Abstract
Purpose
Pre-operative (pre-op) chemoradiation therapy (CRT) improves local control and reduces toxicity more than post-operative (post-op) CRT for the treatment of stages II/III rectal cancer, but studies suggest many patients still receive post-op CRT. We examined patient beliefs, and clinical and provider characteristics associated with receipt of recommended therapy.
Methods
We identified stage II/III rectal cancer patients who had primary site resection and CRT among subjects in the Cancer Care Outcomes Research and Surveillance Consortium, a population- and health system-based prospective cohort of newly diagnosed colorectal cancer patients from 2003 to 2005. Patient surveys and abstracted medical records were used to construct variables and determine sequence of CRT and surgery. Logistic regression was used to model the association between predictors and receipt of pre-op CRT.
Results
Of the 201 patients, 66% received pre-op and 34% received post-op CRT. Those visiting a medical oncologist and/or radiation oncologist prior to a surgeon had a 96% (95% CI, 92% to 100%) predicted probability of receiving pre-op CRT, compared to 48% (95% CI, 41% to 55%) for those visiting a surgeon first. Among those visiting a surgeon first, documentation of recommended staging procedures was associated with receiving pre-op CRT.
Conclusion
Sequence of provider visits and documentation of recommended staging procedures were important predictors of receiving pre-op CRT. Initial multidisciplinary evaluation led to better adherence to CRT guidelines. Further evaluation of provider characteristics, referral patterns and related health system processes should be undertaken to inform targeted interventions to reduce variation from recommended care.
Background
Colorectal cancer is the third leading cause of cancer deaths in the United States and was associated with 51,370 deaths in 2010. Of 141,210 incident cases of colorectal cancer in 2011, 28% (39,510) were rectal.1 Compared to colon cancer, rectal cancer is associated with increased risk of local recurrence and worse overall prognosis.2–5 Rectal cancer therapies are also associated with higher morbidity, including problems with bowel function, urinary and fecal continence, and sexual functioning.
The standard of care for stages II and III rectal cancer previously involved surgical resection and post-operative (post-op) chemoradiation therapy (CRT). However, more recent studies have shown several advantages to pre-operative (pre-op) CRT including improved local control and reduction in toxicity.6–8 Some evidence suggests that pre-op CRT is also associated with more sphincter preserving surgeries than post-op CRT.7 Consequently, national guidelines recommend pre-op CRT for all patients with stage II/III rectal cancer.9
While pre-op CRT is considered the standard of care, many patients still receive post-op CRT. Previous studies of stage II/III rectal cancer patients using SEER or other registry data found rates of pre-op radiation therapy (RT) were increasing as rates of post-op RT were decreasing, but substantial treatment variability remained. Among patients diagnosed with rectal cancer between 2003 and 2005 who received both surgery and RT, 60% received pre-op RT and 40% received post-op RT.10–12 Studies based on SEER data found patients receiving pre-op RT were younger and more likely to be male compared to those receiving post-op RT, and demonstrated geographic variation.10–11 More recent SEER Stat data from 2007 and 2008 indicates that 28% of stage II/III rectal cancer patients who received CRT for rectal cancer still received CRT post-operatively.13
SEER has limited data on potential explanatory variables for this variation. Using data from the Cancer Care Outcomes Research and Surveillance Consortium (CanCORS), the primary objectives of this study were to characterize in more detail the differences between patients who received pre-op CRT and those who received post-op CRT, and the attributes of physicians who practice in any of the participating CanCORS facilities. While the CanCORS cohort spanned a transitional time period from late 2003 through 2005 during which the proportion of stage II/III rectal cancer patients receiving pre-op CRT was approximately 60%, relatively little has changed in recent years with just over 70% receiving pre-op CRT in 2008. Factors associated with receipt of CRT during the CanCORS study period are therefore still very relevant today. In addition, there was at least some awareness of changing guidelines by the beginning of the CanCORS study period, as the National Comprehensive Cancer Network (NCCN) Guidelines incorporated pre-op CRT into their recommended course of treatment in 200314 and multiple trials had begun reporting results at national conferences and in journals in 2003.15–19 We examined patient beliefs, and clinical and provider characteristics associated with receipt of recommended therapy.
