Abstract
Nanoemulsions are adjuvants that enhance antigen penetration in the nasal mucosa, increase cellular uptake of antigens by both epithelial dendritic cells, and promote migration of antigen-loaded dendritic cells to regional lymph nodes within a day of vaccine administration. The objective of this study was to determine whether the W805EC nanoemulsion adjuvant enhances immune response not only by direct uptake of antigen by dendritic cells, but also indirectly, by phagocytosis of antigen-primed, apoptotic, epithelial cells. Consistent with this, we show that exposure of both epithelial cells (TC-1s) and dendritic cells (JAWS II or bone marrow derived dendritic cells (BMDCs)) to nanoemulsion exhibited augmented antigen uptake in cell culture. TC-1 cells subsequently underwent G2/M cell cycle arrest and apoptosis, and when co-cultured with JAWS II or BMDCs were rapidly engulfed by the dendritic cells, which responded by up-regulating dendritic cell maturation marker CD86. Altogether these results suggest that the effectiveness of nanoemulsions as adjuvants stems, at least in part, from the engulfment of antigen-loaded epithelial cells, leading to enhanced antigen processing and a strong and balanced mucosal and systemic immune response.
Keywords: Immunization, antigens, cytokines, antibodies
Introduction
Despite many efforts aimed at developing improved adjuvants, very few have been approved for use in human vaccination [1–4]. Furthermore, most adjuvants are effective in stimulating humoral or cell-mediated immunity (CMI) but not both. For example, alum is pro-inflammatory compounds useful in augmenting Th2 humoral immune responses to bacterial toxoids and other antigens but not in consistently eliciting CMI [5, 6]. This deficiency is significant, since CMI play a crucial role in fighting intracellular pathogens. Other forms of particulate adjuvants (e.g., emulsions, microparticles, iscoms, and liposomes) have been proposed as alternatives to alum, but these adjuvants require additional pro-inflammatory immunostimulants to enhance the immune response [7]. Although liposomes and lipid formulations are capable of priming virus-specific CTLs [8], these formulations tend to be unstable, especially when mixed with antigen. Finally, the identification of innate immune receptors such as toll-like receptor (TLR) has led to the generation of a new class of adjuvants that stimulate CMI [9–12]. While promising, these compounds do not work for all antigens, and often require chemical coupling to antigens to be effective [13, 14]. Thus current adjuvant options fail to meet the need for stable formulations that elicit well-balanced strong humoral and CMI responses to a wide range of antigen types.
Adjuvants for mucosal vaccines are a novel class of compounds that offer unique advantages over both traditional and newer adjuvants. One of potential mucosal adjuvant is heat-labile enterotoxin. However, the enterotoxins raise serious safety issues [15] and therefore the use of enterotoxins as adjuvants in human vaccine formulations at this time is precluded [16].
NEs are oil-in-water emulsions (~400 nm droplet sizes) prepared using surfactants, solvent, soybean oil, and water and were developed as antimicrobial agents [17–21]. Recent studies have documented that NE can also be used as a mucosal adjuvant when mixed with soluble [22–25] or particulate [26–29] antigens. NE induces antigen-specific humoral and CMI responses in mucosal compartments, and protects against challenge with a pathogen [26–29]. The NE has also been shown to stimulate a Th17 response [30]. Finally, the W805EC nanoemulsion adjuvant is well tolerated in animals [24] and humans [31].
Despite these findings, the specific mechanisms by which NE stimulates a robust and balanced immune response are poorly understood. Here we describe a model system for the examination of NE-Ag action on epithelial cells (EC) and dendritic cells (DC). We report that the W805EC NE-Ag vaccine mixture induces antigen uptake and provide evidence that these antigen-primed ECs are engulfed by DCs. Furthermore, we demonstrate both antigen transfer to the DCs, as well as their subsequent maturation. Thus the uptake of antigen by ECs followed by their engulfment by DCs represents an indirect route for antigen acquisition by DCs that may contribute to the remarkable, broad-based adjuvant properties of the W805EC nanoemulsion.
Materials and Methods
Immunization and detection of humoral and CMI immune response
Female C57BL/6 mice 8–12 weeks were purchased from Harlan Sprague Dawley. Anesthetized mice were immunized with 20 μg of OVA mixed either with PBS or 20% W805EC and instilled i.n. at a volume of 10 μL per animal. Mice were immunized on day 0 and then three times at two-week intervals. OVA-specific immunoglobulins were evaluated using ELISA as described previously [29] with some modifications. Plates were coated with ovalbumin diluted in coating buffer (300 ng/well). Splenocytes from immunized mice were treated with OVA (20μg/mL) for48 hours. Supernatants were harvested and tested for the presence of cytokines using cytokine/chemokine LINCOplex kit (Millipore) as described previously [27]. Protocols for animal experiments were approved by the UCUCA at the University of Michigan, Ann Arbor, MI.
Cells
BMDCs were derived as described by Inaba et al. [32]. TC-1 (epithelial) and JAWS II DC lines, both of C57BL/6 origin were purchased from ATCC.
Reagents
The W805EC was provided by NanoBio Corporation (Ann Arbor, MI). OVA was purchased from Hyglos GmbH (Regensburg, Germany).
Antigen uptake by TC-1 cells
The TC-1 cells were incubated overnight with R-PE (40 μg/mL) (AnaSpec, Inc.), or DQ-OVA (20 μg/mL) (Molecular Probes) either with or without 0.05% W805EC. After incubation cells were washed and analyzed using flow cytometry.
