Abstract
B lymphocyte memory generates antibody-secreting cells (ASCs) that represent a source of protective antibodies that may be exploited for therapeutics. Here we vaccinated four donors with Pneumovax23 and produced human monoclonal antibodies (hmAbs) from ASCs. We have cloned 137 hmAbs and the specificities of these antibodies encompass 19 of the 23 serotypes in the vaccine, as well as cell wall polysaccharide (CWPS). Although the majority of the antibodies are serotype specific, 12% cross-react with two serotypes. The Pneumovax23 ASC antibody sequences are highly mutated and clonal, indicating an anamnestic response, even though this was a primary vaccination. Hmabs from 64% of the clonal families facilitate opsonophagocytosis. Although 9% of the total antibodies bind to CWPS impurity in the vaccine, none of these clonal families showed opsonophagocytic activity. Overall, these studies have allowed us to address unanswered questions in the field of human immune responses to polysaccharide vaccines, including the cross-reactivity of individual antibodies between serotypes and the percentage of antibodies that are protective after vaccination with Pneumovax23.
Keywords: Antibody secreting cells, B cell memory, human monoclonal antibodies, Pneumovax, Streptococcus pneumoniae
Introduction
Streptococcus pneumoniae is a ubiquitous human pathogen that causes a range of clinical infections, such as otitis media, pneumonia, meningitis, and bacteremia. The more serious manifestations are especially virulent in immunocompromised and elderly individuals. More than 90 different S. pneumoniae serotypes have been characterized, each having a different capsular polysaccharide structure. These polysaccharides are immunogenic in adults, and the Pneumovax23 vaccine consists of a cocktail of 23 of the most common and/or virulent S. pneumoniae strains. The vaccine is recommended for everyone over the age of fifty, as well as all immunocompromised individuals, to improve seroprotection against these strains. The serology of the response to Pneumovax23, as well as the conjugate vaccine Prevnar (used to immunize children), has been studied in depth with regard to the humoral polyclonal IgG and IgA responses in both sera and saliva (Antilla et al., 1999; Nieminen et al., 1998; Nieminen et al., 1998). The memory and antibody secreting cell (ASC) response to these vaccines has also been previously explored on a cellular level with B cell ELISpot assays and flow cytometry (Nieminen et al., 1998; Clutterbuck et al., 2006; Baxendale et al., 2010), and the presence of both responses after vaccination is now well established. However, utilizing ASCs to produce human monoclonal antibodies provides a novel means to fully elucidate the recall response to pathogen serotypes after vaccination, and provides a window to explore the evolution of past responses.
Antibodies that cross-react with two or more pneumococcal polysaccharides are present in sera both pre- and post-immunization (Lee, C.-J. et al., 1984; Soininen et al., 2000); however, whether this is due to single antibody specificities that are capable of cross-reacting or due to broad polyclonal antibody specificities is not known. Thus, we reasoned that examining this response at the monoclonal level would provide new insight into many aspects of the anti-polysaccharide immune response.
To explore these questions on a per antibody basis we vaccinated patients with the Pneumovax23 vaccine, generated and characterized large numbers of high affinity human monoclonal antibodies to the S. pneumoniae serotypes and cell wall polysaccharide (CWPS) present in the vaccine. Although human monoclonal antibodies to S. pneumoniae have been produced in the past (Baxendale and Goldblatt, 2006; Baxendale et al., 2000; Zhou et al., 2002; Zhou et al., 2004), these previous studies have been limited by two factors: one, they employed Fab expression library screens and two, they employed random production of hybridomas. In addition, previous studies have either focused on one serotype (6B and 23F) or have utilized vaccination with the conjugate vaccine Prevnar that consists of only seven capsular serotypes. In contrast, our technique provides a more cross-sectional characterization of the anti-polysaccharide response at one particular point in time, seven days post vaccination. Prior to monoclonal antibody isolation, ASCs were sorted; thus, every cell used to clone an antibody arose from a memory response to this particular vaccination. This system allows us to shed light on a number of as yet still unanswered questions in the field of polysaccharide immune responses. In this report, we have specifically addressed the percentage of human monoclonal polysaccharide antibodies that cross-react between different serotypes, bind to CWPS, and most importantly facilitate opsonophagocytosis.
Materials and Methods
Immunization and donors
Four donors received Pneumovax23 (Merck, Whitehouse Station, NJ) as standard of care vaccination based upon their age or diagnosis of systemic lupus erythematosus (SLE). Donors PVAX1 and PVAX2 were both Caucasian and without known autoimmune disease; age 62, male, and 61, female, respectively. Two donors were SLE patients: PVAX3, an African American male, age 47, and PVAX4, a Caucasian female, age 45. All protocols were approved by the OMRF Internal Review Board, and patients consented to participate in this study. Blood was drawn (~40–60 ml) into ACD tubes (BD, Franklin Lakes, NJ) by venipuncture seven days post vaccination and was stored no longer than 18 hours before processing.
Cell isolation and flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated from fresh blood using lymphocyte separation medium (Cellgro, Manassas, VA) and suspended in 2% inactivated fetal calf serum in PBS. Cells were then counted and stained within two hours of the isolation. Antibodies used for the staining were anti-CD3 and anti-CD20 conjugated to FITC, anti-CD38 conjugated to APC-Cy5.5, anti-CD27 conjugated to PE, anti-CD19 conjugated to PE-Alexa610 (all from Invitrogen/Caltag, Carlsbad, CA), anti-IgG conjugated to APC (BD Biosciences, San Jose CA), and anti-IgM conjugated to biotin (Southern Biotech, Birmingham, AL) followed by streptavidin-PE-Cy7 (Invitrogen/Caltag). The B cells were bulk sorted (CD3/CD20neg, CD19low, CD38high, CD27very high, IgGpositive) using a Becton-Dickinson FACS Aria cytometer (BD Biosciences, San Jose, CA) and then single cell sorted into 96-well PCR plates with a Cytomation MoFlo cytometer (Dako, Carpinteria, CA).
Single cell RT-PCR and PCR of antibody variable region genes
As detailed in (Smith et al., 2009), the plates receiving the single cells sorted as above contain 10 µl of a hypotonic buffer consisting of 10mM Tris-HCl with 40 U/µl RNase inhibitor (Promega, Madison, WI) in each well. After the sort, plates were immediately frozen on dry ice and stored at −80°C. A One-Step RT-PCR kit (Qiagen, Valencia, CA) was used to amplify VH and VK message using a cocktail of sense primers to the leader regions of each of the gene families and antisense primers to the constant regions of the heavy and kappa chains. Aliquots (1 µl) of the RT-PCR mixture were then amplified in separate heavy and kappa chain PCR reactions to obtain sequences, and separate aliquots (1 µl) were used for the final PCR reactions to incorporate restriction sites for further cloning. The amplified variable regions were then cloned into expression vectors (containing full length IgG1 heavy or kappa constant regions), maxi-prepped (Roche, Indianapolis IN), and co-transfected into the HEK293A cell line using polyethyleneimine (PEI) (Polysciences, Warrington, PA). The transfected cells were allowed to secrete antibodies into serum-free DMEM supplemented with 1% Nutridoma (Roche, Indianapolis, IN) for five days. The secreted antibodies were then purified using protein A-agarose beads (Pierce, Rockford, IL). Antibody purity and integrity were verified by SDS-PAGE and concentrations were obtained with a Nanodrop spectrophotometer (Fisher, Pittsburg, PA).
