Abstract
A multiparticulate product for colon-specific delivery of a small molecule drug has been developed and characterized. Microcrystalline cellulose core beads containing 5-aminosalicylic acid produced by extrusion-spheronization were coated with chitosan and Aquacoat® ECD mixtures according to a factorial design. Coated beads were characterized in terms of drug release, shape, and friability. The optimum formulation was enteric coated and exposed to media simulating conditions in the stomach, small intestine, and colon. Release studies in simulated intestinal fluid revealed that the drug release rate from the coated beads, which were spherical and rugged, depended on the level of chitosan in the coat and the coat thickness. Enlarged pores observed on the surface of the coated beads exposed to the medium containing rat cecal and colonic enzymes are believed to have caused a significant enhancement of the drug release rate compared to the control exposed only to simulated gastric and intestinal fluids. The release mechanisms involved polymer relaxation and dissolved drug diffusion for simulated intestinal fluid and simulated colonic fluid, respectively. From the facilitated drug release in a colonic environment and the inhibition of drug release under gastric and intestinal conditions, it can be concluded that this multiparticulate system demonstrates the potential for colon-specific drug delivery.
Keywords: Biodegradable polymer, chitosan, colonic drug delivery, controlled delivery, excipients, extrusion, formulation, site-specific delivery, spheronization
Introduction
Interest in the design of oral colon-specific drug delivery systems has increased in recent decades. Since the drug is delivered to the site of action to treat local conditions of the colon, such as inflammatory bowel diseases and colon cancer, lower doses may be used to achieve therapeutic levels (Gao et al., 2009; Laroui et al., 2010). Side effects associated with systemic absorption of the drug from the upper part of the gastrointestinal tract may be eliminated or reduced by inhibiting drug release until the delivery device enters the colon where a triggering mechanism initiates release. For example, gastrointestinal, hematological, and general side effects, such as agranulocytosis, toxic epidermal necrolysis, paresthesia, hepatotoxicity, pancreatitis, pulmonary disease, and male infertility have been reported (Goldman and Peppercorn, 1975; Goodacre et al., 1978; Gulley et al., 1979; Levi et al., 1979; Mihas et al., 1978; Peppercorn and Goldman, 1972) following oral administration of sulfasalazine, a prodrug version of 5-aminosalicylic acid (5-ASA, Fig. 1) that is used to treat irritable bowel disease. It has been shown that the colon can allow systemic absorption of peptides, such as calcitonin, vasopressin, insulin, and growth hormone (Fara, 1989; Saffran et al., 1986) because of its longer residence time, reduced proteolytic activity, and greater responsiveness to absorption enhancers (Sinha and Kumria, 2003; Uchiyama et al., 1999) in comparison to the small intestine. The motility pattern in the proximal colon in particular is characterized by antiperistalsis, muscular contractions that cause backward movement of the contents, resulting in a long residence time (Christensen, 1981). It has also been reported that these contents are less viscous than what is found in the rest of the colon (Cummings et al., 1990). This makes the proximal colon a suitable site for drug release.
Figure 1.
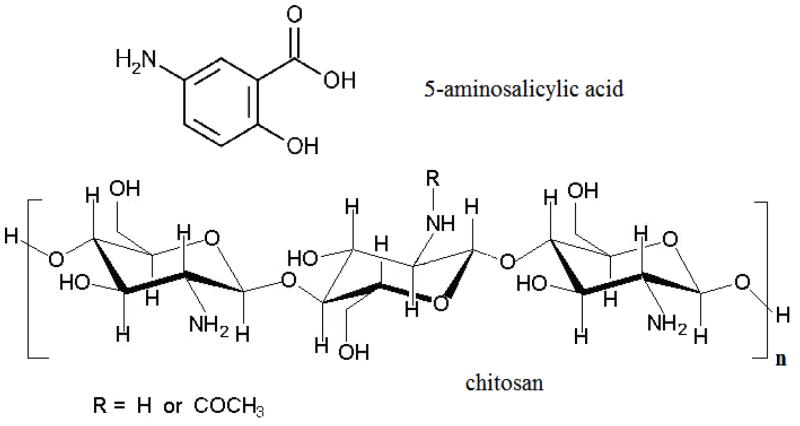
Chemical structures for 5-aminosalicylic acid and chitosan
Various approaches have been taken to achieve colon-specific drug delivery, including time-dependent, pH-dependent, pressure-dependent, and bacteria-dependent delivery systems. The description of these approaches and their respective drawbacks are reviewed elsewhere (Chourasia and Jain, 2003; Kumar and Mishra, 2008; Lamprecht, 2003; Rubinstein, 1995; Van den Mooter, 2006) and will not be discussed here. In general, though, it can be pointed out that lack of specificity in the onset of drug release and premature drug release before the delivery device arrives at the colon are the major disadvantages of some of these systems. Bacteria-dependent delivery systems employ biodegradable polymers that are considered dietary fiber in humans to prevent the release of the drug in the upper gastrointestinal tract (Chourasia and Jain, 2003; Kaushik et al., 2009; Kumar and Mishra, 2008; Lamprecht, 2003; Rubinstein, 1995; Van den Mooter, 2006). Microflora inhabiting the colon secrete enzymes that catalyze degradation reactions for these polymers which in turn cause the subsequent release of the drug (Chourasia and Jain, 2003; Kumar and Mishra, 2008; Lamprecht, 2003; Rubinstein, 1995). The delivery device developed in the present study takes advantage of this feature of the colon.
Coated beads with a combination of ethylcellulose and a biodegradable polymer in the coat have been prepared for colonic drug delivery. Typically the biodegradable polymer is a polysaccharide where a film formed by the polymer alone would be fragile and, due to its ability to swell in water, would release the drug too quickly. Addition of ethylcellulose to the coating material improves the physical and mechanical properties of the lm without affecting the sensitivity of the polysaccharide to microbial enzyme-catalyzed degradation (Leong et al., 2002; Milojevic et al., 1996; Siew et al., 2000), as long as the polymer is at a sufficient concentration. A higher ethylcellulose level in the coat slows the release rate by a greater reduction in the ability of the polysaccharide to swell (Wei et al., 2007). Biodegradable polymers that have been mixed with ethylcellulose and investigated for the purposes of colon-specific delivery include amylose (McConnell et al., 2007; Siew et al., 2004), high amylose maize starch (Eurylon 6 HP-PG) (Karrout et al., 2010), pectin (Wakerly et al., 1997; Wei et al., 2008; Wei et al., 2007), and calcium pectinate (Rubinstein and Radai, 1995; Semdé et al., 2000). Indeed, the ratio of pectin to ethylcellulose in the coat proved to be more important than the coat thickness in terms of controlling drug release (Siew et al., 2000).
In the present study, a mixture of chitosan (Figure 1) and Aquacoat® ECD 30 (an aqueous ethylcellulose pseudolatex dispersion) was used to coat microcrystalline cellulose (MCC) core beads containing a model drug, 5-ASA. The hydrophobic nature of ethylcellulose, the major component of the coat and of Aquacoat, is expected to dramatically reduce the rate of intestinal fluid infiltration into the coated beads to cause time-delayed drug release (Nunthanid et al., 2009; Umprayn et al., 1999). By incorporating chitosan into the coat, the drug release in the colon should occur more readily as chitosan is reported to be susceptible to degradation by microbial enzymes (Dodane and Vilivalam, 1998; McConnell et al., 2008; Thanou and Junginger, 2005; Umadevi et al., 2010; Zhao et al., 2008), although it is not degraded by enzymes found in the human small intestine (Furda, 1983; Ormrod et al., 1998). Such enzymes may include chitinases and chitosanases. Furthermore, chitosan undergoes dissolution in acidic media since the reported average pKa of the polyprotonated chitosan molecule is 6.3–6.5 (Liu et al., 2005; Sadeghi, 2010; Stoilova et al., 1999). The pH in the proximal colon of healthy human subjects was found to be as low as 5.8 probably due to the formation of short chain fatty acids such as acetic, propionic, and butyric acid as a result of microbial fermentation of polysaccharides (Evans et al., 1988; Simon and Gorbach, 1986). A drop in pH to as low as 4.0 was observed in untreated ulcerative colitis patients, while that of some patients receiving treatment was 5.5 (Press et al., 1998). In another study, the pH in the ascending or transverse colon of three ulcerative colitis patients was 2.3, 2.9 and 3.4 (Fallingborg et al., 1993). It is anticipated that this drop in pH at the proximal colon will cause the dissolution of chitosan from the coat, leaving pores that will subsequently ensure a marked increase in the drug release rate. These dual drug release triggering mechanisms, i.e. enzymatic degradation and dissolution of chitosan, are expected to ensure that the delivery system is both effective and successful.