Engulfment of TC-1 cells by DCs and antigen transfer
PKH-26-stained TC-1 cells were treated with either 0.05% W805EC or 1 μM staurosporine overnight. Then TC-1 cells were resuspended to a concentration of 3×105 cells/mL, and mixed with equal number of either JAWS II or BMDC cells and co-cultured for 4 hours at 37°C. Then cells were harvested and analyzed using flow cytometry. To exclude the potential for non-specific binding of fluorescent agents to the exterior of the cells, co-cultures were incubated at 4°C or DCs were either pretreated with 35mM NaN3. In antigen transfer experiments, the TC-1 cells were loaded overnight with R-PE mixed with either W805EC or staurosporine, then washed and incubated with CFSE-stained JAWS II cells. For confocal microscopy, PKH-26-TC-1 cells pretreated with NE were co-incubated with CFSE-JAWS II cells in chambered coverglass for 4 hours at 37°C. Then fixed with paraformaldehyde cells were imaged with a Leica inverted SP5X confocal microscope.
Expression of CD86 differentiation antigen on DCs
The PKH-26-TC-1 cells were incubated with 0.05% W805EC for 6 hours. They were then washed and co-cultured overnight with CFSE-JAWS II cells. As a positive control, JAWS II cells were treated overnight with 10 μg/mL LPS (Salmonella minnesota from List Biological Laboratories, Inc.). The next day, the co-cultured cells were washed and stained with mouse anti-CD86 PE-Cy5-labeled antibody (eBioscience) and analyzed on flow cytometry. To analyze solely JAWS II cells, the red fluorescent TC-1 cells were gated out.
Statistical analysis
Results are presented as the mean ± SD. The data were analyzed by using Wilcoxon signed-rank test, with a significance level of α = 0.05.
Results
Adjuvant activity of W805EC in vivo
Intranasal immunization with W805EC adjuvant produces a humoral immune response
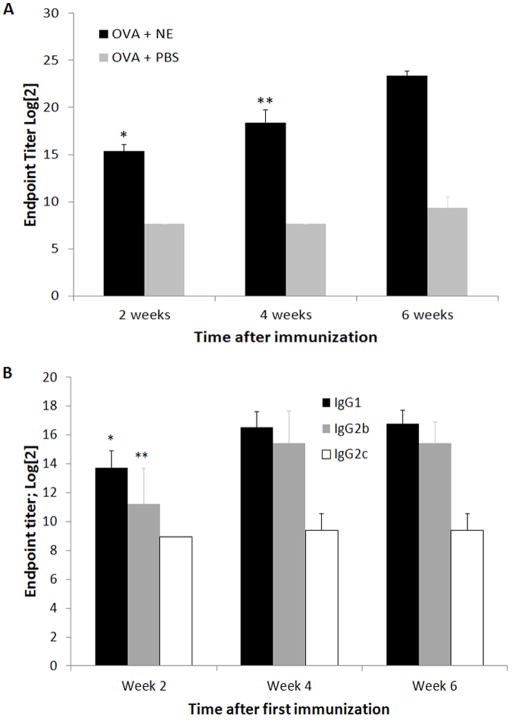
The ability of W805EC to function as a mucosal adjuvant was tested by immunizing mice i.n. with OVA+PBS or OVA+W805EC four times at two-week intervals. Humoral immune response was assessed by measuring end-point titers of OVA-specific IgG (Fig. 1A). The first immunization resulted in an over 2-fold increase in IgG titer compared to control animals (Week 2), with second and third immunizations resulting in further increases of approximately one log each (Week 4 and Week 6). The endpoint titers of IgG1, IgG2b, and IgG2c subclasses of OVA-specific antibodies were evaluated (Fig. 1B). The IgG2a endpoint titer has not been evaluated due to deletion of the Igh-1a gene in C57BL/6 mice which instead express a separate gene for the IgG2c (Igh-1b) heavy chain isotype [33–35]. Both IgG1 and IgG2b subclasses increased between the first and second immunizations, with IgG1 reaching an endpoint titer of approximately log2 16.5 and IgG2b an endpoint titer of log2 15.4 at week 4. In contrast, IgG2c showed an insignificant increase in the endpoint titer.
Figure 1.
Endpoint titer of total OVA specific IgG in sera (A). Mice (8 animals per group) were immunized on day 0 and then three times, two weeks apart. Sera were collected every two weeks. Each additional immunization increased the endpoint titer. Two weeks after the fourth immunization, there was no further increase in endpoint titer (data not shown). Data shown are representative of one of three independent experiments. * - significant difference (p<0.005) in endpoint titer of IgG between groups OVA+NE and OVA+PBS; ** - significant difference (p<0.005) in endpoint titer of IgG between week 2 and week 4 and week 4 and week 6. Endpoint titer of IgG1, IgG2b and IgG2c subclasses of OVA specific antibodies (B). Data shown are representative of one of three independent experiments. * - significant difference (p<0.05) in endpoint titer of IgG1 between week 2 and 4; ** - significant difference (p<0.005) in endpoint titer of IgG2b between week 2 and 4.
Nasal immunization of W805EC adjuvant produces CMI (Th1, Th2 and Th17) response
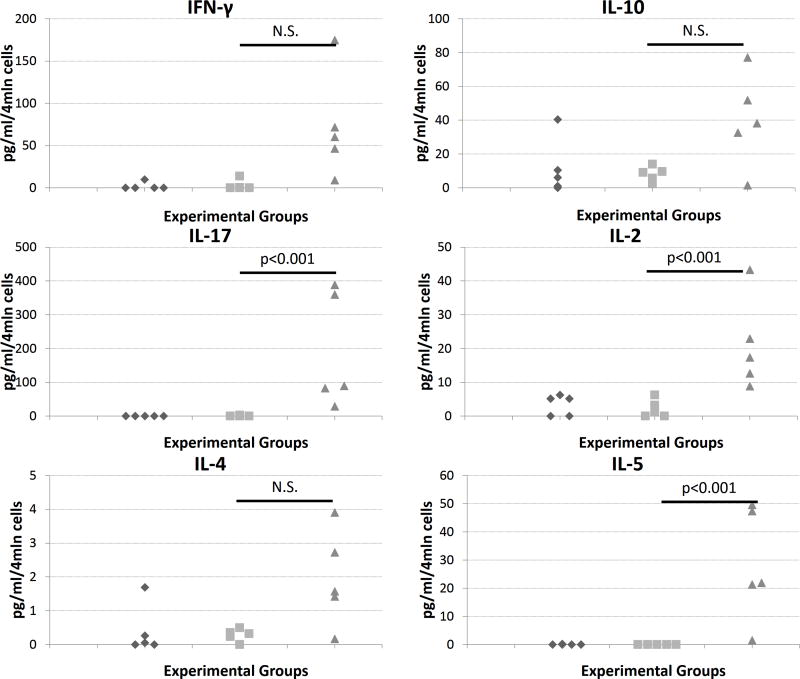
To provide insight into the CMI, splenocytes from immunized mice were re-exposed to the OVA followed by assessment of cytokine response. The animals immunized with OVA+W805EC showed increased production of markers for Th1, Th2, and Th17 cellular response as compared to control animals (Fig. 2).
