Abstract
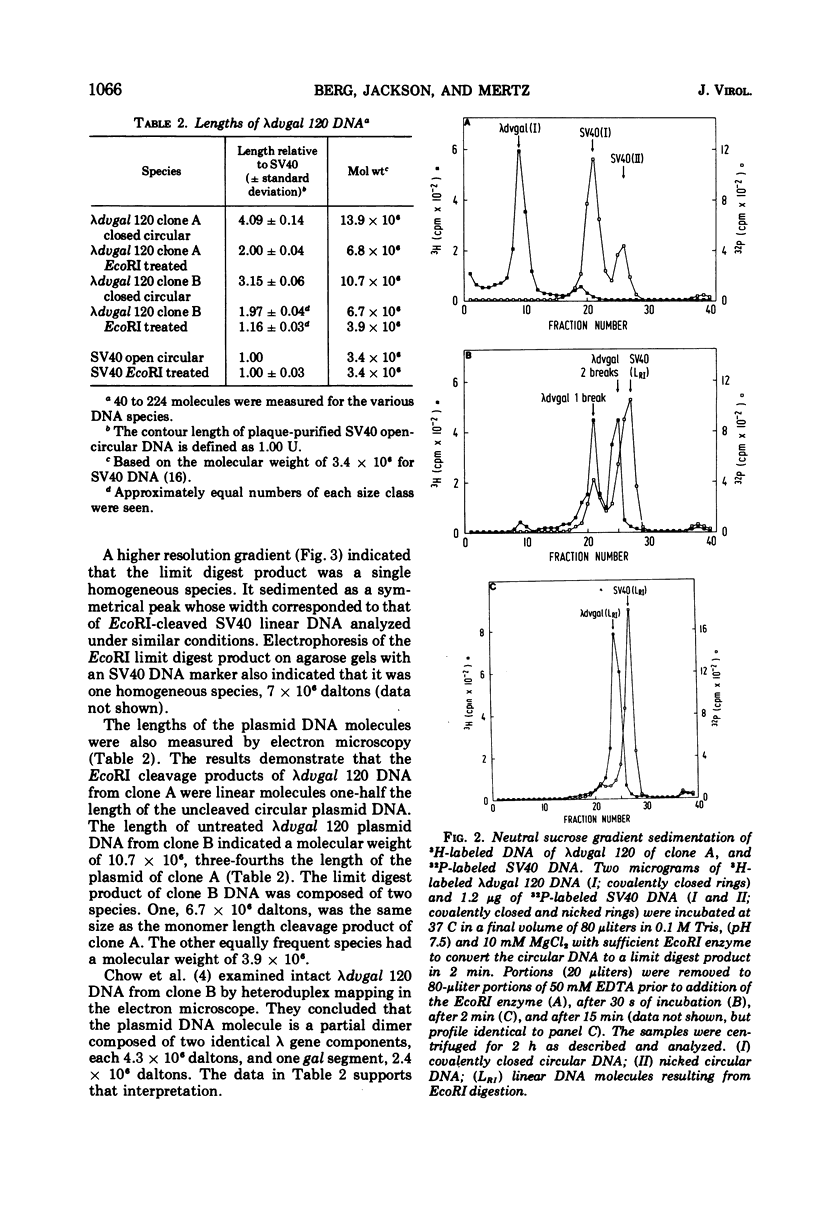
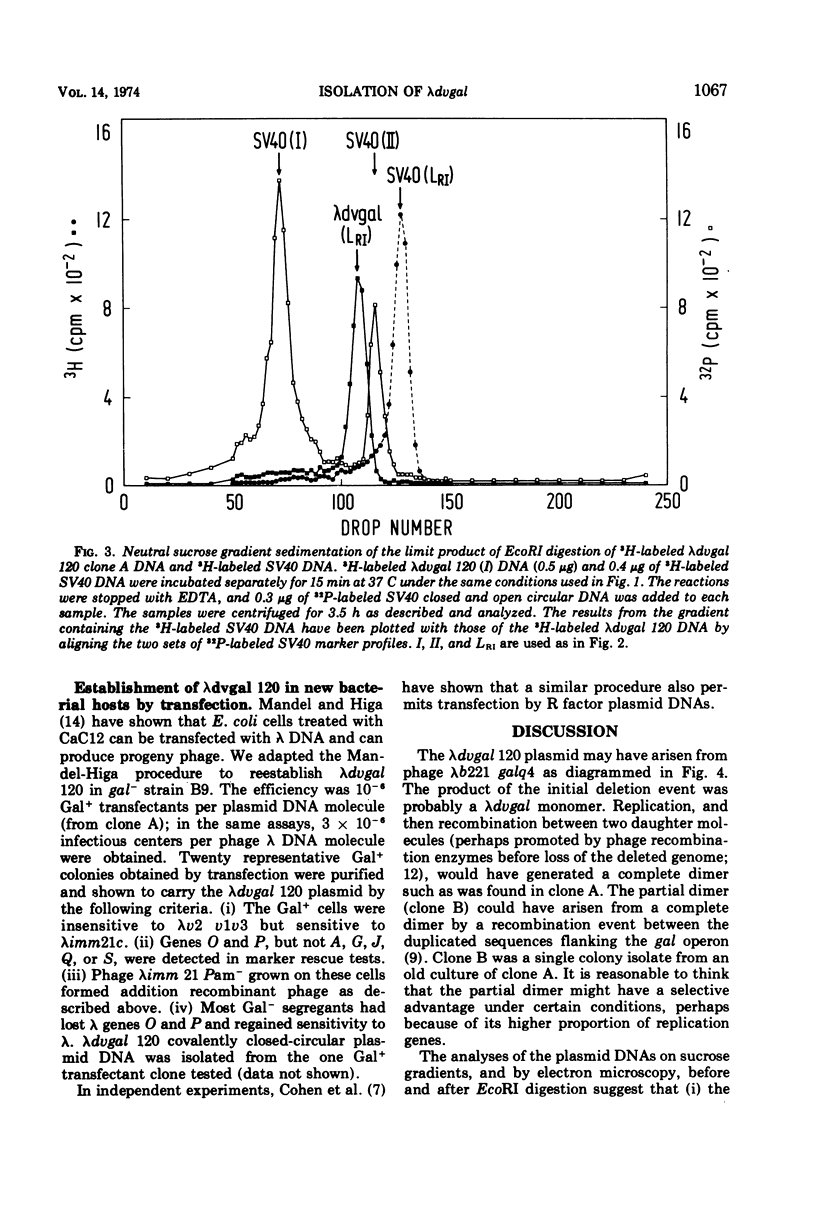
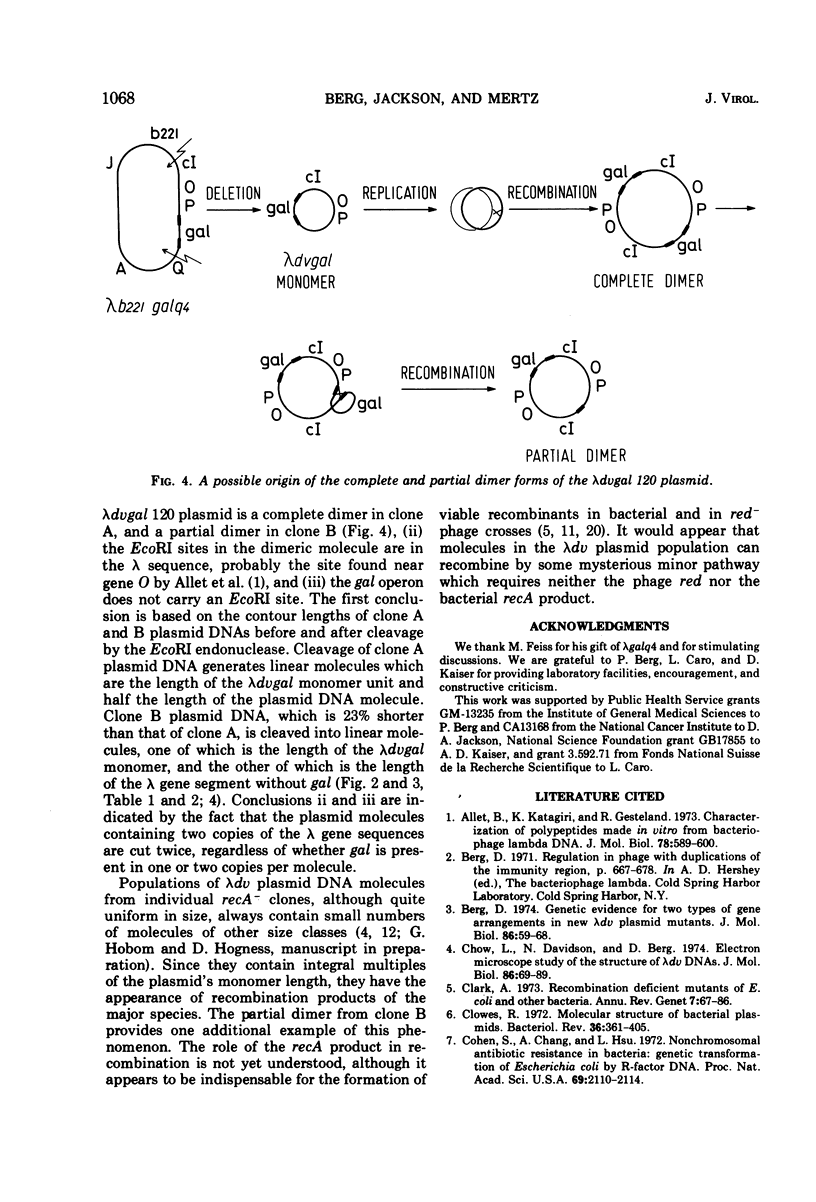
A λdvgal plasmid carrying genes for controlled plasmid replication from phage λ and the bacterial gal operon was isolated as a deletion mutant of phage λgalq4, which carries the gal operon between λ genes P and Q. The plasmid DNA was found in cell extracts as covalently closed circular molecules. The plasmid was characterized by using genetic crosses, digestion with the specific endonuclease EcoRI, sucrose gradient centrifugation, and electron microscopy. In one clone analyzed, the plasmid was a complete dimer (OλPλgalOλPλgal); in a subclone derived from it, the plasmid was a partial dimer with only one copy of gal (OλPλOλPλgal). The partial dimer may be a recombination product of the complete dimer, since test crosses show that the gal and λ sequences in the plasmid can be separated by recombination. Analyses of the EcoRI digests of plasmid DNAs indicated one cleavage site per λ gene sequence and none in the gal operon. A