There are advantages associated with the use of a multiparticulate system such as developed in the present study, as opposed to a conventional monolithic solid dosage form. Beads of less than 3 mm diameter are likely to exit the stomach along with chyme due to their small size (Blok et al., 1991; Meyer et al., 1988; Stotzer and Abrahamsson, 2000), such that they are not subject to gastric emptying variability (Stotzer and Abrahamsson, 2000), a major disadvantage with larger dosage forms such as tablets or capsules intended for controlled drug release. A monolithic solid dosage form could experience a lag time at the ileocecal junction, due to the sphincter muscle known as the ileocecal valve. Just as multiparticulates can pass through the pyloric sphincter to enter the small intestine with little interpatient variability in comparison to monolithic devices, even in the fed state (Davis et al., 1984; Sangekar et al., 1987), a multiparticulate system can surmount the issue of interpatient variability with transit time at the ileocecal junction (Hardy et al., 1985). The use of beads can eliminate dose dumping (Nurulaini and Wong, 2011; Roy and Shahiwala, 2009) and permits more rapid drug release in the colon as a result of their higher overall surface area (Natsume et al., 1991). It is easier to apply a uniform coat to the surface of beads due to their spherical shape (Liao and Lee, 1997; Madamba et al., 2007; Mayo Pedrosa et al., 2007). Moreover, it has been reported that a multiparticulate dosage form is retained longer in the ascending colon than is a single unit dosage form (Hardy et al., 1985; Shirsagar et al., 2011).
Enzymes from rat cecal and colon contents have been added to release media to evaluate a delivery system for its potential in colon-specific drug delivery (Yang, 2008). It has been reported that the rat colon contains much the same microbial contents as the human colon (Hawksworth et al., 1971; Rowland et al., 1983) and that chitosan is indeed degraded by the enzyme systems found there (Zhang and Neau, 2002). In our previous study (Omwancha et al., 2011), these enzymes were used to evaluate the potential for colon-specific delivery by chitosan and ethylcellulose in the coat of a compression-coated tablet. The tablets swelled and ruptured to release the drug when exposed to a release medium with or without enzymes after a lag time that depended on the coat level.
In the present study, we have developed a novel multiparticulate colon delivery system that can effectively suppress drug release until exposed to colonic enzymes in the proximal colon, following the dissolution of an enteric coat under small intestine conditions. It is important that an enteric coat is applied to the surfaces of chitosan/Aquacoat coated beads to prevent dissolution of chitosan under the acidic conditions of the stomach. The enteric coat will also protect chitosan contained in the coat from degradation by chitinase reported to be present in gastric fluid (Paoletti et al., 2007). In this study, the influence of the chitosan content in the Aquacoat coat and the thickness of the coat on bead shape, friability, and in vitro release of 5-ASA in simulated gastric, intestinal, and colonic fluid was examined. An experimental design was employed to reduce the number of experiments and to reveal the existence of two factor interactions on the responses. Modeling of the release kinetics was pursued to elucidate the release mechanism and to study the conditions that could change the release mechanism.
Materials and Methods
5-ASA, obtained from Sigma Chemical Co. (St. Louis, MO), was used as a relevant small molecule model drug. The degree of deacetylation (76%) of a chitosan sample (DCV BioNutritionals, Wilmington, DE) was measured using a literature circular dichroism method (Domard, 1987). Its viscosity average molecular weight (750,000) was determined by a literature viscometric method (Wang et al., 1991) once the degree of deacetylation was known. Avicel PH 101 (microcrystalline cellulose), Avicel RC 591(microcrystalline cellulose containing 11% w/w sodium carboxylmethyl cellulose) and Aquacoat® ECD 30 (an ethylcellulose pseudolatex dispersion) were generous gifts from FMC Corporation (Philadelphia, PA). Dibutyl sebacate (DBS), available from Sigma Chemical Co., was used in the chitosan/Aquacoat mixture as a plasticizer. Eudragit® L30-D from Evonik Degussa Corporation (Parsipanny, NJ) and its plasticizer (triethyl citrate) were used as the enteric coating material. All other chemicals were of analytical grade.
Preparation of core beads
A batch of 300 g consisting of 80% Avicel PH-101, 10% Avicel RC-591, and 10% 5-ASA powders was blended in a Hobart planetary mixer for 5 min before adding 310 ml of water. The wetted mass was then mixed for an additional 5 min and passed through an LCI EXD 60 twin screw extruder (Fuji Paudal Co., Osaka, Japan) equipped with a 1.2 mm axial screen and operated at 30 rpm. The resulting extrudate was immediately transferred to an LCI Q230 Marumerizer (Fuji Paudal Co.) for spheronization at 630 rpm for 5 min to obtain core beads that were dried for 8 h in an oven at 40 °C.
Preparation of Aquacoat/chitosan coating fluid
A 2% w/v chitosan solution prepared in 1% v/v acetic acid was added to an appropriate volume of Aquacoat® ECD 30 and then stirred using a magnetic stirrer for 24 h to form a smooth dispersion. DBS was added in the amount equivalent to 20% w/w of the total solids in the coating mixture before diluting the mixture with de-ionized water to the desired solids content, 5% w/v. This diluted mixture was then stirred for an additional 30 min to ensure sufficient interaction of the plasticizer molecules with those of chitosan and ethylcellulose to lower the minimum film forming temperature and to make the dried coat flexible. This last dilution provides a dispersion of sufficiently low viscosity that no problems are encountered when spraying the fluid through the nozzle of the fluid bed coater.
Coating of the core beads
The coating fluid was sprayed onto the surface of the beads in a Model 0002 fluid bed coater with a Wurster insert (Fluid Air Inc., Aurora, IL) under conditions given in Table 1. Product temperature was approximately 45 °C during coating; the outlet temperature was 43 °C. After application of the fluid was complete, the beads were fluidized in the coater for an additional 20 min to remove water and free acetic acid before curing them for 2 h in a constant temperature oven at 60 °C.
Table 1.
Coating parameters and conditions
| Inlet air flow | 40–45 SCFM* |
| Inlet air temperature | 60 °C |
| Coating fluid flow rate | 4–8 ml/min |
| Atomizing air pressure | 15 psi |
Standard cubic feet per minute
The batch of coated beads that released less than 10% of the loaded drug in simulated intestinal fluid (SIF) was enteric coated using Eudragit® L 30 D-55 following the directions recommended by the manufacturer (Evonik Industries) that included the use of triethyl citrate as a plasticizer, USP grade talc as an anti-tack agent, and de-ionized water.