Figure 2.
Cytokine production by splenocytes obtained from mice immunized with PBS (◆), OVA and PBS (■), and OVA with NE (▲). Data shown are representative of one of three independent experiments. Statistical significance (p<0.005) has been observed for IL-2, IL-17 and IL-5 between groups OVA+PBS vs. OVA+NE.
Adjuvant activity of W805EC in vitro
W805EC promotes antigen uptake by ECs and DCs
The broad based immune response to the W805EC adjuvant led us to consider possible mechanisms for this response. TC-1 cells were treated with either R-PE or DQ-OVA in the presence or absence of W805EC. Treatment of TC-1 cells with R-PE in the presence of W805EC increased the MFI 4 times as compared to cells uploaded with R-PE alone (Fig. 3A). Similar data were obtained when DQ-OVA was used as an antigen; treatment with DQ-OVA+W805EC increased the MFI 2.5 times over that of cells treated with DQ-OVA+PBS (Fig. 3B). Similar data were obtained when BMDCs were treated with OVA-AlexaFluor647+W805EC (Supplemental Figure 2).
Figure 3.
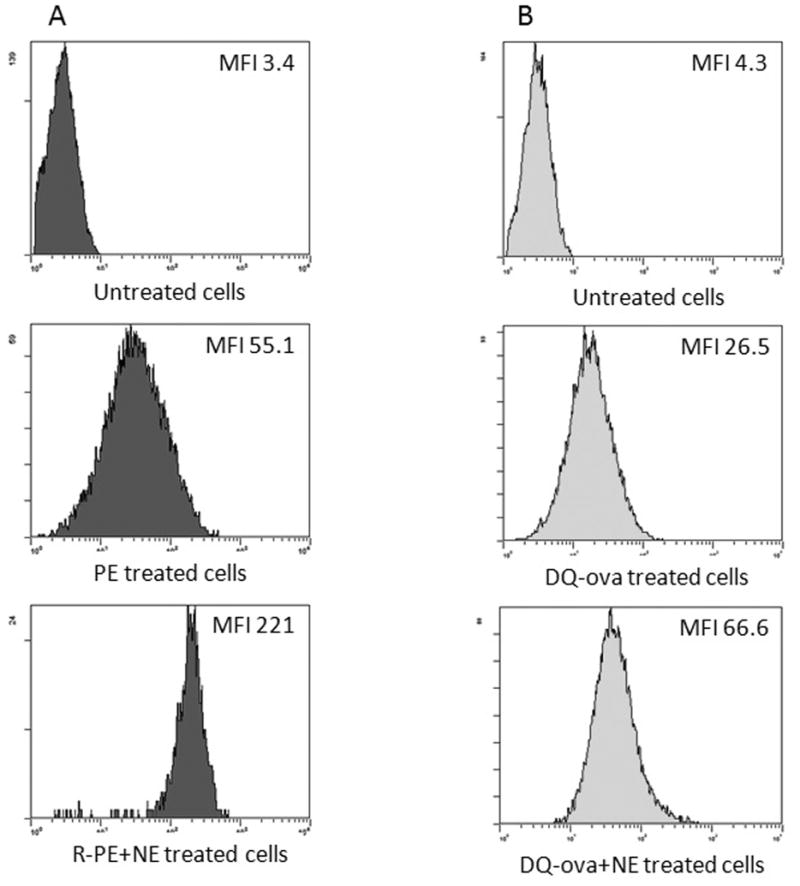
The effect of NE on antigen uptake by TC-1 cells. The cells were incubated with either R-PE (A) or DQ-OVA (B) in the presence or absence of NE for 24 hours and then analyzed using flow cytometry. The experiments were repeated five times (rPE) and four times (DQ-OVA). Significant difference p<0.01 and p<0.03 in the MFI values between the uptake of rPE with and without W805EC (N=5) and DQ-OVA with and without W805EC (N=4), respectively have been observed as tested using Wilcoxon signed-rank test.