Polysaccharide Affinity and avidity ELISAs
To screen for binding, ELISAs were first performed in plates coated with cocktails of five or six S. pneumoniae polysaccharides (ATCC, Manassas, VA), screening all 23 in this manner. Positive binders in this cocktail assay were then re-screened against each of the individual polysaccharides. As cell wall polysaccharide (CWPS) is an impurity in nearly all of the coat polysaccharides (Xu et al., 2005), antibodies that bound to all four groups were further tested on purified cell wall polysaccharide (Miravista Labs, Indianapolis, IN) at a 1:4000 dilution to confirm CWPS binding. Wells were coated with 10 µg of each polysaccharide (or total mixed polysaccharide), blocked with 20% FCS, and developed with anti-human IgG-HRP (Jackson ImmunoResearch, West Grove, PA) and Super Aqua Blue substrate (EBiosciences, San Diego CA). The absorbance was measured at 405nm on a microplate reader (Molecular Devices, Sunnyvale, CA). Antibody affinities (Kd) were calculated by curve fitting analysis of individual ELISA curves plotted from a dilution series of 16 two-fold dilutions of antibody beginning at 10 µg/ml. Binding curves were generated with a saturation binding, non-linear curve fit using GraphPad Prism software. Equilibrium dissociation constants (Kd) values for each hmAb were calculated using the equation Y=Bmax*X/(Kd+X) where Bmax is the maximum number of binding sites, X is the concentration of the antibody and Y is the specific binding. Therefore the reported dissociation constants are equal to the concentration of antibody where half the binding sites are occupied at equilibrium. Each antibody was run in duplicate in at least three unique experiments. The results for each experiment were then averaged to obtain the reported Kd.
For avidity ELISAs, one concentration of antibody was used (1 µg/ml) and an elution step was added before the addition of the conjugate. This elution step used various concentrations of ammonium thiocyanate (8 dilutions ranging from 3M to 0.06M) in PBS, as well as PBS alone. The percent of binding retained was calculated for each dilution of ammonium thiocyanate. These values were plotted versus thiocyanate concentration, and the concentration of thiocyanate resulting in 50% retention (or loss) of binding was calculated by fitting the data to a dose-response/sigmoidal curve with hillslope correction.
Opsonophagocytosis
Opsonophagocytosis assays (OPA) were performed by Flow Applications, Inc. (Okawville, IL) using the methodology of Martinez (Martinez et al., 2002). In brief, S. pneumoniae polysaccharides were covalently conjugated to fluorescent beads. HL60 cells, rabbit complement, and the polysaccharide beads, pre-incubated with antibody, were incubated together and fluorescence of the HL60 cells was measured at a variety of antibody concentrations by flow cytometry. OPA results are expressed as titers, with dilutions starting from 30µg/ml of antibody. Antibodies showing no facilitation of uptake were assigned a titer of 4.
Analysis of sequences and curve fitting
All curve fitting was performed using the GraphPad Prism software, with background subtraction or percent retention values calculated and averaged using Excel. Variable region sequences were analyzed using the International Immunogenetics Information System (IMGT, Montpellier, France, http://imgt.cines.fr/), as well as with in-house software and/or Vector NTI (Invitrogen, Carlsbad, CA). Clonally related antibodies were defined as those having the same VDJ/VJ usage in the heavy and light chains, respectively, and highly related VHDH, DHJH, and VKJK junctions. CDR positions were used as defined by IMGT. Average nucleotide somatic hypermutation values were obtained by analyzing sequences (using IMGT) for the number of nucleotide changes from the germline in each antibody sequence. Resulting per-antibody values were then averaged to obtain average mutation rates per donor. The n value for these analyses included donors previously described (Wrammert et al., 2008): naïve cells from six donors (n=18, 42, 21, 34, 15, 36); IgM germinal center/memory cells from 17 donors (n=56, 158, 18, 91, 17, 10, 16, 30, 19, 28, 11, 36, 29, 13, 22, 20, 64); IgG germinal center/memory cells from 13 donors (n=110, 37, 19, 28, 174, 40, 25, 15, 21, 18, 22, 24, 19, 71); anti-influenza ASCs from 11 donors (n=63, 18, 33, 46, 49, 11, 36, 11, 30, 35, 25). The anti-polysaccharide ASC sequences are from the four donors in this study (PVAX1, 39; PVAX2, 49; PVAX3, 24; PVAX4, 25).
Results
Pneumovax23 induces a strong ASC response
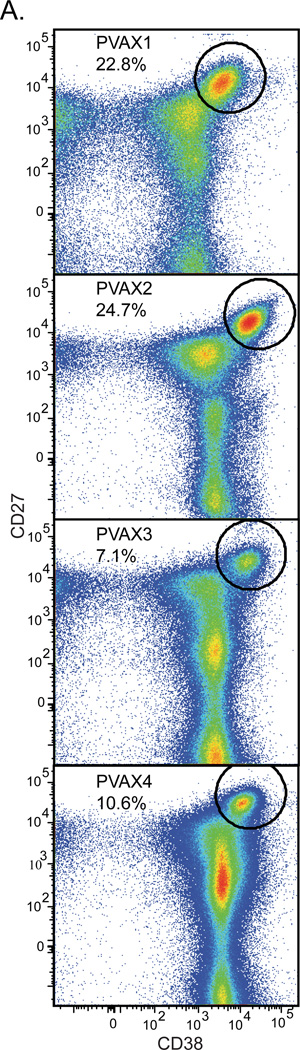
Four individuals were immunized with Pneumovax23. Blood was drawn seven days post vaccination and PBMCs were isolated by Ficoll gradient. The cells were then stained and CD38high/CD27very high ASCs were enumerated. Our previous results using these techniques after influenza vaccination (Wrammert et al., 2008) showed that this population represents an anamnestic ASC burst, which ranged from 1% to 16% of total peripheral blood B cells at day seven (average 6.4%). Pneumovax23 induces an even more robust ASC response (Fig. 1A): the ASC burst from one donor, PVAX2 represented 24.7% of peripheral blood B cells. As this was a primary vaccination for each donor, the average of 16.3% ASCs is impressive. This strong anamnestic response is likely due to the fact that S. pneumoniae is a ubiquitous organism that causes both clinical and subclinical disease among the general population. ASCs were single cell sorted and cloned for making human monoclonal antibodies from antibody secreting cells. This technique has been previously described in detail (Smith et al., 2009; Wrammert et al., 2008; Smith et al., 2012). In total, including non-binding antibodies, 137 antibodies were produced and characterized (PVAX1, n=39; PVAX2, n=49; PVAX3, n=24; PVAX4, n=25).
Figure 1. Pneumovax23 causes a massive ASC burst.
A.) PBMCs were harvested from four donors seven days after vaccination with Pneumovax23. PBMCs were stained and sorted for cells which were CD3 and CD20 negative and CD19 intermediate. The dot plots presented indicate a large ASC burst in all four donors (CD27 very high, CD38 high; circular gate). Averaging the percentage of ASCs from these four donors, 16.3% of total B cells in the peripheral blood are ASCs.
Our previous study of the immune response to influenza vaccination (Wrammert et al., 2008) highlighted the strong clonality of the ASC response to that vaccine; the ASC response to immunization with Pneumovax23 was even more clonally restricted. Table I shows each clonal family, the number of clones isolated from each family, and the average affinity of the family, the serotype that the family binds, the OPA titer of one member, and VH and VK usage. We define the name of the clonal family by a representative antibody as described below.
Table 1.
Summary of S. pneumoniae polysaccharide antibodies.