λdvgal monomer was approximately 6.7 × 106 daltons and the λ gene and gal components were 3.9 × 106 and 2.8 × 106 daltons, respectively. The λdvgal plasmid can be introduced into a new bacterial host by transfection at an efficiency of 10−6 per DNA molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Katagiri K. J., Gesteland R. F. Characterization of polypeptides made in vitro from bacteriophage lambda DNA. J Mol Biol. 1973 Aug 25;78(4):589–600. doi: 10.1016/0022-2836(73)90281-7. [DOI] [PubMed] [Google Scholar]
- Berg D. E. Genetic evidence for two types of gene arrangements in new lambdadv plasmid mutants. J Mol Biol. 1974 Jun 15;86(1):59–68. doi: 10.1016/s0022-2836(74)80007-0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Davidson N. Electron microscope study of the structures of lambdadv DNAs. J Mol Biol. 1974 Jun 15;86(1):69–89. doi: 10.1016/s0022-2836(74)80008-2. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiss M., Adyha S., Court D. L. Isolation of plaque-forming, galactose-transducing strains of phage lambda. Genetics. 1972 Jun;71(2):189–206. doi: 10.1093/genetics/71.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Symons R. H., Berg P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2904–2909. doi: 10.1073/pnas.69.10.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger-Gujer G., Boy de la Tour E., Berg D. E. Transfer of the lambda dv plasmid to new bacterial hosts. Virology. 1974 Apr;58(2):576–585. doi: 10.1016/0042-6822(74)90091-9. [DOI] [PubMed] [Google Scholar]
- Lieb M. Lambda mutants which persist as plasmids. J Virol. 1970 Aug;6(2):218–225. doi: 10.1128/jvi.6.2.218-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Matsubara K., Kaiser A. D. Lambda dv: an autonomously replicating DNA fragment. Cold Spring Harb Symp Quant Biol. 1968;33:769–775. doi: 10.1101/sqb.1968.033.01.088. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuya H., Richardson C. C. Synthesis of bacteriophage lambda DNA in vitro: requirement for O and P gene products. Proc Natl Acad Sci U S A. 1974 May;71(5):1758–1762. doi: 10.1073/pnas.71.5.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]