Treatment of ECs with W805EC promotes engulfment by DCs which leads to indirect antigen uptake
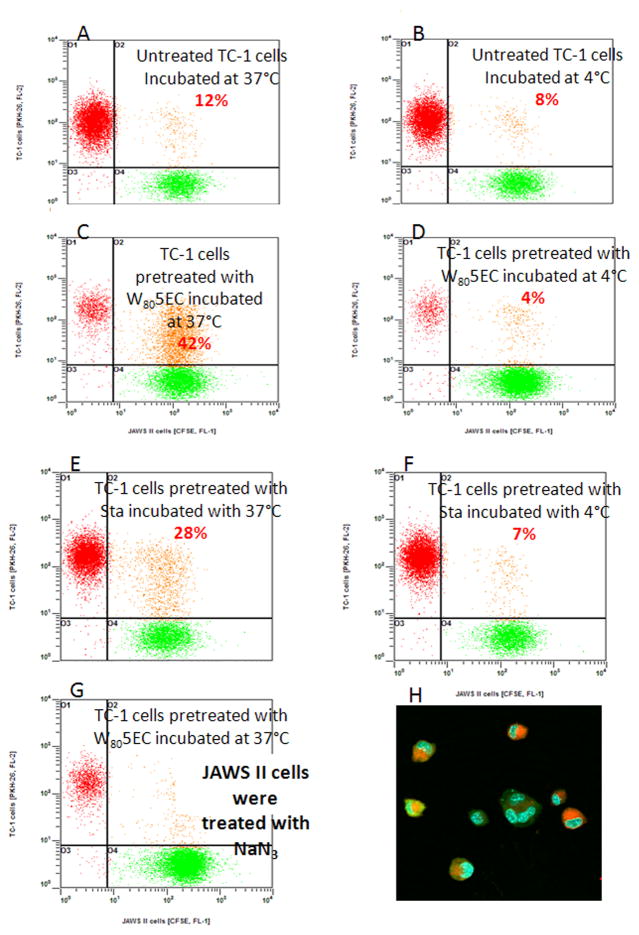
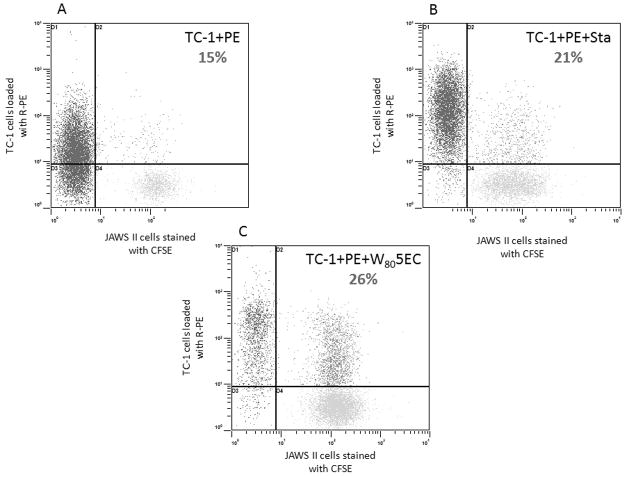
We tested how DCs respond to ECs treated with W805EC using a co-culture system. PKH-25-stained (red fluorescence) TC-1 cells were incubated with W805EC, staurosporine, or vehicle control, and the next day were washed and mixed in equal number with CFSE-stained (green fluorescence) JAWS II cells. When untreated TC-1 cells were incubated with JAWS II cells, double-stained JAWS II cells occurred in 12% of the population (Fig. 4A). In contrast, when TC-1 cells were treated with W805EC, double-stained JAWS II cells increased to 42% (Fig. 4C), indicating a significant increase in the engulfment of ECs when pre-treated with W805EC. Staurosporine-pretreatment of TC-1 cells also increased the percentage of double-stained JAWS II cells, but to a lesser extent (28%) (Fig. 4E). When examined by confocal microscopy, fragments of W805EC-treated TC-1 cells (red) were clearly seen within the JAWS II cells (green) (Fig. 4H). Engulfment of the TC-1 cells did not take place at 4°C (Fig. 4B, D, F) and pretreatment of JAWS II cells with NaN3 also inhibited the process (Fig. 4G). Moreover, non-phagocytic EL-4 cells did not engulf TC-1 cells pretreated with W805EC (data not shown). These studies indicate that engulfment of TC-1 is an active, cell-specific and energy-dependent process. We next investigated whether the engulfment of TC-1 cells may cause antigen transfer to DCs. CFSE-JAWS II cells were incubated with TC-1 cells loaded with R-PE in the presence or absence of W805EC overnight. TC-1 cells were then mixed in equal number with JAWS II cells, incubated for four hours, and analyzed using flow cytometry. When TC-1 cells loaded with R-PE antigen (red) were incubated with CFSE-JAWS II, 15% of CFSE-JAWS II cells were additionally stained with the R-PE (Fig. 5A). In contrast, when the TC-1 cells were also treated with W805EC, the double-stained CFSE-JAWS II cells increased to 26% (Fig. 5C). Staurosporine-treated TC-1 cells also increased the percentage of double-stained JAWS II DCs, to approximately 21% (Fig. 5B).
Figure 4.
NE facilitates engulfment of TC-1 cells by JAWS II cells. JAWS II cells were stained with CFSE (green fluorescence); TC-1 cells were stained with PKH-26 (red fluorescence). TC-1 cells were then incubated overnight with either NE (C, D) or staurosporine (E, F) or were left untreated (A, B). The next day, TC-1 ECs were mixed in equal number with CFSE-stained JAWS II DCs. The JAWS II cells were pretreated with 35 mM NaN3 overnight (G). Mixed cultures were incubated for four hours either at 37°C or 4°C and analyzed using flow cytometry. Confocal microscopy microphotograph of TC-1 cells treated with NE and co-cultured with JAWS II cells (H). Data shown are representative of one of five independent experiments. Significant difference (p<0.05) in the percentage of engulfed TC-1 cells between untreated and treated with 0.05% of W805EC (N=5) has been observed as tested using Wilcoxon signed-rank test.
Figure 5.
NE augments antigen transfer from ECs to DCs. JAWS II cells were stained with CFSE (green fluorescence); TC-1 cells were uploaded overnight with 40 μg/mL R-PE (A), uploaded with R-PE and treated with 1μM staurosporine (B), uploaded with R-PE and treated with 0.05% W805EC (C). The next day TC-1 cells were mixed in equal number with CFSE-stained JAWS II cells and mixed cultures were incubated for four hours at 37°C and analyzed using flow cytometry. The experiments were repeated independently on three occasions. Data shown from a single experiment are representative for all tree experiments performed. Significant difference (p<0.003) in the percentage of JAWS II cells with R-PE antigen transferred from TC-1 cells untreated vs. treated with W805EC (N=6) has been observed as tested using Wilcoxon signed-rank test.