| Antibody | Number of Clones |
Serotype(s) | Kd (M)1 | OPA2 | VH gene | JH gene | Heavy CDR3 | VK gene | JK gene | Kappa CDR3 |
|---|---|---|---|---|---|---|---|---|---|---|
| PVAX1-p2 C01 | 3 | 20 | 1.1E-08 | 512 | VH3-66 | JH6 | AKGVTSFDY | VK3-20 | JK4 | QQFGSSPPDT |
| PVAX1-p2 C04 | 1 | 1 | 2.2E-10 | none | VH3-23 | JH4 | ARDPGIRNGMGV | VK2-30 | JK1 | MQVTHWPRT |
| PVAX1-p2 D02 | 1 | 9N | 3.9E-10 | 256 | VH3-23 | JH4 | AKAHRGDWNNFFDY | VK3-11 | JH4 | QQSGDWPLT |
| PVAX1-p2 D03 | 1 | 19F/19A | 1.2E-08 | 1024 / none | VH4-59 | JH3 | AREWSGFDF | VK3-20 | JK1 | QQYGSLPRT |
| PVAX1-p2 E01 | 3 | 8 | 7.7E-11 | 512 | VH3-7 | JH4 | ARGQWLAF | VK2-30 | JK2 | MQGTHWPYT |
| PVAX1-p3 C02 | 1 | 2 | 1.4E-10 | 4096 | VH3-7 | JH4 | ARGRNNFRH | VK1-33 | JK3 | QQFESFPRT |
| PVAX1-p3 C03 | 1 | 22F | 1.8E-10 | 32 | VH3-66 | JH4 | ARELGVFHSGGDQWLGPLDC | VK3-15 | JK3 | HQYKNWPPMGT |
| PVAX1-p3 G01 | 2 | 2 | 1.4E-10 | 2048 | VH3-49 | JH4 | RWTGGVSFGAY | VK1-5 | JK1 | QQYDIYLT |
| PVAX1-p3 G06 | 1 | 8 | 2.1E-08 | 16 | VH3-74 | JH4 | ARDYYHSVDY | VK2-30 | JK2 | MQGTHWPYT |
| PVAX1-p4 B01 | 2 | 33F | 4.0E-08 | 256 | VH4-59 | JH4 | ARGPDAHKTGY | VK4-1 | JK1 | QQYAATPWT |
| PVAX1-p4 B03 | 1 | 9N/9V | 5.6E-10 | 128 / 128 | VH3-74 | JH4 | ARDSYTSPDY | VK2-30 | JK4 | MQGSHWPLT |
| PVAX1-p4 C01 | 1 | 8 | 9.5E-10 | 128 | VH3-15 | JH3 | TTDNGVKAFDI | VK4-1 | JK3 | HQYYTTPFA |
| PVAX1-p4 G01 | 1 | 6B | 3.1E-10 | 256 | VH3-74 | JH4 | TRGGSGATINY | VK1-39 | JK4 | QQSHSSPLT |
| PVAX1-p6 C01 | 1 | 9V | 3.0E-08 | 256 | VH4-61 | JH4 | ARDRAGIDGYNYYFDY | VK1-5 | JK2 | QQYYSFYT |
| PVAX1-p6 D04 | 1 | CWPS | 4.2E-08 | none | VH1-46 | JH4 | AREVAAEGKAFDY | VK4-1 | JK4 | QQYYTPPLT |
| PVAX1-p6 E03 | 1 | 3 | 8.9E-10 | 128 | VH3-7 | JH3 | ARGQSYPGI | VK3-15 | JK1 | QQYNNWPRT |
| PVAX1-p6 E06 | 1 | 17F/33F | 9.4E-09 | 8 / none | VH4-59 | JH4 | AGRAYSSGYYYLIDY | VK3-15 | JK2 | QHYHNWPPT |
| PVAX2-p3 C04 | 3 | CWPS | 7.9E-11 | none | VH3-30 | JH4 | AKGCSNGGNCFLIDY | VK4-1 | JK4 | QQYYNAPLT |
| PVAX2-p3 C05 | 1 | 4 | 1.8E-10 | 256 | VH3-23 | JH3 | AKGGYYESGTMRAFDI | VK3-11 | JK4 | QQRSNWPAT |
| PVAX2-p3 F03 | 2 | 2 | 1.5E-10 | 4096 | VH3-7 | JH4 | ARGESNFRY | VK1-33 | JK3 | QQFVSFPRT |
| PVAX2-p3 G05 | 9 | 18C | 2.8E-10 | 64 | VH3-7 | JH4 | ARDSTSPARFGY | VK3-20 | JK2 | QHYGTSPPRYT |
| PVAX2-p4 B03 | 1 | 1 | 3.4E-08 | none | VH3-53 | JH4 | ATGGMTSSWYGY | VK4-1 | JK2 | QQYYSTPYT |
| PVAX2-p4 C02 | 5 | 9N/9V | 2.7E-10 | 512 / 8 | VH1-46 | JH4 | SMGPPYCTGGSCYSACDF | VK3-20 | JK2 | QRYGNSPPYT |
| PVAX2-p4 D06 | 5 | 9V | 2.6E-10 | 2048 | VH3-15 | JH5 | TTDIGKGWYTHYPDL | VK4-1 | JK4 | LQYRSAPFT |
| PVAX2-p5 A06 | 2 | CWPS | 5.1E-10 | none | VH3-30 | JH4 | VKEYSWGYYRTADY | VK1-5 | JK1 | QQYSTYPWT |
| PVAX2-p5 B06 | 3 | 1 | 1.4E-10 | none | VH3-74 | JH4 | ARSPGGYFDY | VK3-15 | JK1 | QQYSTWLWT |
| PVAX2-p5 C04 | 1 | 8 | 2.3E-08 | 32 | VH3-15 | JH4 | TTDDLKN | VK1-39 | JK2 | QQRYRIPYS |
| PVAX2-p5 E05 | 1 | 2 | 2.8E-10 | 2048 | VH3-48 | JH6 | ARGRDCYGGNCVIYFHYYGLDV | VK2-28 | JK2 | MRALQTPYT |
| PVAX2-p6 B03 | 3 | CWPS | 6.4E-11 | none | VH3-30 | JH4 | VKESATGWYRTADY | VK1-5 | JK1 | HQYNKYPWT |
| PVAX2-p6 C05 | 1 | 33F | 3.3E-09 | none | VH3-66 | JH3 | ARDIPTTFGIGEAFDI | VK1-5 | JK1 | QQYYSWGT |
| PVAX2-p6 G04 | 1 | 22F | 4.4E-10 | 128 | VH1-46 | JH4 | ARDDSAFDY | VK2-24 | JK1 | MQASQSTWT |
| PVAX2-p7 D03 | 1 | CWPS | 1.8E-09 | none | VH3-30 | JH6 | AKGCSGENCFYMDD | VK4-1 | JK4 | QQCYNAPLT |
| PVAX2-p8 B01 | 1 | 22F | 2.3E-08 | none | VH1-46 | JH4 | TREIGAVVVDATSLGWLGYFDY | VK3-15 | JK1 | QQYNNWPPVT |
| PVAX2-p8 B05 | 2 | 15B | 1.7E-10 | none | VH3-7 | JH4 | AGWGRTQD | VK2-30 | JK2 | MQYTFWPHT |
| PVAX2-p8 E03 | 1 | 23F | 3.3E-08 | none | VH3-30 | JH3 | TKEGAPPGKYAFDI | VK3-11 | JK3 | QHRGEWPPGAT |
| PVAX2-p8 F05 | 1 | 11A | 1.8E-10 | none | VK3-72 | JH3 | LKDSSQYSFDA | VK1-9 | JK4 | QQFKGYPLT |
| PVAX3-p1 A02 | 3 | 5 | 5.1E-10 | 1024 | VH4-59 | JH4 | ARGDGYNFF | VK1-9 | JK2 | QQINSYPRT |
| PVAX3-p1 A03 | 1 | 14/9N | 1.7E-10 | 512 / 32 | VH3-30 | JH5 | AKCGAEDSTTVWLNWFDP | VK3-11 | JK4 | QQRADWPLT |
| PVAX3-p1 B05 | 3 | 5 | 9.5E-10 | none | VH3-23 | JH4 | AKPNYFGSGSPDY | VK3-11 | JK2 | LQCSNWPMYT |
| PVAX3-p1 C04 | 1 | 5 | 2.8E-10 | 2048 | VH4-59 | JH4 | VKEQDYGYYRTADH | VK1-6 | JK2 | QQYDKYPWT |
| PVAX3-p1 E01 | 2 | 9V/9N | 6.2E-11 | 512/256 | VH3-20 | JH3 | VRVAVPAATYTRGNDAFDI | VK1-17 | JK1 | LQHSSFPWT |
| PVAX3-p1 F02 | 1 | 14 | 1.0E-09 | none | VH3-15 | JH4 | TTAHGPVGDH | VK4-1 | JK5 | QQYYTTPSIT |
| PVAX3-p1 G05 | 1 | 15B | 1.6E-10 | none | VH3-7 | JH4 | ARAGGCSSTRCHTTPGFDY | VK4-1 | JK5 | QQYYTTPPIT |
| PVAX3-p2 A02 | 1 | 5 | 1.4E-10 | 512 | VH4-39 | JH3 | ASLSGTNAFDI | VK3-11 | JK1 | QQRSSGRT |
| PVAX3-p2 D04 | 1 | 8 | 7.4E-09 | 256 | VH3-23 | JH4 | AKPRGYSYGYFDY | VK3D-20 | JK2 | QQYGISPRT |
| PVAX3-p3 A02 | 1 | 17F | 2.7E-09 | none | VH3-7 | JH4 | APPARRLDY | VK2-29 | JK1 | MQGTHHPWT |