Data analysis and statistical experimental design
The coat composition and the coat thickness are two critical factors that influence the performance of many controlled release dosage forms (Kramar et al., 2003). A two level 22 factorial design with two factors (chitosan content and coat level) and three center points was generated (Table 2) and the experiments were performed randomly. The percentage coating level represents the percentage mass gain of a batch of core beads that have been coated and dried. The responses included bead shape (Aspect Ratio and Projection Sphericity) and friability. The response data were collected and then analyzed by Design Expert version 7 using analysis of variance (ANOVA). Replicated center points, with each factor set at its middle level, provided additional degrees of freedom for estimation of pure error and the presence or absence of curvature in the responses. Fitting of model equations to release data was accomplished using SigmaStat 3.1 (Systat Software, Inc., Chicago, IL). Assessment of significant differences was conducted at the 95% confidence level.
Table 2.
Two factor, two level factorial design with three center points in coded form and with actual values
| Standard Run Order | Chitosan Content in the Coat* | Coating Level* |
|---|---|---|
| 1 | −1 (8%) | −1 (20%) |
| 2 | −1 (8%) | +1 (60%) |
| 3 | +1 (14%) | −1 (20%) |
| 4 | +1 (14%) | +1 (60%) |
| 5 | 0 (11%) | 0 (40%) |
| 6 | 0 (11%) | 0 (40%) |
| 7 | 0 (11%) | 0 (40%) |
Values in parentheses are the actual levels for the factors
Size and shape of the beads
Sieve analysis of the core and coated beads was performed for 5 min using a stacked nest of United States Standard Sieves with a Retsch Vibrotronic VEI sieve shaker (Brinkmann Instrument Co., Westbury, NY). Although a total bead mass of at least 100 g was studied, a smaller sample of beads (30 g) was screened each time to avoid screen blinding. Core and coated beads in the 14/20 mesh cut (1.41–0.84 mm) and 12/18 mesh cut (1.68–1.00 mm), respectively, were used in further processing to control the size and size distribution.
The shape of coated beads was assessed using the Sympatec QICPIC Dynamic Image Analysis System (Clausthal-Zellerfeld, Germany) with Windox 5.0 software. A VIBRI/L vibratory feeder channeled the beads into the RODOS/L high-speed dry-sample disperser that creates a particle flow of up to 100 m/s through a Venturi tube. As a result, the beads were properly dispersed and aerosolized by centrifugal forces caused by velocity gradients. In this equipment, motion blur is minimized by utilizing a pulsed laser light source with a very short exposure time of approximately 1 ns. This light source works synchronously with a high-speed digital camera to provide clear images. To obtain the bead sphericity with the QICPIC, the Windox software calculates the ratio of the perimeter of a circle that has the same projected area as the bead image to the measured perimeter of the image. The aspect ratio (AR) was calculated as the ratio of the minimum and maximum Feret diameters. It follows then that the values of sphericity and AR must fall in the range 0–1, where the image of a perfect sphere would have both sphericity and AR equal to 1.
Friability studies
The friability of each batch of coated beads was assessed in triplicate by placing 1 g of coated beads from the 12/18 mesh cut along with twenty-five 3 mm glass beads in a model DF-1W friabilator (Distek Inc., North Brunswick, NJ) that rotated the beads 100 times in a vertical motion at 25 rpm, dropping them six inches (15.24 cm) at the top of each rotation. The beads were screened with sieve No. 10 to separate the glass beads and with sieve No. 18 to remove any particles formed during the test. Friability was then obtained by expressing the loss of mass of the coated beads as a percentage.
Microscopy
The surface characteristics of coated beads exposed or not exposed to a release medium were evaluated using a Philips FEI XL-30 FEG-ESEM environmental scanning electron microscope (ESEM) equipped with a standard gaseous secondary electron detector and moisture at 0.7 Torr in the sample chamber as a secondary electron amplifier. The beads were secured to ESEM stubs with carbon black tape and placed on a stainless steel sample holder surface. The sample holders were then mounted on the Peltier stage. The beads were examined with a working distance of 7.5–7.8 mm, an accelerating voltage of 10 kV and a spot size of 3. The ESEM data were analyzed by Scandium software.
In vitro release studies
Release studies were performed for 12 h with six replicates in simulated intestinal fluid without added enzymes (SIF, pH 6.8, 0.05 M phosphate buffer) since there are no enzymes in the human small intestine that degrade chitosan (Furda, 1983). Accurately weighed beads placed in USP type I baskets were rotated at 100 rpm in vessels containing 900 ml of SIF at 37 °C. Samples of 3 ml were withdrawn at regular intervals and analyzed for 5-ASA content by UV spectrophotometry at 330 nm.
The potential for colon-specific drug delivery of the batches of beads that released less than 10% of 5-ASA in SIF over 6 h was assessed by exposing beads from the same batch to simulated gastric fluid (SGF, 0.1 N HCl) for 2 h, SIF for 6 h, following which the beads were transferred to 20 ml of a solution containing rat cecal and colonic enzymes in a shaker bath maintained at 37 °C and 100 rpm. Aliquots of 300 μl from the solution containing cecal and colonic enzymes were withdrawn with replacement at regular intervals and assayed for 5-ASA content following precipitation of proteins using methanol and using a literature pre-column derivatization method that N-alkylated 5-ASA by reaction with propionic anhydride (Hussain et al., 1998). The HPLC system was a Perkin Elmer series 200 pump, autosampler, and column oven as well as a Spectra Physics Analytical fluorescence detector (excitation at 315 nm; emission at 430 nm) and a UV/Vis detector (absorbance at 310 nm). A Phenomenex C18 column (150 × 4.60 mm, Hypersil 5 μm DDS) was protected by a Phenomenex guard column with the same stationary phase. The mobile phase consisted of 0.1 M acetic acid, acetonitrile, and triethylamine (93.02:6.63:0.35) with a 1.5 ml/min flow rate.
Preparation of cecal and colonic medium
Male Sprague Dawley rats weighing 300–400 g were anesthetized under isoflurane and sacrificed by decapitation before harvesting the cecum and colon contents. The contents were then subjected to a literature differential centrifugation technique at 0–4 °C (Prizont and Konigsberg, 1981; Zhang and Neau, 2002). The contents were first weighed in centrifuge tubes and then diluted with 0.05 M ice cold isotonic phosphate buffer at pH 6.8. The mixture was centrifuged at 500 g for 15 min to remove debris. Supernatants were then centrifuged at 15,000 g for another 30 min in a refrigerated centrifuge (0–4 °C) and pellets deposited at the bottom of the centrifuge tubes were discarded to obtain a clear supernatant containing the extracellular microbial enzymes. Studies have shown that the extracellular enzymes were far more effective at chitosan degradation than the cell-associated enzymes were (Zhang and Neau, 2001). The protein content in the supernatant was determined using a Micro BCA protein assay with bovine serum albumin as the protein standard. Dilution of the protein content to 3 mg/ml was accomplished with phosphate buffer prior to use of the mixture, named simulated cecal and colonic fluid (SCF), in the release study to evaluate the potential for colon-specific drug delivery.
Results
Friability
Friability results shown in Table 3 indicate that coated beads were very rugged (< 1% friability). Although each batch of beads was rugged enough that this response did not require modeling, ANOVA revealed that the model equation describing the data was insignificant (p>0.05).
Table 3.
Results for coated bead shape (aspect ratio and projection sphericity) and friability
| Responses | |||
|---|---|---|---|
| Bead Shape | Friability (%) | ||
| Standard Run Order | Aspect Ratio | Projection Sphericity | Mean (s.e.) |
| 1 | 0.92 | 0.94 | 0.28 (0.25) |
| 2 | 0.92 | 0.93 | 0.35 (0.30) |
| 3 | 0.92 | 0.94 | 0.07 (0.07) |
| 4 | 0.92 | 0.93 | 0.28 (0.24) |
| 5 | 0.93 | 0.94 | 0.33 (0.33) |
| 6 | 0.92 | 0.94 | 0.28 (0.17) |
| 7 | 0.92 | 0.94 | 0.27 (0.27) |
Shape of coated beads
The aspect ratio and sphericity results, as presented in Table 3, indicate that the bead shape is not affected by the chitosan level in the coat or by the coating thickness. The values of sphericity and aspect ratio are so consistent and very close to each other that modeling was not required.