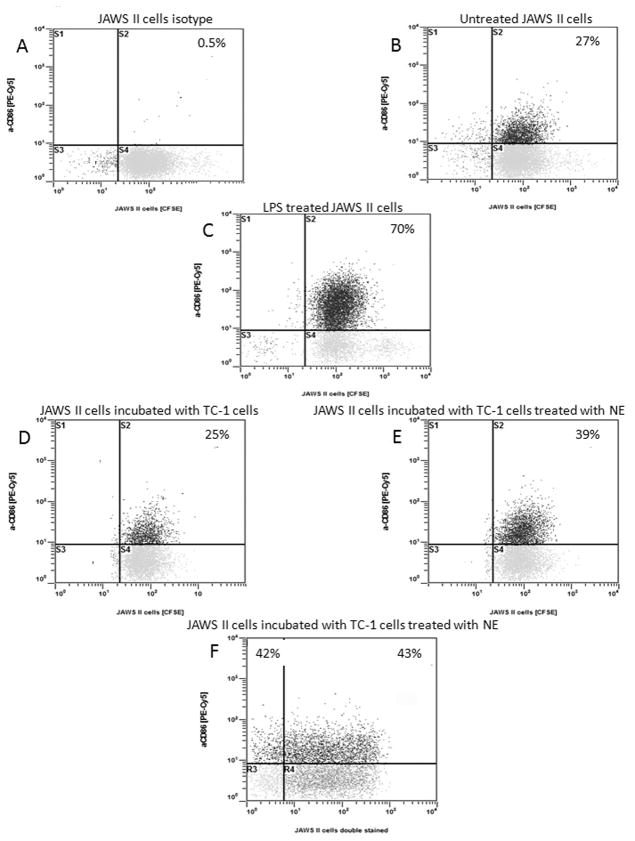
Co-culture of JAWS II cells and TC-1 cells treated with W805EC leads to up-regulation of CD86 on JAWS II cells
PKH-26-TC-1 cells were treated with 0.05% W805EC, washed and mixed with an equal number of CFSE-JAWS II cells. Co-cultured cells were incubated overnight and analyzed using flow cytometry. As shown in Figure 6B, JAWS II cells showed baseline CD86 antigen expression on approximately 27% of cells. LPS treatment (a positive control) up-regulates expression of CD86 on JAWS II cells to 79% (Fig. 6C). Co-culture of the JAWS II cells with untreated TC-1 cells had no effect on the expression of CD86 (Fig. 6D). In contrast, co-culture of the JAWS II with TC-1 cells pretreated with W805EC increased the expression of CD86 antigen in approximately 39% of JAWS II cells (Fig. 6E). There was no difference in CD86 expression between JAWS II cells that had or had not engulfed TC-1 cells (upper right quadrant vs. upper left quadrant in Figure 6F).
Figure 6.
Detection of expression of CD86 antigen on JAWS II cells. A) JAWS II cells stained with isotype control antibody. B) Untreated JAWS II cells stained with anti-CD86-PE-Cy5 antibody. C) JAWS II cells stimulated with 10 μg/mL LPS for 24 hours and stained with anti-CD86-PE-Cy5 antibody (positive control). D) JAWS II cells co-cultured with TC-1 cells and stained with anti-CD86-PE-Cy5 antibody. E) JAWS II cells co-cultured with TC-1 cells pretreated with W805EC and stained with anti-CD86-PE-Cy5 antibody. F) Shows equal percentage of JAWS II cells expressing CD86 antigen between two subsets of JAWS II cells: those which phagocytosed TC-1 cells (the upper right quadrant) and those which did not (the upper left quadrant). To exclusively analyzed JAWS II cells, red fluorescent TC-1 cells were gated out. Cells were treated and stained with antibodies on two independent occasions. Significant difference (p<0.01) in the percentage of anti-CD86 expressed on JAWS II cells co-cultured with untreated TC-1 vs. treated with W805EC (N=5) has been observed as tested using Wilcoxon signed-rank test.
NE causes cell-cycle arrest at G2/M phase and apoptosis of epithelial cells
Since phagocytes engulf apoptotic and necrotic cells [36, 37], we next investigated whether increased engulfment of W805EC-treated TC-1 cells could be attributable to cell-cycle aberration and apoptosis. We indeed observed an increase of percentage of cells in the G2/M phase after treatment with W805EC (Supplemental Figure 3).
To detect apoptosis W805EC-treated TC-1 cells were stained with annexin V (apoptosis) and PI (necrosis), and analyzed by flow cytometry. Treatment of TC-1 cells with W805EC resulted in approximately 8% early apoptotic cells at 48 hr (annexin V fluorescence alone) and 65% late apoptotic cells (annexin V and PI fluorescence). At 72 hr time-point the fraction of late apoptotic cells increased to 88% and no early apoptotic cells were recorded (Supplemental Figure 4C). In contrast, staurosporine-treated cells (Supplemental Figure 4B) showed only 16% late apoptotic/necrotic cells, while one-third of the cells were early apoptotic after 48-hour treatment. At 72 hour, the fraction of late apoptotic/necrotic cells increased up to 63%. RT-PCR analysis of genes involved in cell-cycle arrest and apoptosis showed that the pattern of gene expression in W805EC-treated and staurosporine-treated cells was different (Supplemental Table 1 and Figure 5).
Discussion
In this study potential nanoemulsion adjuvant for mucosal vaccine has been evaluated both in vivo and in vitro. The potential drawback of mucosal vaccine is how to avoid uptake by the olfactory nerve in the upper part of the nose and cause brain damage [38]. In our pilot unpublished study we addressed this issue. We examined whether one of the active components of NE – CPC can penetrate olfactory tissue and brain after intranasal instillation of 10 μl 20% W805EC. We did not detect any trace of CPC in brain using HPLC method (Paul Makidon – personal communication).
To evaluate the function of W805EC as a mucosal vaccine adjuvant we immunized C57BL/6 mice either with OVA+PBS or OVA+W805EC. A significant titer of specific IgG was observed two weeks after a single immunization with OVA+W805EC and further increased following subsequent immunizations (Fig. 1A). Since IgG2a isotype (Igh-1a) is not expressed in the C57BL/6 strain due to deletion of the Igh-1a gene [33] only IgG2b and IgG2c were evaluated. Evaluation of immunoglobulin subclasses showed that endpoint titers of both IgG1 and IgG2b increased after each additional immunization, while the endpoint titer of the IgG2c subclass did not increase after multiple immunizations, which may indicate a lack of Th1 response (Fig. 1B) [35].