Materials and Methods
CanCORS is a population and health-system based cohort study including approximately 4,713 adults with newly diagnosed colorectal cancer recruited between 2003 and 2005 from geographically diverse populations and healthcare systems. It contains detailed information on the acute treatment phase, including surgery, chemotherapy and RT regimens, and information on the clinical and patient-reported outcomes experienced by the patient.20–22
CanCORS participants with stage II or III rectal adenocarcinoma who had primary site resection, RT and chemotherapy initiated within 180 days of surgery, no recurrence within 180 days of diagnosis and no history of prior cancer were studied. Medical record information was used to assign American Joint Committee on Cancer collaborative stage.23 Surgery, RT and chemotherapy information was extracted from medical records, which covered 30 days prior to and at least 15 months after diagnosis. Medical record information was reviewed to determine dates of service, radiation dose ( (i) <4500 cGy, (ii) 4500 – 5040 cGy, and (iii) ≥5040 cGy), surgical approach (abdominal perineal resection (APR), lower anterior resection (LAR), other), chemotherapy regimens [(i) 5-fluorouracil (5-FU), leucovorin and/or capecitabine with no other agents; (ii) regimens including agents other than 5-FU, leucovorin or capecitabine, such as irinotecan, oxaliplatin or bevacizumab; or (iii) unspecified], Adult Comorbidity Evaluation – 27 (ACE-27) comorbidity indicators,24 documentation of recommended staging procedures [computed tomography (CT) of the abdomen and chest, and magnetic resonance imaging (MRI) of the pelvis or endorectal ultrasound (EUS)], and tumor size and location.
Sequence of visits to the medical oncologist and/or radiation oncologist and the surgeon was also captured. Medical record abstractors were instructed to record all dates associated with patient-provider contacts. For analytic purposes, new consultations, clinical office follow-up notes, emergency department/urgent care clinic visits, radiation treatment visits (with provider contact), chemotherapy, transfusion, or other intravenous medication administration (with physician contact) and patient-to-provider phone calls were considered to be visits. If no visit to a medical oncologist, a radiation oncologist or a surgeon was recorded prior to the initiation of their respective therapies/procedures, the first date of treatment was used as the first visit date for that corresponding provider type.
Sociodemographic information (age, sex, education, income, race, income), health insurance status, patient beliefs and treatment preferences were obtained from self-reported baseline patient surveys.21
Data Analysis
Univariate statistics, Chi-square test, Fisher’s exact test and Wilcoxon rank sum test were used to compare the pre-op and post-op CRT groups on key variables. Logistic regression was used to model the association between predictors and receipt of pre-op CRT, and obtain both covariate-adjusted predicted proportions and predicted marginal counts of subjects receiving pre-op CRT.25
The set of candidate predictive variables included age, gender, race, income, health insurance (none vs. covered by at least one insurer/payer), education (no 4-year college vs. at least some 4-year college), marital status (lives alone vs. married or lives with partner), stage of cancer (II vs. III), CanCORS research site affiliation, sequence of physician visits (surgeon first vs. medical and/or radiation oncologist first), documentation of staging procedures, comorbidities (ACE-27 categories and individual conditions), tumor size and location and patient treatment beliefs and preferences. Age and cancer stage were considered key predictors and were not candidates for removal in a backward variable selection procedure, used to eliminate non-informative or redundant predictors. Sensitivity analyses limited to patients with documented provider visits prior to treatment were performed.