In vitro release studies
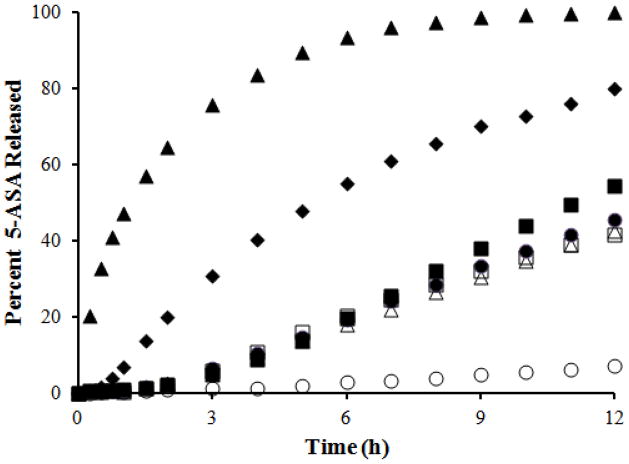
Figure 2 presents data for drug release from the core and coated beads in SIF over 12 h. In general, these results show that an increase in the coating thickness and a decrease in chitosan level in the coat caused a reduction in the drug release rate. Based on these results, the bead product containing 8% chitosan in the coat and a 60% coating level suppressed the release of 5-ASA in SIF and thus qualified for further studies. It can also be observed that a zero order drug release rate in SIF can be achieved using any of several multiparticulate delivery systems developed in the present study. The percentage of the drug released after 6 h in SIF was modeled to obtain a mathematical expression for the effect of the coded values of the two factors varied in the product, namely the chitosan content in the coat (A) and the coat level (B). The following equation was obtained:
| (1) |
that indicates that an increase in the chitosan content can encourage release in SIF, whereas an increase in the coat level will hinder it. ANOVA showed that this model was significant (p = 0.0172). It also revealed that each of the main factor effects was significant (p ≤ 0.0204). The curvature and lack of fit were insignificant (p = 0.4116 and 0.0884, respectively), indicating that the linear model presented above describes the data in the design space.
Figure 2.
Release of 5-ASA in SIF over 12 h from core beads (▲) and coated beads with different chitosan levels (CH) in the coat and coat levels (CL) as follows: 14% CH and 20% CL (◆), 8% CH and 20% CL (■), 11% CH and 40% CL (●), 11% CH and 40% CL (△), 11% CH and 40% CL (◇), 14% CH and 60% CL (□), and 8% CH and 60% CL (○).
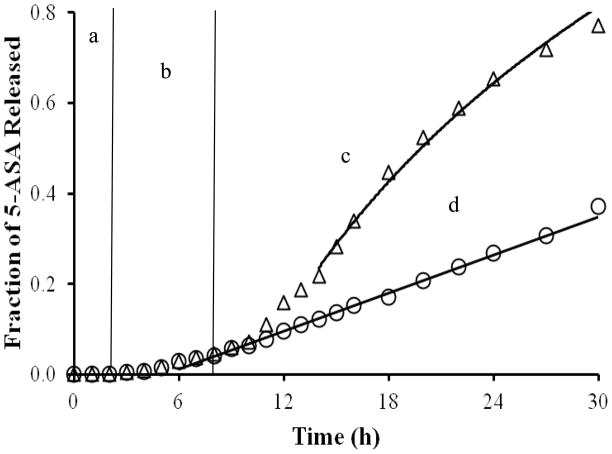
A successful colon-specific drug delivery system must suppress the release of the drug as it moves through the stomach and the small intestine and should allow adequate release upon entering the colon. With this in mind, the bead product from the formulation that qualified for further studies based on release results in SIF was enteric coated. It was then exposed to SGF for 2 h, followed by 6 h in SIF and 22 h in SCF. The same coated bead product acted as the control by exposure to SGF for 2 h and SIF for 28 h, but no exposure to cecal and colonic enzymes. The enteric coat applied to the surface of the coated beads successfully suppressed drug release for the entire 2 h in SGF as shown in Figure 3. It is clear from the results shown in Figure 3 that the bead product exposed to a solution containing rat cecal and colonic enzymes released 81% of the drug over 30 h. In contrast, the control released 26% of the incorporated drug over the same time period. This represents a threefold increase in drug release due to the enzymatic degradation of the chitosan contained in the coat of the coated bead product.
Figure 3.
Release profile of the coated bead product under conditions simulating a. the stomach, b. and c. the small intestine, and d. the colon. The triangles represent coated beads that have been exposed to SGF for 2 h, SIF for 6 h, and then SCF for 22 h. The diamonds represent the coated beads that have been exposed to SGF for 2 h and then to SIF for 28 h. A predictive curve for each profile overlays the corresponding data.
Microscopy
The ESEM micrographs at 1K and 5K magnification of coated beads not exposed to a release medium, to SIF, or to SIF and then SCF are shown in Figure 4. From these images, it is evident that there is a lack of pores on the surfaces of the beads that were not exposed to a release medium. The pores on the surfaces of those beads exposed to SIF and then SCF are obviously larger than those observed on the surfaces of the beads exposed to SIF alone.
Figure 4.
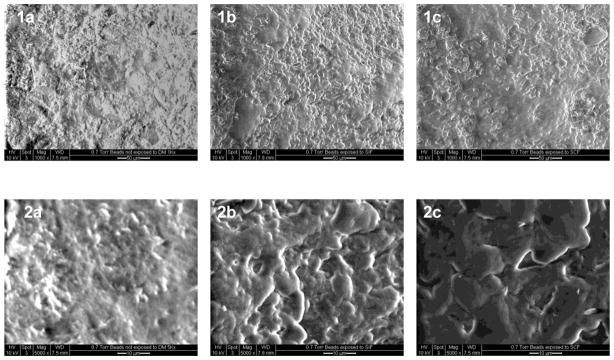
ESEM micrographs for coated beads at 1K magnification for 1a, 1b and 1c and 5K magnification for 2a, 2b and 2c. Beads in 1a and 2a have not been exposed to a release medium, 1b and 2b have been exposed to SIF, and 1c and 2c have been exposed to SIF and SCF.
Discussion
Friability studies were conducted to ensure that coated beads prepared in this study were rugged enough to withstand harsh processing conditions such as those experienced during packaging. Statistical analysis revealed that the level of chitosan contained in the coat and the coat level did not significantly affect friability of the coated beads. Aspect ratios and sphericity scores revealed that neither the chitosan content nor the coat level affected the roundness of the coated beads, confirming the uniformity of the coat applied to the surfaces of these beads.
Upon arrival at the small intestine, the enteric coat will dissolve and then the chitosan/ethylcellulose coat will be exposed to physiological fluid with a near neutral pH. Since each chitosan chain in the coat has a number of amine groups protonated by acetic acid, such that the polymer is actually present as chitosan acetate, some of the amine groups will be deprotonated at this physiological pH and those that remain protonated might or might not be sufficient to allow the polymer to dissolve. For this reason, some drug release at a slow rate under conditions simulating the small intestine can be expected because pores could open up in the coat when chitosan dissolves. Alternatively, if not dissolving under these conditions, chitosan could still encourage release by taking up water and swelling. It is possible that the swollen chitosan allows a sufficiently low tortuosity that a low release rate for 5-ASA is possible in the small intestine. Any bead product that released less than 10% of the drug in SIF over a 6 h study was considered eligible for further studies. The 6 h time period was based on the small intestine transit time that is reasonably consistent (Liu et al., 2010), even when diarrhea or constipation is diagnosed (Waller, 1975).