| PVAX3-p3 A04 | 1 | 4 | 3.8E-08 | none | VH3-74 | JH4 | ARSNAGHEA | VK4-1 | JK4 | QQYYSTPLT |
| PVAX3-p3 B03 | 1 | 20 | 1.5E-09 | none | VH1-46 | JH4 | ARDIPHANLDY | VK1-17 | JK1 | LQHTTFPWT |
| PVAX3-p3 C03 | 1 | 33F | 1.1E-09 | 128 | VH3-23 | JH4 | VKDRVPPGDVPGDF | VK3-11 | JK5 | QQRRTWPPLT |
| PVAX4-p1 A01 | 2 | 23F | 2.5E-09 | none | VH3-48 | JH6 | ATLLLRDNQLDV | VK2-30 | JK1 | MQGTHWRT |
| PVAX4-p1 A06 | 1 | CWPS | 7.9E-10 | none | VH3-33 | JH4 | VKEQGFGYYRTADY | VK1-5 | JK1 | HQYDKYPWT |
| PVAX4-p1 B01 | 2 | 15B/14 | 2.0E-10 | 256 / 256 | VH4-59 | JH3 | ARRNDFNI | VK3-20 | JK3 | QQYGSSPFT |
| PVAX4-p1 C03 | 1 | 17F/33F | 2.9E-10 | none | VH3-23 | JH4 | SIWWGTSVQYPLVLDY | VK3D-15 | JK5 | QQYSKWPPIT |
| PVAX4-p1 C04 | 1 | CWPS | 2.0E-09 | none | VH3-30 | JH5 | VKEQDYGYYRTADH | VK1-5 | JK1 | QQYDKYPWT |
| PVAX4-p1 D02 | 5 | 5 | 2.0E-10 | none | VH4-61 | JH4 | ARGHGFNAY | VK3-20 | JK1 | QQYGNSPRT |
| PVAX4-p1 D04 | 1 | 6B | 8.8E-11 | 512 | VH3-15 | JH4 | TTVRNMADLSLNH | VK3-20 | JK1 | QQYDDSRWT |
| PVAX4-p2 A01 | 1 | 18C | 4.2E-09 | none | VH3-48 | JH4 | ATGNRGSLPRR | VK2D-28 | JK2 | MQALRSPYT |
| PVAX4-p2 C04 | 1 | 33F | 4.9E-09 | none | VH3-7 | JH4 | VRDGWDTFFDS | VK2-30 | JK2 | MQGRYWPYT |
| PVAX4-p2 D03 | 1 | 19A/19F | 1.1E-09 | none / 8192 | VH3-74 | JH4 | VNFQLG | VK3-20 | JK1 | QQYGNSPRT |
| PVAX4-p2 E04 | 1 | 8 | 5.1E-10 | 1024 | VH3-30-3 | JH5 | ARAEYCSPGDCFLIDT | VK2-30 | JK1 | MQGTHWRT |
| PVAX4-p2 F01 | 1 | CWPS | 9.6E-10 | none | VH3-33 | JH4 | LRGNPPSSPTDY | VK1-16 | JK4 | QQYNSYPLT |
| PVAX4-p2 G01 | 1 | 5 | 1.4E-09 | none | VH3-23 | JH6 | AKVVYSRPPMDV | VK1D-39 | JK1 | QQSYSTPWT |
| PVAX4-p2 G06 | 1 | 17F | 4.8E-11 | 128 | VH3-7 | JH4 | ARASRETGEPY | VK2-30 | JK1 | MQATHWPWT |
reported ELISA affinities are the averaged affinities of all of the clones in the clonal family
reported OPA titers are for one representative member of each clonal family (a titer = 4 is reported as "none")
The polysaccharide antibodies produced from ASCs are high affinity and are highly mutated resulting from an anamnestic response
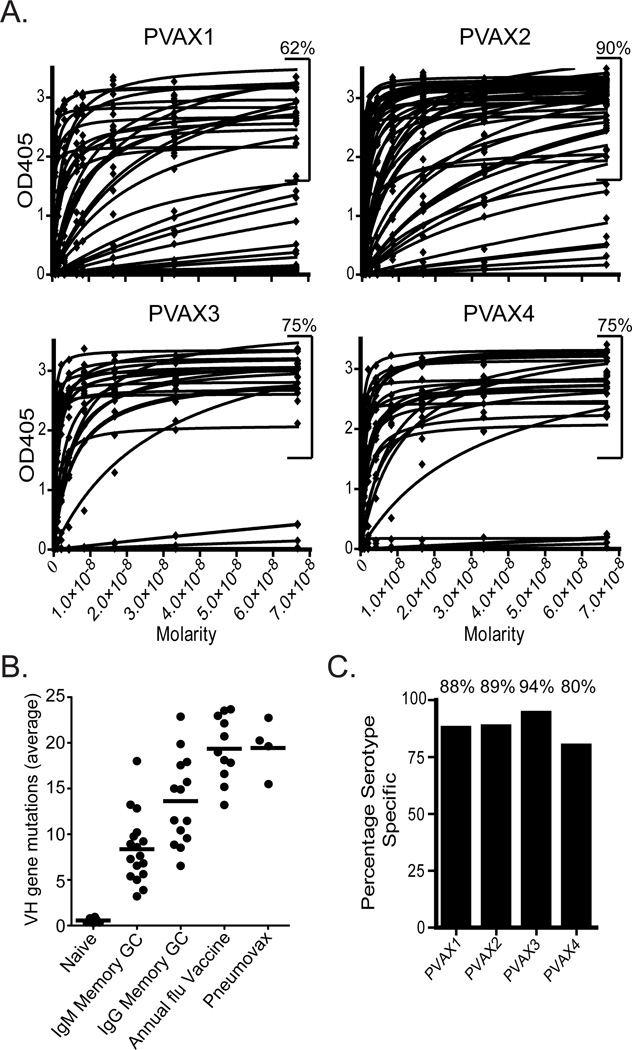
Polysaccharide ELISA curves of individual antibodies against all serotypes and CWPS are shown in Fig. 2A. An arbitrary cutoff at an OD405 of 1.5 was used to separate high to moderate affinity antibodies from low to non-binding antibodies. Percentages were calculated using this cutoff as a means to determine the fraction of antibodies that had significant binding. Averaged across the four donors, 76% of the antibodies (PVAX1, 62%; PVAX2, 90%; PVAX3 75%; PVAX4, 75%) bound to S. pneumoniae serotype polysaccharide or cell wall polysaccharide from the vaccine (Fig. 2A).
Figure 2. Binding, somatic hypermutation and serotype specificity of hmAbs from Pneumovax23.
A.) An average of 76% (PVAX1, 62%; PVAX2, 90%; PVAX3, 75%; PVAX4, 75%) of the antibodies expressed bind to S. pneumoniae capsule or cell wall polysaccharide by ELISA. ELISA curves represent the binding of each antibody to a mixure of purified S. pneumoniae polysaccharides. B.) Each data point is the average frequency of somatic mutations (nucleotide) per sequence from each donor (n values in Methods). On average, the anti-polysaccharide ASCs had accumulated a similar number of mutations as anti-influenza ASCs after seasonal influenza vaccination (Wrammert et al., 2008). (GC are germinal center populations. The four averages of the Pneumovax23 donors are from the study, the others are from (Wrammert et al., 2008).) C.) The percentage of the total number of antibodies that bind to a single serotype is plotted for each donor. While a significant percentage of antibodies are cross-reactive (12%), most of the antibodies produced are specific to a single serotype.
As we described previously (Wrammert et al., 2008), the ASC recall response to the influenza vaccine is highly mutated, even more so than in a typical IgG germinal center memory cell. We hypothesized that this was due to the repeated nature of the annual vaccine, as well as frequent exposure to various influenza strains. We compared the frequency of mutations among different B cell populations using current and previously published data (see Fig. 2B). On average, the anti-polysaccharide ASCs examined in this study had accumulated a similar number of mutations as anti-influenza ASCs after seasonal influenza vaccination (Wrammert et al., 2008). This is particularly interesting because for each donor, this was a primary vaccination. If the donors were truly naïve to these polysaccharide antigens, the ASC response is predicted to have been smaller and the sequences of the antibodies would show less mutation. Thus, this vaccine likely elicited an anamnestic response which can only arise from previous infection or exposure to S. pneumoniae strains.