Cytokine production in OVA+W805EC immunized mice was also evaluated. An increase in cytokine production as compared to the OVA+PBS group was observed for IFN-γ, IL-10, IL-17, IL-2, IL-4 and IL-5 (Fig. 2). This is interesting, because it demonstrates that the cells secrete Th1 markers (IL-2 and IFN-γ) despite the lack of elevated IgG2c antibodies. Furthermore, increasing levels of IL-4, IL-5 and IL-10 cytokines (Th2), and IL-17 cytokine (Th17) were also observed. Th17 response is implicated in clearing pathogens during host defense reactions and in inducing tissue inflammation in autoimmune disease [39]. The cytokine evaluation has been performed using splenocytes but similar pattern of cytokine production was noticed when lymphocytes isolated from cervical and inguinal lymph nodes were evaluated (data not shown). These results are consistent with previous studies that used HIV gp120 soluble antigen with W805EC for mucosal immunization in BALB/c mice [23].
To further define the function of the W805EC we examined mechanisms of NE adjuvant activity with respect to antigen uptake by ECs, antigen transfer to DCs and apoptosis. A study by our group has recently demonstrated that i.n. delivery of a W805EC-based vaccine results in the rapid uptake and internalization of antigen by both ECs and DCs present in the nasal mucosa and draining LNs [40] suggesting the possibility that the uptake of antigen not only by DCs, but also by ECs, may be an important aspect of the adjuvant activity of W805EC. In current study, we documented that W805EC promoted direct antigen uptake in both EC and DC cells. One possibility is that positively charged W805EC-Ag particles bind to cell membranes electrostatically and are delivered to the interior of the cell by endocytosis. Once inside the cell, the NE-Ag complex may then fuse with lysosomes to hydrolyze or to break down the complex. This hypothesis is supported by experiments using DQ-OVA, which only becomes fluorescent after hydrolysis inside lysosomes (Fig. 3B).
Whether antigen loaded into W805EC-treated ECs later gains access to DCs is an important question, since uptake of antigen by ECs followed by engulfment and secondary antigen uptake by DCs would represent indirect route for presentation of antigen to the immune system. To examine the effect of W805EC on antigen transfer from EC to DC cells, we applied a co-culture system. We demonstrate, first, that DCs engulf W805EC-treated TC-1 cells, but not untreated control cells (Fig. 4A, C). This suggested that W805EC may mediate antigen transfer from TC-1 cells to JAWS II cells. It was confirmed by the uptake of antigen by JAWS II cells from TC-1 cells that had been exposed to NE-Ag (Fig. 5).
Remarkably, W805EC-treated TC-1 cells induced maturation of the DCs. The W805EC-treated TC-1 cells enhance the expression of the CD86 antigen on the JAWS II cells (Fig. 6). This is consistent with a recent report documenting that exposure to emulsion-pretreated cells induced the maturation of DCs, resulting in enhanced surface expression of MHC class II molecules and the up-regulation of co-stimulatory molecules [41]. The CD86 receptor on the surface of DCs provides important co-stimulatory signals to augment and sustain a T-cell response via an interaction with CD28 [42–44]. Since NE-treated ECs affect rapid increase of CD86+ DCs it is plausible that NE contributes directly or indirectly to both the maturation and activation of DCs. Since phagocytes readily engulf apoptotic cells [36, 37], we investigated whether increased engulfment of W805EC-treated TC-1 cells is associated with induction of cell-cycle arrest and apoptosis. We found that W805EC treatment does indeed lead to significant G2/M arrest (Supplemental Figure 3), to a remarkable degree similar to staurosporine. However, the process blocking mitotic entry by NE and staurosporine appear different (Supplemental Figure 5).
The dead or dying cells generate danger signals that stimulate migration of APCs, facilitate antigen uptake, and induce the maturation of DCs [45–47]. APCs are thought to engulf apoptotic cells and subsequently load antigens on MHC class I and II and trigger downstream antigen-specific immune responses [48, 49]. Necrotic cells, on the other hand, serve as natural adjuvants to activate DCs by endogenous signals [50]. However, many recent studies have demonstrated that the mode of cell death does not impact uptake and presentation of cell-associated antigen by DCs or their maturation [51]. We used annexin V and PI to distinguish early- and late-apoptotic cells treated with NE (Supplemental Figure 4). We observed that NE-treated cells became increasingly double-stained (annexin V/PI) over time. This observation indicates that most of the cells either succumb to apoptotic necrosis, oncotic necrosis, or both. It is consistent with data obtained by Lecoeur, et al [52], who documented that the external PS exposure has not been exclusive to apoptotic cells, but also occurs in oncotic cells. Interestingly, RT-PCR analysis of genes involved in apoptosis showed that after treatment of TC-1 cells with NE three out of six genes down-regulated were genes coding caspases, directly involved in apoptosis (Supplemental Table 1). Altogether these results are consistent with data obtained by Yang, et al [53–55], who documented that apoptotic and necrotic effects of emulsion-based adjuvants play a pivotal role in antigen delivery and presentation.
In conclusion, we showed that exposure of both epithelial cells and DCs to nanoemulsion exhibited augmented antigen uptake. Epithelial cells (TC-1) subsequently underwent apoptosis, and when co-cultured with DC cells were rapidly engulfed by latter cells, which responded by up-regulating DC maturation marker CD86. Altogether these results suggest that the effectiveness of nanoemulsions as adjuvants may stem, at least in part, from the engulfment of antigen-loaded epithelial cells, leading to enhanced antigen processing and a strong and balanced mucosal and systemic immune response. However, we have not documented yet whether the NE promotes their migration to lymph nodes, followed by the presentation of antigen to effector cells and thereby induces an unusually strong and balanced mucosal and systemic immune response. This is the focus of our ongoing studies.
Supplementary Material
Highlights.