Drug release rate in SIF could be modulated by varying either the level of chitosan in the coat or the coating thickness. An increase in drug release rate was obtained when the chitosan level in the coat was increased or the coating thickness decreased. Since chitosan is hydrophilic, it hydrates and attempts to swell in the presence of SIF. Due to its hydrophobic character, ethylcellulose hinders the entry of intestinal fluid into the coat. Likely because it swells less than chitosan, ethylcellulose also hinders the swelling of hydrated chitosan, as demonstrated with amylose-ethylcellulose films (Siew et al., 2000). The presence of a lag time prior to drug release, as shown in Figure 2, acknowledges the slow entry of the dissolution medium into the coated beads as well as successful formation of a continuous film on the surfaces of the core beads. There is no lag time associated with the release profile for uncoated beads. Hydrated and swollen chitosan provides less tortuous pathways, and dissolved chitosan offers open pores, through which drug molecules can readily diffuse. This explains the observed increase in drug release rate with higher chitosan content in the coat. A reduction in drug release rate with an increase in the coating level results from an increase in the length of the diffusion layer in the coat. Since a desired formulation in colon specific delivery is one that effectively retards or eliminates the release of the drug in SIF, the bead product containing 8% chitosan in the coat and coated at 60% coating level that released less than 3% of the incorporated drug over 6 h in SIF qualified for further studies.
To evaluate the potential of this formulation for colon specific drug delivery, release studies were conducted under conditions mimicking the stomach, small intestine, and colon. From the results depicted in Figure 3, 5-ASA release was effectively suppressed until the coated beads were exposed to SCF. The presence of relatively large pores on the surface of those beads exposed to SCF as seen in the ESEM images (Figure 4, 2c vs. 2a or 2b) confirms the assertion of chitosan degradation when exposed to rat cecal and colonic enzymes. The pores on the surfaces of those beads exposed to SIF and then SCF are obviously larger than those observed on the surfaces of the beads exposed to SIF alone, although these pores might not be seen visually or by optical microscopy (Wei et al., 2010). This would explain the increase in 5-ASA release rate observed with the beads exposed to a medium containing rat cecal and colonic enzymes.
Tozaki et al. reported that chitosan capsules released more 5-ASA in a medium containing rat cecal and colonic enzymes compared to the control that was exposed to phosphate buffered saline containing no enzymes (Tozaki et al., 2002). The delivery system developed in the present study is superior to those chitosan capsules because of the advantages, highlighted in the introduction, of using multiparticulates as opposed to single unit dosage forms to minimize interpatient variability in gastrointestinal transit times. In addition, the production methods for the present products are easily accomplished in the manufacturing setting.
The most elementary drug release mechanism is dissolved drug diffusion out of a matrix delivery device that remains essentially intact during drug release. The Higuchi equation describes the drug release rate under this condition:
| (1) |
where Mt/M∞ is the fraction of the drug released at time t and k1 is the release rate constant estimated by fitting the equation to the release data. Some suggest that this equation should be applied only up to 75% (Carstensen, 1993) or even 60% cumulative drug released (Siepmann and Peppas, 2001a, b). However, based on the fact that chitosan is a hydrophilic polymer that can become hydrated and swollen, it is likely that a contributing mechanism to drug release is polymer relaxation. Peppas and Sahlin (1989) presented an equation that describes the contributions of these two mechanisms to the fraction of drug released at time t, Mt/M∞:
| (2) |
where represents the contribution from drug diffusion and k2t is the contribution from polymer hydration and swelling. A lag time must be acknowledged in the present study at least because the beads spent two hours in SGF where fluid entry and subsequent drug release was inhibited by the enteric coat. Adding the lag time to the equation gives:
| (3) |
A linear relationship is evident in the 7–27 h data for the control beads that were exposed to SGF for 2 h and then SIF (Fig. 3). It is not surprising, then, that fitting equation 3 to this range of data gives k1 equal to 1.32 × 10−8 which is not significant (p = 1.000). Limiting the equation to the second term:
| (4) |
results in a significant model equation (p < 0.001), a significant k2 and tlag (p < 0.001) and a good fit to the data (R2 = 0.998). The coefficient k2 equals 0.0140 (0.0001773) and tlag equals 5.08 (0.147) h where the values in parentheses are the standard error of the estimate. This means that, after a lag time of a little more than five hours, release of drug is dominated by the polymer relaxation mechanism, because chitosan is hydrating and swelling to provide a less tortuous and fluid-filled pathway through which dissolved drug can diffuse.
For the beads exposed to SGF, SIF, and then SCF, it is apparent that a deviation from the control data takes place at 10 h and the subsequent data is nonlinear. A fit of equation 3 to the 14–27 h data yields k2 equal to 1.66 × 10−11 that is not significant (p = 1.000). Dropping the second term on the right-hand side of equation 3 gives:
| (5) |
and fitting the equation again to the data yielded a significant model equation (p < 0.001), a significant k1 and tlag (p < 0.001), and an excellent fit to the data (R2 = 0.999). The value of k1 is 0.193 (0.00141) and tlag is 12.8 (0.0726) h. From this fit to the data, it is clear that the drug release mechanism is dominated by diffusion of dissolved drug out of the device. This is not surprising since, as chitosan is hydrated and swollen, enzyme-catalyzed degradation of chitosan between 10 and 14 h provides a fluid-filled pore that offers less resistance to drug diffusion than does a region of hydrated and swollen polymer. In Figure 3, predicted data using this equation can describe release data in the presence of rat cecal and colonic enzymes, and the equation for the control data describes its respective experimental data.
Since drug release rates due to pore formation are correlated to the surface area of the coat that has been eliminated, the essentially consistent drug diffusion mechanism over the 14–27 h time period indicates that the pores have formed by 14 hours in the release study. Further pore formation is essentially non-existent, likely due to product inhibition or degradation of the enzymes.
Liu et al. (2007) reported the influence of the level of chitosan in a cellulose acetate coat on in vitro budesonide release from coated tablets in simulated intestinal and colonic fluids. Utilizing differing amounts of chitosan in chitosan/cellulose acetate coating levels of 10, 12, and 14% w/w with Eudragit® L100-55 as an enteric coat, drug release in simulated gastric and intestinal fluids was prevented. Based on tablets coated at 12 % w/w, exposure for 2 h to simulated gastric fluid, for 4 h to simulated intestinal fluid, and for 18 h to simulated colonic fluid resulted in approximately 70 and 40 % of the drug released from tablets where the coat contained 25 and 15 % w/w chitosan. This is comparable to the 66 % of 5-ASA released after 24 h in the present study by the optimum batch of coated beads (Fig. 3). Since Liu et al. demonstrated the influence of the level of chitosan in the coat on the extent of drug release under simulated colonic conditions, this was not pursued in the present study.