A large majority of polysaccharide antibodies produced from ASCs bind to a single serotype
Of the antibodies from the four donors that bound to polysaccharide (76% of the total), an average of 88% were serotype specific (Fig. 2C) (PVAX1, 88%; PVAX2, 90%; PVAX3, 94%; PVAX4, 80%). The observation that 88% of the antibodies produced by currently circulating ASCs specifically bound carbohydrate epitopes, discriminating between very closely related structures, reinforces the well-known specificity of the antibody repertoire.
Antibodies to structurally similar serotypes cross-react but may also be serotype specific
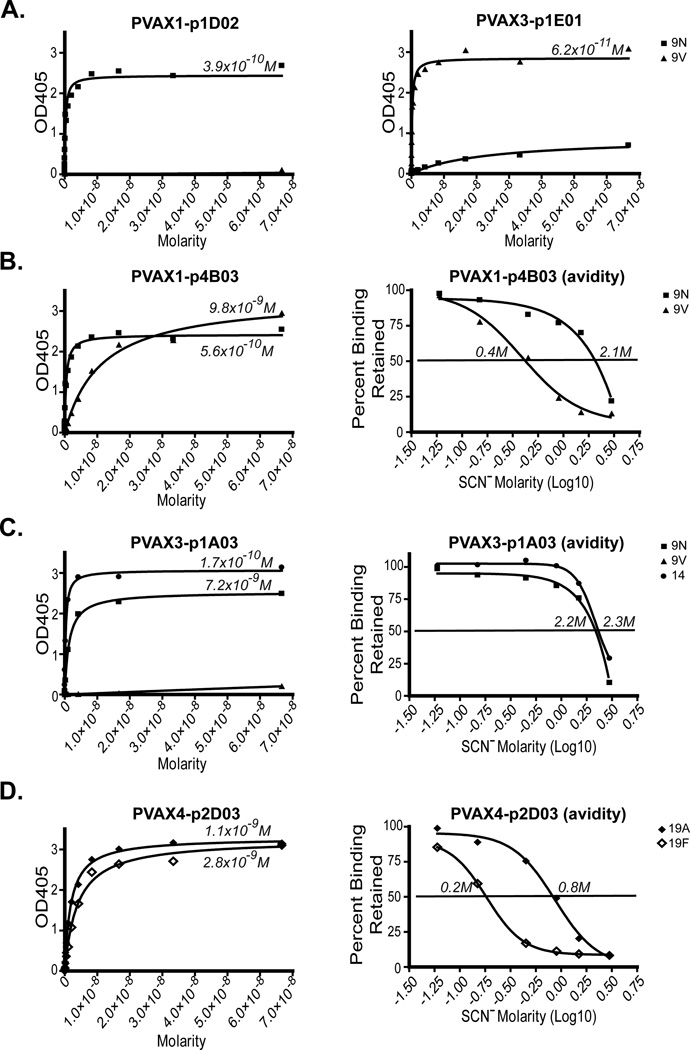
Although most of the antibodies studied were serotype specific, 12% of the antibodies we characterized bound two serotypes. Of the antibodies that bound two serotypes, several antibodies bound one pair of polysaccharides, 9N and 9V. These two carbohydrates have very similar non-branched structures, which differ only by a substitution of one of four D-Glc in the 9N chain repeat with a D-Gal in 9V. Thus, it was not unexpected that some antibodies cross-reacted with both serotypes. However, we observed a wide variety of 9N and 9V binding antibodies, some of which cross-reacted and some that did not. For example, PVAX1-p2D02 and PVAX3-p1E01 antibodies were mono-specific for 9N and 9V, respectively (Fig. 3A), and showed significant OPA titers (256 and 512 respectively, Table I). PVAX1-p4B03, however, bound both serotypes, favoring 9N by five fold in both affinity and avidity (Fig. 3B), yet had identical OPA titers against each serotype (128). One antibody specific for 9N, PVAX3-p1A03, did not cross-react with 9V, but did cross-react with serotype 14 polysaccharide (Fig. 3C), with similar affinity and avidity, and exhibited an OPA titer against both serotypes, an observation that is difficult to explain only on the basis of carbohydrate sequence. Several of these cross-reacting antibodies were from the same donor, demonstrating a variety of antibodies to a specific serotype within a single individual. Serotypes 19A and 19F also have very similar structures differing in that 19F has a D-Glc in a 1–2 linkage and 19A has a 1–3 linkage. The antibody PVAX4-p2D03 bound to both 19A and 19F with nearly equivalent affinities (Fig. 3D), although there was a four-fold difference in avidity (favoring 19A).
Figure 3. An individual can produce multiple antibodies to the same serotype, some of which are specific and others of which cross-react.
A.) Binding of PVAX1-p2D02 and PVAX3-p1E01 to serotypes 9N and 9V. B.) Binding affinity and avidity measurements of PVAX1-p4B03 to serotypes 9N and 9V. C.) Binding affinity and avidity measurements of PVAX3-p1A03 to serotypes 9N, 9V, and 14. D.) Binding affinity and avidity measurements of PVAX4-p2D03 to serotypes 19A and 19F. Affinities (Kd’s) are expressed in molarity. The avidity is equal to the concentration of ammonium thiocyanate causing a 50% reduction (or retention) of binding.
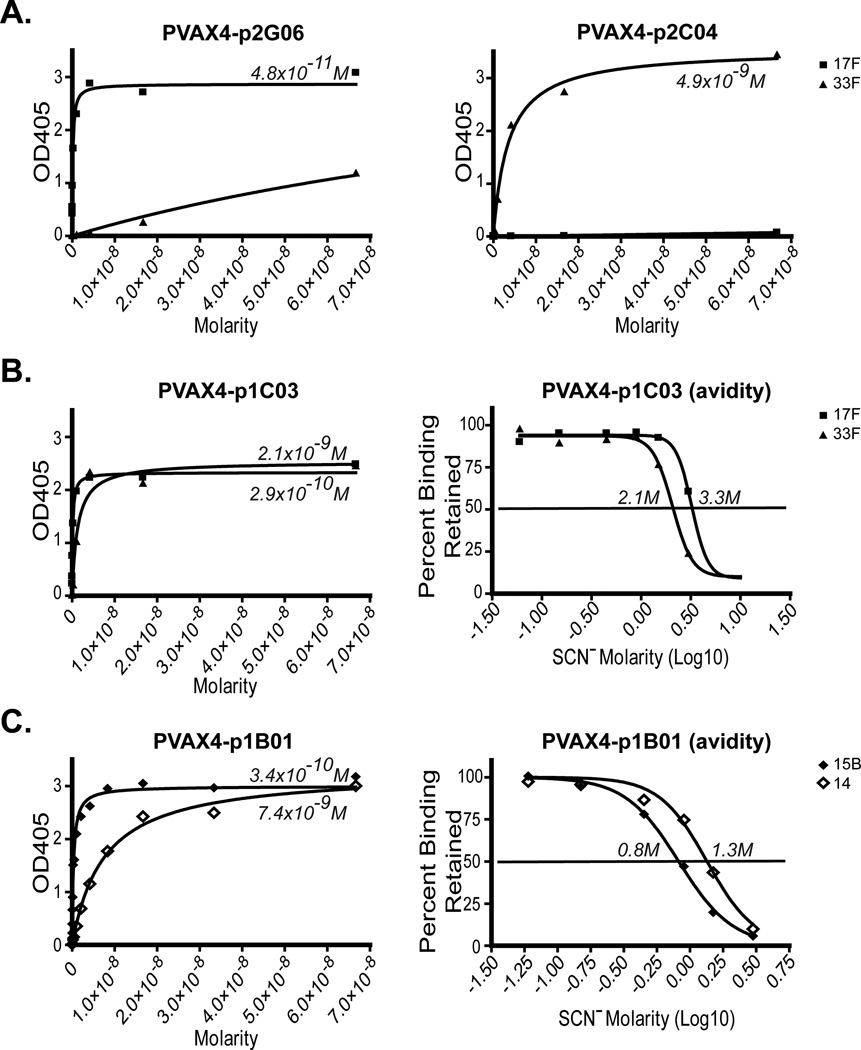
We also detected cross-reactivity between serotypes 17F and 33F (Fig. 4A and 4B) and 15B and 14 (Fig. 4C). While PVAX4-p2G06 and PVAX4-p2C04 were mono-specific for 17F and 33F, respectively (Fig. 4A), PVAX4-p1C03 (from the same donor; Fig. 4B) cross-reacted with both serotypes. The affinity of PVAX4-p1C03 for 33F was an order of magnitude better than the affinity for 17F, however, the avidities were similar. Of the antibodies shown in Figs 4A and 4B, only PVAX4-p2G06 showed an OPA titer. The affinity of antibody PVAX4-p1B01 was almost an order of magnitude higher for 15B than 14, although it showed a two-fold higher avidity for 14 and had identical OPA titers against each serotype. Overall, it was evident that although cross-reactions between two serotypes may be detected in serum, 85% of the actual antibodies making up this response are specific to only one polysaccharide. We encountered no antibodies that reacted with more than two serotypes with a measureable affinity or avidity.