A descriptive analysis of a random sample of surgeons, radiation oncologists and medical oncologists who cared for CanCORS patients in the previous 12 months, including those with colorectal cancer was also performed. Methods and instruments used in relation to the CanCORS physician survey have been previously described.26
Results
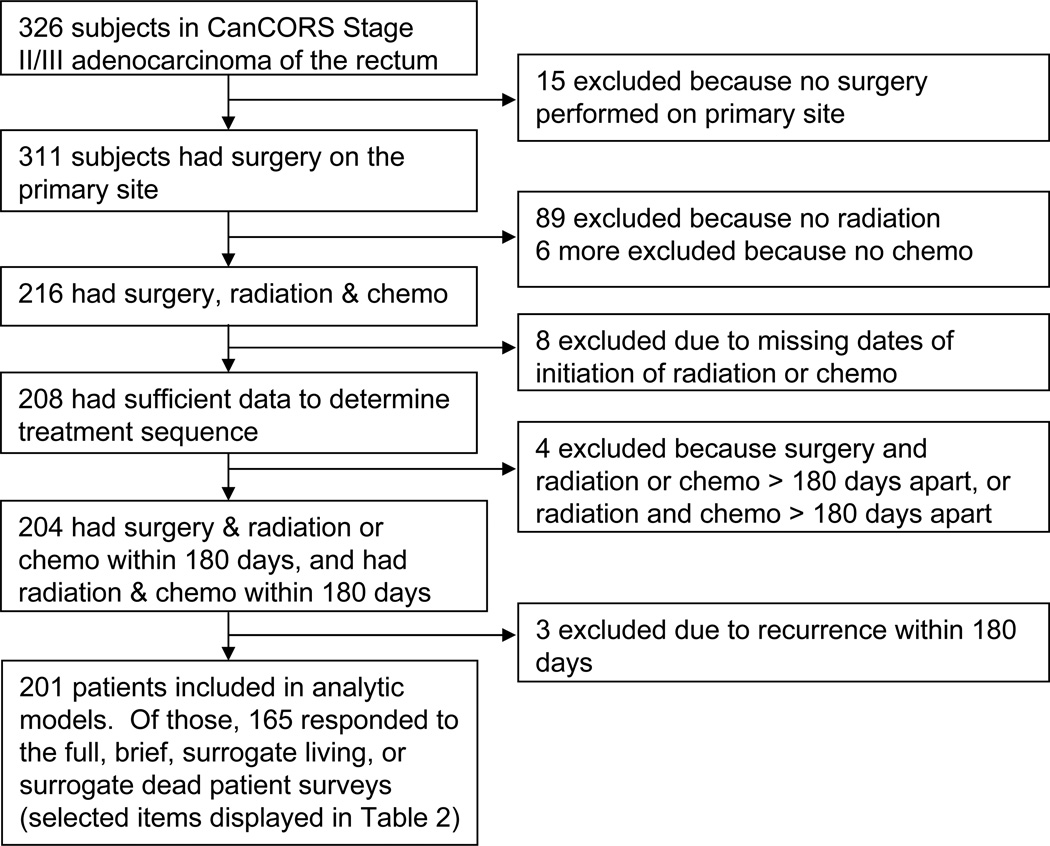
Of the 311 CanCORS patients with stages II/III rectal adenocarcinoma and primary site resection, 201 met all inclusion criteria (Figure 1). The characteristics of pre-op and post-op CRT groups are described in Table 1. Higher proportions of those receiving pre-op CRT had stage II disease and documentation of a pre-treatment MRI of the pelvis or rectal EUS and CT of the chest. Those receiving post-op CRT had a higher proportion of tumors located at the rectosigmoid junction, significantly larger tumors and angina/coronary artery disease. There were no differences in demographics, such as age, gender, ethnicity, marital status, education, income or insurance status, between groups.
Figure 1.
Flowchart of study participants.
Table 1.