In a recent study by Wei et al. (Wei et al., 2010), pellets containing 5-ASA, lactose, and MCC were coated with a mixture of chitosan and Kollicoat SR 30D to achieve site-specific delivery to the colon. Kollicoat SR 30D is a pseudolatex dispersion of a different hydrophobic polymer, poly(vinylacetate). Chitosan was between 2 and 5% of the coat, based on 27 g of poly(vinylacetate) per 100 ml of Kollicoat SR 30 D, with 15–25% coating levels. They suggested that the improved release rate when rat cecal contents were added to simulated intestinal fluid confirms that chitosan is indeed degraded by rat cecal enzymes. For the product they considered suitable (3% chitosan with a 25% coat level), approximately 50% of the incorporated drug is released after 10 h of exposure to a medium with 4% w/v rat cecal contents. While the percentage of 5-ASA released in SCF (Figure 3) is comparable after 10 h of exposure to rat cecal and colonic enzymes, the difference between this release profile and that of the control at the same time point is far greater than was observed by Wei at al. In that article, there was no report that their coat was plasticized or cured. The coat in the present study was cured immediately after the coat was applied to facilitate coalescence of the plasticized coat, leading to formation of a homogeneous and continuous film. Coated beads exhibit cracking and chipping of the coat over time if a plasticizer is not included in the coating material, and the release profile is prone to change with time if the coat was not cured. The release profiles become altered because coalescence of the polymer particles is incomplete immediately after coating, especially when the coating is based on aqueous based pseudolatex dispersions, such as Aquacoat® ECD and Kollicoat SR 30D (Hutchings et al., 1994; Shao et al., 2002). During curing, polymer particles are thermodynamically encouraged to form the most stable arrangement within the coat. As recommended by the manufacturer of Aquacoat® ECD, curing the coated beads for 2 h in a constant temperature oven at 60 °C was completed in the present study to ensure reproducibility of the release profiles.
The reliability of the delivery system developed in the present study lies in the fact that drug release in the colon will be triggered by (i) degradation of chitosan by enzymes secreted by bacteria inhabiting the colon and/or (ii) dissolution of chitosan in the low pH of the proximal colon due to polysaccharide fermentation or disease. As a result of either mechanism or both mechanisms, release medium filled pores will be formed, leading to facilitated drug release in the colon.
Conclusion
Proper selection of the coating thickness and the chitosan level in the coat can minimize drug release in simulated intestinal fluid. Since different formulations provided zero order release in SIF, these coated bead products can also be used to achieve controlled release of drugs in the small intestine. Beads coated with chitosan/Aquacoat proved to be susceptible to the action of rat cecal and colonic enzymes and demonstrated their potential for colon-specific drug delivery. ESEM images confirmed the formation of larger pores as the means by which the drug release rate was improved in simulated colonic fluid.
Acknowledgments
The authors would like to thank Richard D. Bruce, Senior Scientist, Johnson & Johnson (Spring House, PA), for access to and training on the use of the QICPIC and ESEM equipment. The NIH is acknowledged for financial support of this research (GM084428-01). We are grateful to Brenda Pinto and FMC Corporation (Philadelphia, PA) for the generous gifts of Aquacoat® 30D, Avicel® PH-101, and Avicel® RC-591.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blok D, Arndt J, De Haan F, Vermeij P, Junginger H, Pauwels E. Scintigraphic investigation of the gastric emptying of 3 mm pellets in human volunteers. Int J Pharm. 1991;73:171–176. [Google Scholar]
- Carstensen JT. Lancaster: Technomic. 1993. Pharmaceutical Principles of Solid Dosage Forms. [Google Scholar]
- Chourasia M, Jain S. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharmacol Sci. 2003;6:33–66. [PubMed] [Google Scholar]
- Christensen J. Motility in the colon. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 1. Raven Press; New York: 1981. pp. 445–471. [Google Scholar]
- Cummings J, Banwell J, Segal I, Coleman N, Englyst H, Macfarlane G. The amount and composition of large bowel contents in man. J Gastroenterol. 1990;98:A408. [Google Scholar]
- Davis SS, Hardy JG, Taylor MJ, Whalley DR, Wilson CG. The effect of food on the gastrointestinal transit of pellets and an osmotic device (Osmet) Int J Pharm. 1984;21:331–340. [Google Scholar]
- Dodane V, Vilivalam VD. Pharmaceutical applications of chitosan. Pharm Sci Technol Today. 1998;1:246–253. [Google Scholar]
- Domard A. Determination of N-acetyl content in chitosan samples by cd measurements. Int J Biol Macromol. 1987;9:333–336. [Google Scholar]
- Evans D, Pye G, Bramley R, Clark A, Dyson T, Hardcastle J. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallingborg J, Christensen LA, Jacobsen BA, Rasmussen SN. Very low intraluminal colonic pH in patients with active ulcerative colitis. Dig Dis Sci. 1993;38:1989–1993. doi: 10.1007/BF01297074. [DOI] [PubMed] [Google Scholar]
- Fara J. Colonic drug absorption and metabolism. In: Prescott LF, Nimmo WS, editors. Novel Drug Delivery and Its Therapeutic Application. Chichester: Wiley; 1989. pp. 103–112. [Google Scholar]
- Furda I. Aminopolysaccharides - their potential as dietary fiber. In: Furda I, editor. Unconventional sources of dietary fiber. Washington, DC: ACS; 1983. pp. 105–122. [Google Scholar]
- Gao SQ, Sun Y, Kope ková P, Peterson CM, Kope ek J. Antitumor efficacy of colon specific HPMA copolymer/9 aminocamptothecin conjugates in mice bearing human colon carcinoma xenografts. Macromol Biosci. 2009;9:1135–1142. doi: 10.1002/mabi.200900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman P, Peppercorn M. Drug therapy: Sulfasalazine. N Engl J Med. 1975;293:20–23. doi: 10.1056/NEJM197507032930105. [DOI] [PubMed] [Google Scholar]
- Goodacre R, Ali M, Vanderlinden B, Hamilton J, Castelli M, Seaton T. Hemolytic anemia in patients receiving sulfasalazine. Digestion. 1978;17:503–508. doi: 10.1159/000198156. [DOI] [PubMed] [Google Scholar]
- Gulley R, Mirza A, Kelly C. Hepatotoxicity of salicylazosulfapyridine: a case report and review of the literature. Am J Gastroenterol. 1979;72:561–564. [PubMed] [Google Scholar]
- Hardy J, Wilson C, Wood E. Drug delivery to the proximal colon. J Pharm Pharmacol. 1985;37:874–877. doi: 10.1111/j.2042-7158.1985.tb04992.x. [DOI] [PubMed] [Google Scholar]
- Hawksworth G, Drasar B, Hill M. Intestinal bacteria and the hydrolysis of glycosidic bonds. J Med Microbiol. 1971;4:451–459. doi: 10.1099/00222615-4-4-451. [DOI] [PubMed] [Google Scholar]
- Hussain F, Ajjan R, Moustafa M, Anderson J, Riley S. Simple method for the determination of 5-aminosalicylic and N-acetyl-5-aminosalicylic acid in rectal tissue biopsies. J Chromatogr B Biomed Appl. 1998;716:257–266. doi: 10.1016/s0378-4347(98)00323-5. [DOI] [PubMed] [Google Scholar]