Figure 4. Characterization of cross-reactive antibodies to serotypes 15B and 14, and to 17F and 33F.
A.) Binding of PVAX4-p2G06 and PVAX4-p2C04 to serotypes 17F or 33F. B.) Binding affinity and avidity measurements of PVAX4-p1C03 to serotypes 17F and 33F. C.) Binding affinity and avidity measurements of PVAX4-p1B01 to serotypes 15B and 14.
Antibodies induced by CWPS impurities in the vaccine have similar sequences and do not facilitate opsonophagocytosis
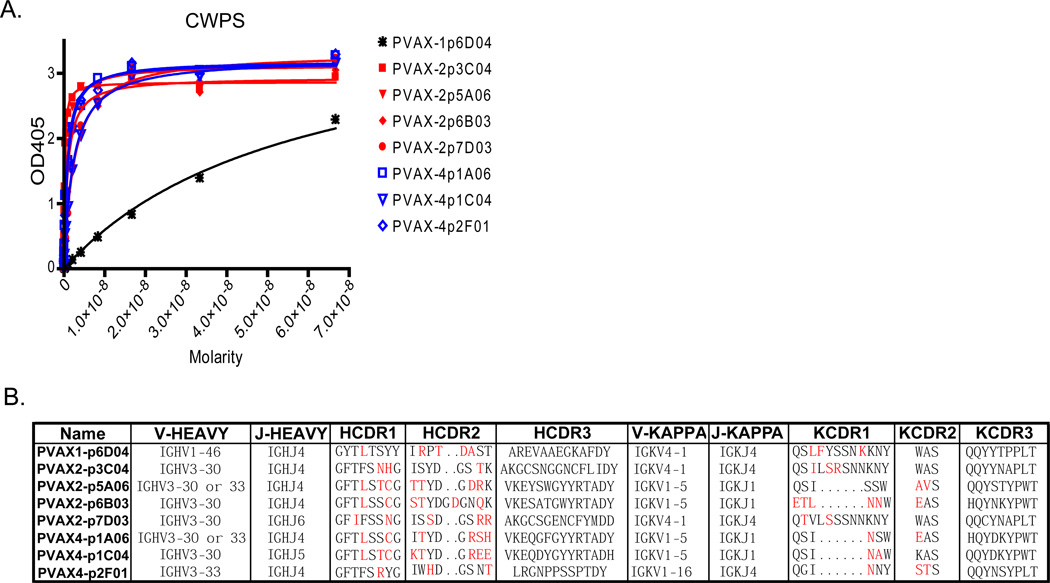
Pneumovax23 is known to have CWPS, which is a carryover impurity from the purification of the polysaccharides (Xu et al., 2005). We were able to isolate antibodies to CWPS from three of the four donors. Including all members of clonal families, 13 of the 137 total antibodies characterized bound to CWPS (9.5%), including 9 of 44 from donor PVAX2 (20.4%). This is important to note because even though 7 of the 8 clonal families have better than nanomolar affinities (Fig. 5A), none of the CWPS clonal families showed OPA activity. Thus, 20.4% of the antibodies produced by PVAX2 are not capable of facilitating opsonophagocytosis and may be irrelevant bystanders produced by this impurity in the vaccine. Close examination of the sequences of the CWPS antibodies showed remarkable similarities even in unique donors (Fig. 5B). All seven of the high affinity families used VH3-30/33. PVAX1-p6D04 had low affinity (~40nM) and was the only member of the group not to use VH3-30 or VH3-33 (see Fig. 5B). These VH3 gene families are difficult to distinguish among highly mutated sequences, and even with significantly mutated HCDR1 and HCDR2, the antibodies encoded by the genes shown in Fig. 5B have similar affinities for CWPS. Kappa usage was also conserved with all CWPS antibodies using either VK4-1, VK1-5, or VK 1–16.
Figure 5. Binding and V gene usage analysis of CWPS antibodies.
A.) Binding affinity measurements of representative antibodies from each clonal family to CWPS. B.) Variable heavy and kappa gene usage for each of the CWPS clonal families. Red amino acids in the H and KCDRs indicate a mutation from germline.
Each donor displays an anamnestic fingerprint of antibody serotype specificities of which 64% facilitate opsonophagocytosis
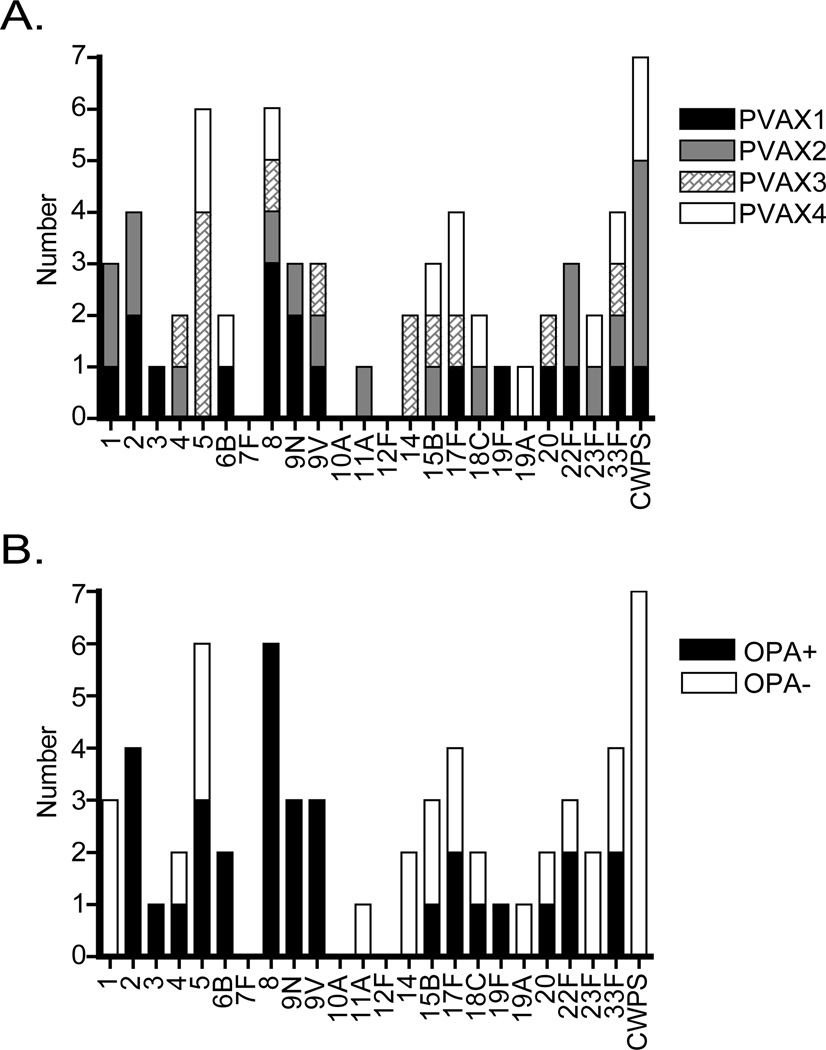
By displaying all four donors on a single histogram and reducing clonally related antibodies to a count of 1 (Fig. 6A), it is evident that the hmAbs isolated from each donor create a unique fingerprint with three donors binding 9V, 15B, and 17F, and only serotypes 8 and 33F being bound by all four donors. Also, no subject in the study produced an antibody that bound to serotypes 7F, 10A, or 12F.