Facilitates antigen uptake by epithelial and dendritic cells
Promotes engulfment of epithelial by dendritic cells
Augments antigen transfer from epithelial to dendritic cells
Induces maturation of dendritic cells
Contributes to cell cycle arrest and apoptosis
Acknowledgments
Source of Funding: This project has been funded in whole or in part with Federal funds from the National Institute for Allergy and Infectious Disease, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272200900031C.
The authors wish to express thanks to Dr. Wendy Banka for her assistance in revision of the manuscript.
Abbreviations used in this article
- EC
epithelial cell
- LDH
lactate dehydrogenase cytotoxicity assay kit
- NE
nanoemulsion
- PI
propidium iodide
- PS
phosphatidylserine
- XTT
cell proliferation assay kit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361(25):2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 2.De Gregorio E, Tritto E, Rappuoli R. Alum adjuvanticity: unraveling a century old mystery. Eur J Immunol. 2008;38(8):2068–2071. doi: 10.1002/eji.200838648. [DOI] [PubMed] [Google Scholar]
- 3.Gupta RK, Siber GR. Adjuvants for human vaccines--current status, problems and future prospects. Vaccine. 1995;13(14):1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 4.Skowronski DM, Janjua NZ, De Serres G, Hottes TS, Dickinson JA, Crowcroft N, Kwindt TL, Tang P, Charest H, Fonseca K, et al. Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009. BMJ. 2011;342:c7297. doi: 10.1136/bmj.c7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta RK, Rost BE, Relyveld E, Siber GR. Vaccine Design: The Subunit and Vaccine Approach. New York: Plenum; 1995. [Google Scholar]
- 6.Wijburg OL, van den Dobbelsteen GP, Vadolas J, Sanders A, Strugnell RA, van Rooijen N. The role of macrophages in the induction and regulation of immunity elicited by exogenous antigens. Eur J Immunol. 1998;28(2):479–487. doi: 10.1002/(SICI)1521-4141(199802)28:02<479::AID-IMMU479>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Vajdy M, Srivastava I, Polo J, Donnelly J, O’Hagan D, Singh M. Mucosal adjuvants and delivery systems for protein-, DNA- and RNA-based vaccines. Immunology & Cell Biology. 2004;82 (6):617–627. doi: 10.1111/j.1440-1711.2004.01288.x. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Krishnan S, Lenzen G, Magne R, Gomard E, Guillet JG, Levy JP, Meulien P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. European Journal of Immunology. 1993;23(7):1719–1722. doi: 10.1002/eji.1830230749. [DOI] [PubMed] [Google Scholar]
- 9.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239:178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11(4 Suppl):S63–68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 11.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109(9):3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Q, Egelston C, Gagnon S, Sui YJ, Belyakov IM, Klinman DM, Berzofsky JA. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010;120(2):607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101(11):4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 14.Watts C, Zaru R, Prescott AR, Wallin RP, West MA. Proximal effects of Toll-like receptor activation in dendritic cells. Curr Opin Immunol. 2007;19(1):73–78. doi: 10.1016/j.coi.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Liang SA, Hajishengallis G. Heat-Labile Enterotoxins as Adjuvants or Anti-Inflammatory Agents. Immunol Invest. 2010;39(4–5):449–467. doi: 10.3109/08820130903563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajishengallis G, Arce S, Gockel CM, Connell TD, Russell MW. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J Dent Res. 2005;84(12):1104–1116. doi: 10.1177/154405910508401205. [DOI] [PubMed] [Google Scholar]
- 17.Hamouda T, Baker JR., Jr Antimicrobial mechanism of action of surfactant lipid preparations in enteric Gram-negative bacilli. J Appl Microbiol. 2000;89(3):397–403. doi: 10.1046/j.1365-2672.2000.01127.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamouda T, Hayes MM, Cao Z, Tonda R, Johnson K, Wright DC, Brisker J, Baker JR., Jr A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus species. J Infect Dis. 1999;180(6):1939–1949. doi: 10.1086/315124. [DOI] [PubMed] [Google Scholar]
- 19.Hamouda T, Myc A, Donovan B, Shih AY, Reuter JD, Baker JR., Jr A novel surfactant nanoemulsion with a unique non-irritant topical antimicrobial activity against bacteria, enveloped viruses and fungi. Microbiol Res. 2001;156(1):1–7. doi: 10.1078/0944-5013-00069. [DOI] [PubMed] [Google Scholar]
- 20.LiPuma JJ, Rathinavelu S, Foster BK, Keoleian JC, Makidon PE, Kalikin LM, Baker JR., Jr In vitro activities of a novel nanoemulsion against Burkholderia and other multidrug-resistant cystic fibrosis-associated bacterial species. Antimicrob Agents Chemother. 2009;53(1):249–255. doi: 10.1128/AAC.00691-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myc A, Vanhecke T, Landers JJ, Hamouda T, Baker JR., Jr The fungicidal activity of novel nanoemulsion (X8W60PC) against clinically important yeast and filamentous fungi. Mycopathologia. 2002;155(4):195–201. doi: 10.1023/a:1021129710316. [DOI] [PubMed] [Google Scholar]
- 22.Bielinska AU, Janczak KW, Landers JJ, Makidon P, Sower LE, Peterson JW, Baker JR., Jr Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect Immun. 2007;75(8):4020–4029. doi: 10.1128/IAI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bielinska AU, Janczak KW, Landers JJ, Markovitz DM, Montefiori DC, Baker JR., Jr Nasal immunization with a recombinant HIV gp120 and nanoemulsion adjuvant produces Th1 polarized responses and neutralizing antibodies to primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2008;24(2):271–281. doi: 10.1089/aid.2007.0148. [DOI] [PubMed] [Google Scholar]