- Hutchings D, Kuzmak B, Sakr A. Processing considerations for an EC latex coating system: influence of curing time and temperature. Pharm Res. 1994;11:1474–1478. doi: 10.1023/a:1018960310144. [DOI] [PubMed] [Google Scholar]
- Karrout Y, Neut C, Siepmann F, Wils D, Ravaux P, Deremaux L, Flament MP, Dubreuil L, Lemdani M, Desreumaux P, Siepmann J. Enzymatically degraded Eurylon 6 HP-PG: Ethylcellulose film coatings for colon targeting in inflammatory bowel disease patients. J Pharm Pharmacol. 2010;62:1676–1684. doi: 10.1111/j.2042-7158.2010.01165.x. [DOI] [PubMed] [Google Scholar]
- Kaushik D, Sardana S, Mishra DN. Implications of biodegradable and bioadhesive systems in colon delivery. Int J Pharm Sci Drug Res. 2009;1:55–62. [Google Scholar]
- Kramar A, Turk S, Vrecer F. Statistical optimisation of diclofenac sustained release pellets coated with polymethacrylic films. Int J Pharm. 2003;256:43–52. doi: 10.1016/s0378-5173(03)00061-9. [DOI] [PubMed] [Google Scholar]
- Kumar P, Mishra B. Colon targeted drug delivery systems - an overview. Curr Drug Delivery. 2008;5:186–198. doi: 10.2174/156720108784911712. [DOI] [PubMed] [Google Scholar]
- Lamprecht A. Multiparticulate systems in the treatment of inflammatory bowel disease. Curr Drug Targets: Inflammation Allergy. 2003;2:137–144. doi: 10.2174/1568010033484188. [DOI] [PubMed] [Google Scholar]
- Laroui H, Dalmasso G, Nguyen HTT, Yan Y, Sitaraman SV, Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. J Gastroenterol. 2010;138:843–853. doi: 10.1053/j.gastro.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Leong C, Newton J, Basit A, Podczeck F, Cummings J, Ring S. The formation of colonic digestible films of amylose and ethylcellulose from aqueous dispersions at temperatures below 37 C. Eur J Pharm Biopharm. 2002;54:291–297. doi: 10.1016/s0939-6411(02)00121-2. [DOI] [PubMed] [Google Scholar]
- Levi A, Fisher A, Hughes L, Hendry W. Male infertility due to sulphasalazine. Lancet. 1979;314:276–278. doi: 10.1016/s0140-6736(79)90292-7. [DOI] [PubMed] [Google Scholar]
- Liao Y, Lee D. Slow release from a coated sphere with slight deformations of coating film and drug matrix. J Pharm Sci. 1997;86:92–100. doi: 10.1021/js960143c. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang XG, Nie SF, Wei LL, Zhou LL, Liu H, Tang R, Pan WS. Chitosan-based controlled porosity osmotic pump for colon-specific delivery system: Screening of formulation variables and in vitro investigation. Int J Pharm. 2007;332:115–124. doi: 10.1016/j.ijpharm.2006.09.038. [DOI] [PubMed] [Google Scholar]
- Liu HY, Pi XT, Zheng XL, Hou WS, Cui JG. Pharmacokinetics of aminophylline delivered to the small intestine and colon using remote controlled capsules. Chin Med J (Engl) 2010;123:320–325. [PubMed] [Google Scholar]
- Liu W, Sun S, Cao Z, Zhang X, Yao K, Lu WW, Luk K. An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials. 2005;26:2705–2711. doi: 10.1016/j.biomaterials.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Madamba MC, Mullett WM, Debnath S, Kwong E. Characterization of tablet film coatings using a laser-induced breakdown spectroscopic technique. AAPS PharmSciTech. 2007;8:184–190. doi: 10.1208/pt0802041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo Pedrosa M, Alvarez Lorenzo C, Lacík I, Martinez Pacheco R, Concheiro A. Sustained release pellets based on poly(N isopropyl acrylamide): Matrix and in situ photopolymerization coated systems. J Pharm Sci. 2007;96:93–105. doi: 10.1002/jps.20708. [DOI] [PubMed] [Google Scholar]
- McConnell EL, Murdan S, Basit AW. An investigation into the digestion of chitosan (noncrosslinked and crosslinked) by human colonic bacteria. J Pharm Sci. 2008;97:3820–3829. doi: 10.1002/jps.21271. [DOI] [PubMed] [Google Scholar]
- McConnell EL, Tutas J, Mohamed MAM, Banning D, Basit AW. Colonic drug delivery using amylose films: The role of aqueous ethylcellulose dispersions in controlling drug release. Cellulose. 2007;14:25–34. [Google Scholar]
- Meyer J, Elashoff J, Porter-Fink V, Dressman J, Amidon G. Human postprandial gastric emptying of 1–3-millimeter spheres. J Gastroenterol. 1988;94:1315–1325. doi: 10.1016/0016-5085(88)90669-5. [DOI] [PubMed] [Google Scholar]
- Mihas AA, Goldenberg DJ, Slaughter RL. Sulfasalazine toxic reactions. J Am Med Assoc. 1978;239:2590–2591. doi: 10.1001/jama.239.24.2590. [DOI] [PubMed] [Google Scholar]
- Milojevic S, Newton JM, Cummings JH, Gibson GR, Louise Botham R, Ring SG, Stockham M, Allwood MC. Amylose as a coating for drug delivery to the colon: Preparation and in vitro evaluation using 5-aminosalicylic acid pellets. J Control Rel. 1996;38:75–84. [Google Scholar]
- Natsume H, Sugibayashi K, Morimoto Y. In vitro release profile of mitomycin C from albumin microspheres: Extrapolation from macrospheres to microspheres. Pharm Res. 1991;8:185–190. doi: 10.1023/a:1015883818651. [DOI] [PubMed] [Google Scholar]
- Nunthanid J, Luangtana-anan M, Sriamornsak P, Limmatvapirat S, Huanbutta K, Puttipipatkhachorn S. Use of spray-dried chitosan acetate and ethylcellulose as compression coats for colonic drug delivery: Effect of swelling on triggering in vitro drug release. Eur J Pharm Biopharm. 2009;71:356–361. doi: 10.1016/j.ejpb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Nurulaini H, Wong TW. Design of in situ dispersible and calcium cross-linked alginate pellets as intestinal-specific drug carrier by melt pelletization technique. J Pharm Sci. 2011;100:2248–2257. doi: 10.1002/jps.22459. [DOI] [PubMed] [Google Scholar]
- Omwancha W, Kouba C, Yelamanchili S, Neau SH. Colon-specific drug delivery using ethylcellulose and chitosan in the coat of compression-coated tablets. Drug Dev Ind Pharm. 2011;37:945–953. doi: 10.3109/03639045.2010.551773. [DOI] [PubMed] [Google Scholar]
- Ormrod DJ, Holmes CC, Miller TE. Dietary chitosan inhibits hypercholesterolaemia and atherogenesis in the apolipoprotein E-deficient mouse model of atherosclerosis. Atherosclerosis. 1998;138:329–334. doi: 10.1016/s0021-9150(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Paoletti MG, Norberto L, Damini R, Musumeci S. Human gastric juice contains chitinase that can degrade chitin. Ann Nutr Metab. 2007;51:244–251. doi: 10.1159/000104144. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Sahlin JJ. A simple equation for the description of solute release. III Coupling of diffusion and relaxation. Int J Pharm. 1989;57:169–172. [Google Scholar]
- Peppercorn MA, Goldman P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J Pharmacol Exp Ther. 1972;181:555–562. [PubMed] [Google Scholar]
- Press A, Hauptmann I, Hauptmann L, Fuchs B, Fuchs M, Ewe K, Ramadori G. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 1998;12:673–678. doi: 10.1046/j.1365-2036.1998.00358.x. [DOI] [PubMed] [Google Scholar]
- Prizont R, Konigsberg N. Identification of bacterial glycosidases in rat cecal contents. Dig Dis Sci. 1981;26:773–777. doi: 10.1007/BF01309607. [DOI] [PubMed] [Google Scholar]
- Rowland IR, Mallett AK, Wise A. A comparison of the activity of five microbial enzymes in cecal content from rats, mice, and hamsters, and response to dietary pectin. Toxicol Appl Pharmacol. 1983;69:143–148. doi: 10.1016/0041-008x(83)90130-8. [DOI] [PubMed] [Google Scholar]