Figure 6. Analysis of the “anamnestic fingerprint” of each donor and opsonophagocytic titer of each clonal family.
A.) Histogram showing serotype specificity fingerprint for all clonal families from all four donors. B.) Histogram showing the opsonophagocytic potential of each clonal family. Open bars are OPA negative (titer = 4), black bars are OPA positive (titer > 4).
The antibody clonal families detailed above are also shown in Fig. 6B and scored as either OPA+ (titer >4) or OPA- (titer =4). Overall, most clonal families show a 50% chance of facilitating opsonophagocytosis. Remarkably, all six clonal families that bind to serotype 8 and all four that bind to serotype 2 facilitate OPA, as well as all antibodies that bind to either 9N or 9V, whether or not they are specific to one serotype or cross-react with both. Serotypes for which no OPA-active antibodies were detected, with the exception of CWPS, are for the most part poorly represented in this study (serotypes 1, 11A, 14, 19A, and 23F).
Discussion
In this study, we analyzed the human IgG anamnestic response to Pneumovax23 immunization, on a per antibody basis, using antibody secreting cells (ASCs) that emerged seven days post vaccination as a source for the production of monoclonal antibodies. An analysis of these polysaccharide-specific monoclonal antibodies allowed a detailed study of the human antibody repertoire to this vaccine. This analysis also provided insight into the specificities and protective capacity of each antibody.
In an earlier study (Wrammert et al., 2008), we found that the magnitude of the anamnestic response following influenza vaccination was such that an average of 6% of total B cells were ASCs, yet some donors made poor to non-existent responses. Using these same techniques, some vaccines (notably Anthrax AVA) routinely result in very poor induction of a protective response (Smith et al., 2012). Here we report that Pneumovax23 invoked a two- to four-fold more robust response than the strongest responses induced in some of our influenza donors, suggesting that these polysaccharides are exceptionally efficient at triggering a memory response. Earlier studies (Nieminen et al., 1998; Nieminen et al., 1998; Clutterbuck et al., 2006) also detected antibody secreting cells seven days following vaccination with both the polysaccharide and conjugate vaccines, averaging over 100 serotype specific cells per million PBMCs. Our own ELISpot results were similar to these previous reports (data not shown), but the overall magnitude of the IgG ASC response as determined by flow cytometry was nonetheless surprising.
Our study has greatly increased the number of reported human monoclonal antibodies against S. pneumoniae that have been characterized with respect to binding and repertoire usage. These anti-polysaccharide antibodies are as highly mutated as antibodies that arise from repeated seasonal influenza vaccination. In comparing V gene usage in our antibodies to the previous reports, we observed similar trends. For example, Baxendale (Baxendale and Goldblatt, 2006; Baxendale et al., 2000) suggests that VH3-48 likely contributes to an antigen binding domain that prefers epitopes from serotypes 23F and 18C, as the two VH3-48 family antibodies they characterized bound those two serotypes. Similarly, Zhou found VH3-48 in a serotype 23F study (Zhou et al., 2002), but not a serotype 6B study (Zhou et al., 2004). Similarly, two of three VH3-48 clonal families (Table 1) characterized in our study also bind these two serotypes. We have also characterized a VH3-48 which bound serotype 2 (PVAX2-p5E05), a case of a VH3-48 binding a different serotype. We also observed remarkable similarity among antibodies which bound cell wall polysaccharide (CWPS) (Fig. 5B). Examining only the high affinity antibodies from seven clonal families derived from two unique donors, we found that these antibodies use either VH3-30 or the closely related VH3-33. The CDR3s also showed remarkable similarity (PVAX2-p6B03, VKESATGWYRTADYW; PVAX2-p5A06, VKEYSWGYYRTADY; PVAX4-p1A06, VKEQGFGYYRTADY; PVAX4-p1C04, VKEQDYGYYRTADH). Thus, the chemical simplicity of repeated polysaccharide sequences seems to induce similar V gene family usage among unique individuals.
Although avidity has been shown to be an important correlate with protection (Anttila et al., 1999; Harris et al., 2007; Usinger and Lucas, 1999), thiocyanate ELISA is not commonly performed on monoclonal antibodies. We utilized it here because there are several complications in determining affinity by fitting simple ELISA curves. These include the magnified effects of small antibody concentration errors on affinities, uncertainty whether or not the antigen binding interaction is univalent or bivalent, and coating plates with large units of repeating epitopes. It is also possible that poly-reactive antibodies from SLE donors (and occasionally healthy controls) may interact with antigens outside of the binding site. These effects are minimized in the thiocyanate avidity ELISA system. Figs. 3D and 4C show data for antibodies which affinity and avidity ELISA binding measurements do not correlate. Both of these antibodies are from PVAX4 who is an SLE patient, and both antibodies are poly-reactive. We are currently exploring interesting antibodies such as these in more detail, but in these cases, thiocyanate avidity is a more reliable measure of the antibody-carbohydrate interaction.
Serum cross-reactivity is typically determined by depleting the serum with a particular serotype carbohydrate and then observing binding of the serotypes remaining in the serum. Soininen (Soininen et al., 2000), for example, found remarkable serum cross-reactivity to a variety of pneumococcal serotypes, especially in unvaccinated individuals. However, these assays require careful calibration, as well as pre-adsorption with CWPS and other polysaccharides to remove nonspecific reactivity, which is especially common in unvaccinated individuals (Marchese et al., 2006). Modern updates to this method using microarray printing and reading technology (Pickering et al., 2007), for example, have greatly improved the reliability of these assays; yet until our study, one could not be certain whether observed cross-reactivity was due to actual cross-reactive individual antibodies, or the polyclonal nature of serum antibodies.
Our study has addressed these ambiguities. Park (Park et al., 2009) describes cross-serotype monoclonal antibodies, deducing the common linear carbohydrate structure to which the antibodies were binding. Other reports (Baxendale and Goldblatt, 2006; Baxendale et al., 2000; Zhou et al., 2002; Zhou et al., 2004) do not specify cross-reactive antibodies, although those produced from Fab libraries were only panned with the serotype of interest. Our experiments characterized a large number of anti-pneumococcal human monoclonal antibodies, and although most of the antibodies are serotype specific, 12% were not. The explanation for the cross-reactivity of several of the monoclonal antibodies we characterized is clearly not as simple as finding similar primary polysaccharide structures. While 9N/9V and 19A/19F are structurally similar, 17F and 33F, and 14 and 15B do not have similar primary structures. Pickering (Pickering et al., 2007) found that 9V could inhibit 9N binding, 15B inhibited 14 binding, 19F strongly inhibited 19A binding and 33F strongly inhibited 17F binding, all matching our observed results (Fig. 3 and 4). Interestingly, the converse is not typically the case (14 does not inhibit 15B and 17F does not inhibit 33F), but this is likely an affinity issue. Using our results to illustrate this point, it is unlikely that PVAX1-p4B03 binding to 9N could be inhibited by adding 9V polysaccharide because the affinity of PVAX1-p4B03 for 9N is much higher than its affinity for 9V. Overall, we can say with confidence that a component of the serum cross-reactivity observed in these studies is, indeed, due to individual monoclonal antibodies that bind to at least two different serotypes.
While the analysis of these human monoclonal antibodies helps to elucidate the basic anamnestic response, it may also serve a therapeutic purpose. As many of our current treatments can become ineffective due to antibiotic resistance, it is important to consider passive immunotherapeutics that can safely target pathogens. Several previous reports (Casal et al., 2002; Yuste et al., 2002) have explored the effects of specific antibodies in a mouse sepsis model. Remarkably, administering hyperimmune serum during an infection was able to reduce by eight-fold the amount of antibiotic required for the mouse to recover. This synergistic effect might be effectively used in treating difficult or invasive infections, such as empyema, or bacteremia in immunocompromised individuals. In addition to the myriad of treatment possibilities of fully human monoclonal antibodies, the drastically decreased risk of anaphylactic shock and of anti-treatment immune responses suggests that monoclonal antibody therapies may become as important in infectious diseases as they are currently in autoimmune settings.