- 24.Makidon PE, Bielinska AU, Nigavekar SS, Janczak KW, Knowlton J, Scott AJ, Mank N, Cao Z, Rathinavelu S, Beer MR, et al. Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS One. 2008;3(8):e2954. doi: 10.1371/journal.pone.0002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makidon PE, Knowlton J, Groom JV, 2nd, Blanco LP, LiPuma JJ, Bielinska AU, Baker JR., Jr Induction of immune response to the 17 kDa OMPA Burkholderia cenocepacia polypeptide and protection against pulmonary infection in mice after nasal vaccination with an OMP nanoemulsion-based vaccine. Med Microbiol Immunol. 2010;199(2):81–92. doi: 10.1007/s00430-009-0137-2. [DOI] [PubMed] [Google Scholar]
- 26.Bielinska AU, Chepurnov AA, Landers JJ, Janczak KW, Chepurnova TS, Luker GD, Baker JR., Jr A novel, killed-virus nasal vaccinia virus vaccine. Clin Vaccine Immunol. 2008;15(2):348–358. doi: 10.1128/CVI.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamouda T, Chepurnov A, Mank N, Knowlton J, Chepurnova T, Myc A, Sutcliffe J, Baker JR., Jr Efficacy, immunogenicity and stability of a novel intranasal nanoemulsion-adjuvanted influenza vaccine in a murine model. Hum Vaccin. 2010;6(7):585–594. doi: 10.4161/hv.6.7.11818. [DOI] [PubMed] [Google Scholar]
- 28.Lindell DM, Morris SB, White MP, Kallal LE, Lundy PK, Hamouda T, Baker JR, Jr, Lukacs NW. A novel inactivated intranasal respiratory syncytial virus vaccine promotes viral clearance without Th2 associated vaccine-enhanced disease. PLoS One. 2011;6(7):e21823. doi: 10.1371/journal.pone.0021823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myc A, Kukowska-Latallo JF, Bielinska AU, Cao P, Myc PP, Janczak K, Sturm TR, Grabinski MS, Landers JJ, Young KS, et al. Development of immune response that protects mice from viral pneumonitis after a single intranasal immunization with influenza A virus and nanoemulsion. Vaccine. 2003;21(25–26):3801–3814. doi: 10.1016/s0264-410x(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 30.Bielinska AU, Gerber M, Blanco LP, Makidon PE, Janczak KW, Beer M, Swanson B, Baker JR., Jr Induction of Th17 cellular immunity with a novel nanoemulsion adjuvant. Crit Rev Immunol. 2010;30(2):189–199. doi: 10.1615/critrevimmunol.v30.i2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanberry LR, Simon JK, Johnson C, Robinson PL, Morry J, Flack MR, Gracon S, Myc A, Hamouda T, Baker JR., Jr Safety and immunogenicity of a novel nanoemulsion mucosal adjuvant W805EC combined with approved seasonal influenza antigens. Vaccine. 2012;30(2):307–316. doi: 10.1016/j.vaccine.2011.10.094. [DOI] [PubMed] [Google Scholar]
- 32.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jouvin-Marche E, Morgado MG, Leguern C, Voegtle D, Bonhomme F, Cazenave PA. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics. 1989;29(2):92–97. doi: 10.1007/BF00395856. [DOI] [PubMed] [Google Scholar]
- 34.Martin RM, Lew AM. Is IgG2a a good Th1 marker in mice? Immunol Today. 1998;19(1):49. doi: 10.1016/s0167-5699(97)87499-3. [DOI] [PubMed] [Google Scholar]
- 35.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212(2):187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 36.Brouckaert G, Kalai M, Krysko DV, Saelens X, Vercammen D, Ndlovu M, Haegeman G, D’Herde K, Vandenabeele P. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol Biol Cell. 2004;15 (3):1089–1100. doi: 10.1091/mbc.E03-09-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 38.Levine MM, Sztein MB. Vaccine development strategies for improving immunization: the role of modern immunology. Nat Immunol. 2004;5(5):460–464. doi: 10.1038/ni0504-460. [DOI] [PubMed] [Google Scholar]
- 39.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual Review of Immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 40.Makidon PE, Belyakov IM, Janczak KW, Lenders J, Bielinska AU, Groom JV, Baker JR., Jr Nanoemulsion mucosal adjuvant uniquely activates cytokine production be nasal ciliated epithelium and induces dendritic cells trafficking. Eur J Immunol. 2012 doi: 10.1002/eji.201142346. (in print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen SS, Yang YW. Antigen delivery for cross priming via the emulsion vaccine adjuvants. Vaccine. 2012;30(9):1560–1571. doi: 10.1016/j.vaccine.2011.12.120. [DOI] [PubMed] [Google Scholar]
- 42.Lanzavecchia A, Lezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96(1):1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 43.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 44.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 45.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392(6671):86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 46.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188(7):1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 49.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195(10):1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5(11):1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 51.Lohmann C, Muschaweckh A, Kirschnek S, Jennen L, Wagner H, Hacker G. Induction of tumor cell apoptosis or necrosis by conditional expression of cell death proteins: analysis of cell death pathways and in vitro immune stimulatory potential. J Immunol. 2009;182(8):4538–4546. doi: 10.4049/jimmunol.0803989. [DOI] [PubMed] [Google Scholar]
- 52.Lecoeur H, Prevost MC, Gougeon ML. Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane: a reconsideration of the specificity of the annexin V/propidium iodide assay. Cytometry. 2001;44(1):65–72. doi: 10.1002/1097-0320(20010501)44:1<65::aid-cyto1083>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 53.Yang YW, Wu CA, Morrow WJ. The apoptotic and necrotic effects of tomatine adjuvant. Vaccine. 2004;22(17–18):2316–2327. doi: 10.1016/j.vaccine.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 54.Yang YW, Wu CA, Morrow WJ. Cell death induced by vaccine adjuvants containing surfactants. Vaccine. 2004;22(11–12):1524–1536. doi: 10.1016/j.vaccine.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 55.Yang YW, Shen SS. Enhanced antigen delivery via cell death induced by the vaccine adjuvants. Vaccine. 2007;25(45):7763–7772. doi: 10.1016/j.vaccine.2007.08.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.