- Roy P, Shahiwala A. Multiparticulate formulation approach to pulsatile drug delivery: Current perspectives. J Control Rel. 2009;134:74–80. doi: 10.1016/j.jconrel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Rubinstein A. Approaches and opportunities in colon-specific drug delivery. Crit Rev Ther Drug Carrier Syst. 1995;12:101–149. doi: 10.1615/critrevtherdrugcarriersyst.v12.i2-3.10. [DOI] [PubMed] [Google Scholar]
- Rubinstein A, Radai R. In vitro and in vivo analysis of colon specificity of calcium pectinate formulations. Eur J Pharm Biopharm. 1995;41:291–295. [Google Scholar]
- Sadeghi M. Synthesis and swelling behaviors of graft copolymer based on chitosan-g-poly (AA-co-HEMA) Int J Chem Eng Appl. 2010;1:354–358. [Google Scholar]
- Saffran M, Kumar GS, Savariar C, Burnham JC, Williams F, Neckers DC. A new approach to the oral administration of insulin and other peptide drugs. Science. 1986;233:1081–1084. doi: 10.1126/science.3526553. [DOI] [PubMed] [Google Scholar]
- Sangekar S, Vadino WA, Chaudry I, Parr A, Beihn R, Digenis GA. Evaluation of the effect of food and specific gravity of tablets on gastric retention time. Int J Pharm. 1987;35:187–191. [Google Scholar]
- Semdé R, Amighi K, Devleeschouwer MJ, Moës AJ. Effect of pectinolytic enzymes on the theophylline release from pellets coated with water insoluble polymers containing pectin HM or calcium pectinate. Int J Pharm. 2000;197:169–179. doi: 10.1016/s0378-5173(99)00465-2. [DOI] [PubMed] [Google Scholar]
- Shao ZJ, Morales L, Diaz S, Muhammad NA. Drug release from Kollicoat SR 30D-coated nonpareil beads: evaluation of coating level, plasticizer type, and curing condition. AAPS PharmSciTech. 2002;3:87–96. doi: 10.1208/pt030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirsagar SJK, Bhalekar MR, Shukla GN, Mohapatra SK. Development and evaluation of multiparticulate colon targeted drug delivery system by combined approach of pH and bacteria. Int J Pharm Tech Res. 2011;3:1139–1149. [Google Scholar]
- Siepmann J, Peppas NA. Mathematical modeling of controlled drug delivery. Adv Drug Deliv Rev. 2001a;48:137–138. doi: 10.1016/s0169-409x(01)00111-9. [DOI] [PubMed] [Google Scholar]
- Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliv Rev. 2001b;48:139–57. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- Siew LF, Basit AW, Newton JM. The properties of amylose-ethylcellulose films cast from organic-based solvents as potential coatings for colonic drug delivery. Eur J Pharm Sci. 2000;11:133–139. doi: 10.1016/s0928-0987(00)00098-1. [DOI] [PubMed] [Google Scholar]
- Siew LF, Man SM, Newton JM, Basit AW. Amylose formulations for drug delivery to the colon: a comparison of two fermentation models to assess colonic targeting performance in vitro. Int J Pharm. 2004;273:129–134. doi: 10.1016/j.ijpharm.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Simon GL, Gorbach SL. The human intestinal microflora. Dig Dis Sci. 1986;31:147–162. doi: 10.1007/BF01295996. [DOI] [PubMed] [Google Scholar]
- Sinha V, Kumria R. Microbially triggered drug delivery to the colon. Eur J Pharm Sci. 2003;18:3–18. doi: 10.1016/s0928-0987(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Stoilova O, Koseva N, Manolova N, Rashkov I. Polyelectrolyte complex between chitosan and poly (2-acryloylamido-2-methylpropanesulfonic acid) Polym Bull. 1999;43:67–73. [Google Scholar]
- Stotzer PO, Abrahamsson H. Human postprandial gastric emptying of indigestible solids can occur unrelated to antral phase III. Neurogastroenterol Motil. 2000;12:415–419. doi: 10.1046/j.1365-2982.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- Thanou M, Junginger H. Pharmaceutical applications of chitosan and derivatives. In: Dimitriu S, editor. Polysaccharides: Structural Diversity and Functional Versatility. 2. New York: Marcel Dekker; 2005. pp. 661–677. [Google Scholar]
- Tozaki H, Odoriba T, Okada N, Fujita T, Terabe A, Suzuki T, Okabe S, Muranishi S, Yamamoto A. Chitosan capsules for colon-specific drug delivery: Enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J Control Rel. 2002;82:51–61. doi: 10.1016/s0168-3659(02)00084-6. [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Sugiyama T, Quan YS, Kotani A, Okada N, Fujita T, Muranishi S, Yamamoto A. Enhanced permeability of insulin across the rat intestinal membrane by various absorption enhancers: Their intestinal mucosal toxicity and absorption-enhancing mechanism of n-lauryl-beta-D-maltopyranoside. J Pharm Pharmacol. 1999;51:1241–1250. doi: 10.1211/0022357991776976. [DOI] [PubMed] [Google Scholar]
- Umadevi SK, Thiruganesh R, Suresh S, Reddy KB. Formulation and evaluation of chitosan microspheres of aceclofenac for colon-targeted drug delivery. Biopharm Drug Dispos. 2010;31:407–427. doi: 10.1002/bdd.722. [DOI] [PubMed] [Google Scholar]
- Umprayn K, Chitropas P, Amarekajorn S. Development of terbutaline sulfate sustained-release coated pellets. Drug Dev Ind Pharm. 1999;25:477–491. doi: 10.1081/ddc-100102198. [DOI] [PubMed] [Google Scholar]
- Van den Mooter G. Colon drug delivery. Expert Opin Drug Deliv. 2006;3:111–125. doi: 10.1517/17425247.3.1.111. [DOI] [PubMed] [Google Scholar]
- Wakerly Z, Fell J, Attwood D, Parkins D. Studies on drug release from pectin/ethylcellulose film-coated tablets: A potential colonic delivery system. Int J Pharm. 1997;153:219–224. [Google Scholar]
- Waller SL. Differential measurement of small and large bowel transit times in constipation and diarrhoea: A new approach. Gut. 1975;16:372–378. [PMC free article] [PubMed] [Google Scholar]
- Wang W, Bo S, Li S, Qin W. Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int J Biol Macromol. 1991;13:281–285. doi: 10.1016/0141-8130(91)90027-r. [DOI] [PubMed] [Google Scholar]
- Wei H, Li Fang F, Min B, Yong Zhen C, Bai X, Qing D, Feng W, Min Q, De Ying C. Chitosan/Kollicoat SR 30D film coated pellets of aminosalicylates for colonic drug delivery. J Pharm Sci. 2010;99:186–195. doi: 10.1002/jps.21810. [DOI] [PubMed] [Google Scholar]
- Wei H, Qing D, De Ying C, Bai X, Li Fang F. In vitro and in vivo studies of pectin/ethylcellulose film coated pellets of 5 fluorouracil for colonic targeting. J Pharm Pharmacol. 2008;60:35–44. doi: 10.1211/jpp.60.1.0005. [DOI] [PubMed] [Google Scholar]
- Wei H, Qing D, De-Ying C, Bai X. Pectin/Ethylcellulose as film coatings for colon-specific drug delivery: Preparation and in vitro evaluation using 5-fluorouracil pellets. J Pharm Sci Technol. 2007;61:121–130. [PubMed] [Google Scholar]
- Yang L. Biorelevant dissolution testing of colon-specific delivery systems activated by colonic microflora. J Control Rel. 2008;125:77–86. doi: 10.1016/j.jconrel.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Zhang H, Neau SH. In vitro degradation of chitosan by a commercial enzyme preparation: Effect of molecular weight and degree of deacetylation. Biomaterials. 2001;22:1653–1658. doi: 10.1016/s0142-9612(00)00326-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Neau SH. In vitro degradation of chitosan by bacterial enzymes from rat cecal and colonic contents. Biomaterials. 2002;23:2761–2766. doi: 10.1016/s0142-9612(02)00011-x. [DOI] [PubMed] [Google Scholar]
- Zhao XL, Li KX, Zhao XF, Pang DH, Chen DW. Study on colon-specific 5-Fu pH-enzyme Di-dependent chitosan microspheres. Chem Pharm Bull (Tokyo) 2008;56:963–968. doi: 10.1248/cpb.56.963. [DOI] [PubMed] [Google Scholar]