In conclusion, the generation of hmAbs from ASCs seven days after vaccination with Pneumovax23 has given us greater insight into the anti-polysaccharide memory response in ways that conventional serology cannot. The overall anamnestic response is massive; the antibodies are predominantly serotype specific and facilitate opsonophagocytosis. Although this was a primary vaccination, the anamnestic character of the response indicates that the subjects were previously primed, presumably a result of the ubiquitous occurrence of S. pneumoniae. Furthermore, antibodies to CWPS have restricted V genes usage and do not facilitate opsonophagocytosis. In general, human monoclonal antibodies to the most widespread S. pneumoniae serotypes represent novel diagnostics for rapid serotyping and potentially passive immunotherapeutics for the treatment of resistant strains.
Acknowledgments
We thank J. Donald Capra, MD for his helpful discussions and critical reading of this manuscript. We also thank our donors for this study, as well as our clinical staff Virginia Roberts and Jeremy Levin. We thank Lori Garman for editing the manuscript and Jennifer VanDeventer, Jacob Bass and Diana Hamilton for their technical assistance. This research has been supported by National Institutes of Health Grants P20RR015577, P20RR015577-10S1, P30RR031152, P30AR053483, U19AI062629 and contract HHSN266200500026C (N01-AI500026).
Abbreviations
- ASC
antibody secreting cell
- CWPS
cell wall polysaccharide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anttila M, Voutilainen M, Jäntti V, Eskola J, Käyhty H. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin. Exp. Immunol. 1999;118:402–407. doi: 10.1046/j.1365-2249.1999.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale HE, Davis Z, White HN, Spellerberg MB, Stevenson FK, Goldblatt D. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur. J. Immunol. 2000;30:1214–1223. doi: 10.1002/(SICI)1521-4141(200004)30:4<1214::AID-IMMU1214>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Baxendale HE, Goldblatt D. Correlation of Molecular Characteristics, Isotype, and In Vitro Functional Activity of Human Antipneumococcal Monoclonal Antibodies. Infect. Immun. 2006;74:1025–1031. doi: 10.1128/IAI.74.2.1025-1031.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale HE, Johnson M, Keating SM, Ashton L, Burbidge P, Woodgate S, Southern J, Miller E, Goldblatt D. Circulating pneumococcal specific plasma and memory B cells in the elderly two years after pneumococcal conjugate versus polysaccharide vaccination. Vaccine. 2010;28:6915–6922. doi: 10.1016/j.vaccine.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Casal J, Aguilar L, Jado I, Yuste J, Giménez MJ, Prieto J, Fenoll A. Effects of Specific Antibodies against Streptococcus pneumoniae on Pharmacodynamic Parameters of β-Lactams in a Mouse Sepsis Model. Antimicrob. Agents Chemother. 2002;46:1340–1344. doi: 10.1128/AAC.46.5.1340-1344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck EA, Salt P, Oh S, Marchant A, Beverley P, Pollard AJ. The kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM197 conjugate vaccine. Immunol. 2006;119:328–337. doi: 10.1111/j.1365-2567.2006.02436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Tsao H, Ashton L, Goldblatt D, Fernsten P. Avidity of the Immunoglobulin G response to a Neisseria meningitides group C polysaccharide conjugate vaccine as measured by inhibition and chaotropic enzyme-linked immunosorbent assays. Clin. Vacc. Immunol. 2007;14:397–403. doi: 10.1128/CVI.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-J, Koizumi K, Henrichsen J, Perch B, Lin CS, Egan W. Capsular polysaccharides of nongroupable streptococci that cross-react with pneumococcal group 19. J. Immunol. 1984;133:2706–2711. [PubMed] [Google Scholar]
- Marchese RD, Jain N, Antonello J, Mallette L, Butterfield-Gerson K, Raab J, Burke P, Schulman C, Adgate H, Sikkema D, Chirmule N. Enzyme-linked immunosorbent assay for measuring antibodies to pneumococcal polysaccharides for the Pneumovax 23 vaccine: Assay operating characteristics and correlation to the WHO international assay. Clin. Vacc. Immunol. 2006;13:905–912. doi: 10.1128/CVI.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Pilishvili T, Barnard S, Caba J, Spear W, Romero-Steiner S, Carlone GM. Opsonophagocytosis of fluorescent polystyrene beads coupled to Neisseria meningitides serogroup A, C, Y, or W135 polysaccharide correlates with serum bactericidal activity. Clin. Diagn. Lab. Immunol. 2002;9:485–488. doi: 10.1128/CDLI.9.2.485-488.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen T, Käyhty H, Virolainen A, Eskola J. Circulating antibody secreting cell response to parenteral pneumococcal vaccines as an indicator of a salivary IgA antibody response. Vaccine. 1998;16:313–319. doi: 10.1016/s0264-410x(97)00162-x. [DOI] [PubMed] [Google Scholar]
- Nieminen T, Eskola J, Käyhty H. Pneumococcal conjugate vaccination in adults: circulating antibody secreting cell response and humoral antibody responses in saliva and in serum. Vaccine. 1998;16:630–636. doi: 10.1016/s0264-410x(97)00235-1. [DOI] [PubMed] [Google Scholar]
- Park S, Parameswar A, Demchenko A, Nahm M. Identification of a simple chemical structure associated with protective human antibodies against multiple pneumococcal serogroups. Infect. Immun. 2009;77:3374–3379. doi: 10.1128/IAI.00319-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering JW, Hoopes J, Groll M, Romero H, Wall D, Sant H, Astill M, Hill H. A 22-plex chemiluminescent microarray for pneumococcal antibodies. Am. J. Clin. Pathol. 2007;128:23–31. doi: 10.1309/781K5W6QH7JH2TMA. [DOI] [PubMed] [Google Scholar]
- Soininen A, Van Den Dobbelsteen G, Oomen L, Käyhty H. Are the Enzyme Immunoassays for Antibodies to Pneumococcal Capsular Polysaccharides Serotype Specific? Clin. Diagn. Lab. Immunol. 2000;7:468–476. doi: 10.1128/cdli.7.3.468-476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 2009;4:372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Crowe SR, Garman L, Guthridge CJ, Muther JJ, McKee E, Farris AD, Guthridge JM, Wilson PC, James JA. Human monoclonal antibodies generated following vaccination with AVA provide neutralization by blocking furin cleavage but not by preventing oligomerization. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.03.002. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usinger WR, Lucas AH. Avidity as a Determinant of the Protective Efficacy of Human Antibodies to Pneumococcal Capsular Polysaccharides. Infec. Immun. 1999;67:2366–2370. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng N, Mays I, Garman L, Helms C, James J, Air G, Capra JD, Ahmed R, Wilson PC. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Abeygunawardana C, Ng AS, Sturgess AW, Harmon BJ, Hennessey JP., Jr Characterization and quantification of C-polysaccharide in Streptococcus pneumoniae capsular polysaccharide preparations. Anal. Biochem. 2005;336:262–272. doi: 10.1016/j.ab.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Yuste J, Jado I, Giménez MJ, Aguilar L, Molder F, Fenoll A, Casal J. Modification of bacteraemia by specific antibodies and relation with mortality in a pneumococcal mouse sepsis model. Clin. Exp. Immunol. 2002;128:411–415. doi: 10.1046/j.1365-2249.2002.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Lottenbach KR, Barenkamp SJ, Lucas AH, Reason DC. Recurrent Variable Region Gene Usage and Somatic Mutation in the Human Antibody Response to the Capsular Polysaccharide of Streptococcus pneumoniae Type 23F. Infect. Immun. 2002;70:4083–4091. doi: 10.1128/IAI.70.8.4083-4091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Lottenbach KR, Barenkamp SJ, Reason DC. Somatic Hypermutation and Diverse Immunoglobulin Gene Usage in the Human Antibody Response to the Capsular Polysaccharide of Streptococcus pneumoniae Type 6B. Infect. Immun. 2004;72:3505–3514. doi: 10.1128/IAI.72.6.3505-3514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]