humbert IA, Poletto CJ, Saxon KG, Kearney PR, Crujido L, Wright-Harp W, Payne J, Jeffries N, Sonies BC, Ludlow CL. The effect of surface electrical stimulation on hyolaryngeal movement in normal individuals at rest and during swallowing. Journal of Applied Physiology. 2006;101:1657–1663. doi: 10.1152/japplphysiol.00348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kays S, Robbins J. Effects of sensorimotor exercise on swallowing outcomes relative to age and age-related disease. Seminars in Speech and Language. 2006;27:245–259. doi: 10.1055/s-2006-955115. [DOI] [PubMed] [Google Scholar]
- 56.Suiter DM, Leder SB, Ruark JL. Effects of neuromuscular electrical stimulation on submental muscle activity. Dysphagia. 2006;21:56–60. doi: 10.1007/s00455-005-9010-7. [DOI] [PubMed] [Google Scholar]
- 57.Harris LR, Churchward MA, Butt RH, Coorssen JR. Assessing detection methods for gel-based proteomic analyses. Journal of Proteome Research. 2007;6:1418–1425. doi: 10.1021/pr0700246. [DOI] [PubMed] [Google Scholar]
- 58.Huckabee ML, Doeltgen S. Emerging modalities in dysphagia rehabilitation: neuromuscular electrical stimulation. New Zealand Medical Journal. 2007;120:U2744. [PubMed] [Google Scholar]
- 59.Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal Dysphagia. Dysphagia. 2007;22:1–10. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pae EK, Hyatt JP, Wu J, Chien P. Short-term electrical stimulation alters tongue muscle fibre type composition. Archives of Oral Biology. 2007;52:544–551. doi: 10.1016/j.archoralbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, Taylor AJ. The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine and Rehabilitation. 2007;88:150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: preliminary evidence of prevalence, risk factors, and socioemotional effects. Annals of Otology, Rhinology, and Laryngology. 2007;116:858–865. doi: 10.1177/000348940711601112. [DOI] [PubMed] [Google Scholar]
- 63.Shaw GY, Sechtem PR, Searl J, Keller K, Rawi TA, Dowdy E. Transcutaneous neuromuscular electrical stimulation (VitalStim) curative therapy for severe dysphagia: myth or reality? Annals of Otology, Rhinology, and Laryngology. 2007;116:36–44. doi: 10.1177/000348940711600107. [DOI] [PubMed] [Google Scholar]
- 64.Volz LM, Mann LB, Russell JA, Jackson MA, Leverson GE, Connor NP. Biochemistry of Anterior, Medial, and Posterior Genioglossus Muscle in the rat. Dysphagia. 2007;22:210–214. doi: 10.1007/s00455-006-9075-y. [DOI] [PubMed] [Google Scholar]
- 65.Bülow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23:302–309. doi: 10.1007/s00455-007-9145-9. [DOI] [PubMed] [Google Scholar]
- 66.Carnaby-Mann GD, Crary MA. Adjunctive neuromuscular electrical stimulation for treatment-refractory dysphagia. Annals of Otology, Rhinology, and Laryngology. 2008;117:279–287. doi: 10.1177/000348940811700407. [DOI] [PubMed] [Google Scholar]
- 67.Connor NP, Ota F, Nagai H, Russell JA, Leverson GE. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. Journal of Speech, Language, and Hearing Research. 2008;51:818–827. doi: 10.1044/1092-4388(2008/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagai H, Russell JA, Jackson MA, Connor NP. Effect of aging on tongue protrusion forces in rats. Dysphagia. 2008;23:116–121. doi: 10.1007/s00455-007-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robbins J, Butler SG, Daniels SK, Gross RD, Langmore S, Lazarus CL, Martin-Harris B, McCabe D, Musson N, Rosenbek JC. Swallowing and dysphagia rehabiliation: Translating principles of neural plasticity into clinically oriented evidence. Journal of Speech, Language, and Hearing Research. 2008;51:S276–S300. doi: 10.1044/1092-4388(2008/021). [DOI] [PubMed] [Google Scholar]
- 70.Bogaardt H, van Dam D, Wever NM, Bruggeman CE, Koops J, Fokkens WJ. Use of neuromuscular electrostimulation in the treatment of dysphagia in patients with multiple sclerosis. Annals of Otology, Rhinology, and Laryngology. 2009;118:241–246. doi: 10.1177/000348940911800401. [DOI] [PubMed] [Google Scholar]
- 71.Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender KR. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. Journal of Speech, Language, and Hearing Research. 2009;52:732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim KB, Lee HJ, Lim SS, Choi YI. Neuromuscular electrical and thermal-tactile stimulation for dysphagia caused by stroke: a randomized controlled trial. Journal of Rehabilitation Medicine. 2009;41:174–178. doi: 10.2340/16501977-0317. [DOI] [PubMed] [Google Scholar]
- 73.Lehnen AM, Leguisamo NM, Pinto GH, Markoski MM, De Angelis K, Machado UF, Schaan B. The beneficial effects of exercise in rodents are preserved after detraining: a phenomenon unrelated to GLUT4 expression. Cardiovascular Diabetology. 2010;9:67. doi: 10.1186/1475-2840-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson AM, Connor NP. Effects of electrical stimulation on neuromuscular junction morphology in the aging rat tongue. Muscle & Nerve. 2011;43:203–211. doi: 10.1002/mus.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia. 2011;26:256–263. doi: 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]