Characteristics of patients with stages II or III rectal cancer by chemoradiation therapy (CRT)—surgery sequence status (pre-operative: pre-op; post-operative: post-op)
| Variable |
Total N=201* |
Pre-op CRT (N=133) |
Post-op CRT (N=68) |
p† |
|---|---|---|---|---|
| Research site affiliation | 0.69 | |||
| Cancer Research Network (Health Maintenance Organization population) | 22% | 26% | ||
| Veterans Affairs Health Care System | 18% | 15% | ||
| Population-based Registry | 60% | 59% | ||
| Female gender | 32% | 34% | 0.83 | |
| Lives alone | 39% | 35% | 0.60 | |
| Ethnicity | ||||
| White | 60% | 59% | 0.96 | |
| African American | 14% | 15% | ||
| Hispanic or Latino | 9% | 7% | ||
| Asian | 10% | 9% | ||
| Other | 8% | 10% | ||
| Age group (years) | ||||
| <55 | 36% | 37% | 0.72 | |
| 55–64 | 33% | 28% | ||
| >=65 | 31% | 35% | ||
| Stage III | 42% | 57% | 0.04 | |
| Comorbidities‡ (ACE-27 categories & selected conditions) | ||||
| None | 38% | 38% | ||
| Mild | 38% | 38% | ||
| Moderate | 17% | 16% | ||
| Severe | 8% | 7% | 0.99 | |
| Hypertension | 32% | 38% | 0.35 | |
| Angina/Coronary Artery Disease | 8% | 19% | 0.02 | |
| Arrhythmias | 4% | 0% | 0.17 | |
| Peripheral Artery disease | 5% | 3% | 0.45 | |
| Hepatic disease | 4% | 1% | 0.37 | |
| Obesity | 7% | 10% | 0.38 | |
| Respiratory | 7% | 9% | 0.60 | |
| Stomach/intestine | 8% | 4% | 0.31 | |
| Alcohol abuse | 29% | 18% | 0.07 | |
| Psychiatric disease | 7% | 7% | 1.00 | |
| Staging Procedures | ||||
| Computed Tomography (CT)-abdomen/pelvis | 88% | 78% | 0.06 | |
| Computed Tomography (CT)--chest | 29% | 13% | 0.01 | |
| Magnetic Resonance Imaging (MRI)--pelvis or Endorectal Ultrasound (EUS) | 49% | 25% | <0.01 | |
| Median tumor size (interquartile range) in mm (N=144) | 40 (25,50) | 50 (35,60) | 0.03 | |
| Tumor located at rectosigmoid junction (vs. rectum NOS) (N=199) | 11% | 27% | <0.01 | |
| Visited a medical or radiation oncologist before a surgeon | 44% | 4% | <0.01 | |
| Visited a medical or radiation oncologist and a surgeon prior to initiation of treatment | 71% | 13% | <0.01 | |
Total subjects=201 unless otherwise specified.
p values calculated based on Chi-square, Fisher’s exact or Wilcoxon rank sum tests as appropriate.
Comorbidities displayed if at least 5 people in one group had documentation of the condition, and were categorized according to the Adult Comorbidity Evaluation – 27 (ACE-27) indicators.
The two groups differed significantly in the sequence of their provider visits. Forty-four percent of those who received pre-op CRT visited a medical or radiation oncologist prior to visiting a surgeon, whereas only 4% of those who received post-op CRT visited a medical or radiation oncologist prior to visiting a surgeon, and 71% of the pre-op group visited a surgeon and a medical or radiation oncologist prior to receiving any treatment compared to 13% of the post-op group.
As illustrated in Table 2, there were no significant differences between those who received pre-op and post-op CRT in their preferred decision-making roles and beliefs related to RT, surgery and chemotherapy, with two exceptions. A higher proportion of people who received post-op CRT reported that RT would very likely or somewhat likely cure their cancer. Conversely, a higher proportion of people who received pre-op CRT reported that the RT would very likely or somewhat likely help with the symptoms they were having from their cancer. Almost all respondents reported receiving high quality care from their providers, with no differences by pre-op/post-op CRT status.
Table 2.
Decision making roles, beliefs, perceptions of quality and preferences of rectal cancer patients related to radiation therapy, chemotherapy and surgery by CRT—surgery sequence status
| Radiation Therapy |
Chemotherapy | Surgery | ||||
|---|---|---|---|---|---|---|
| Variable | Pre-op CRT |
Post-op CRT |
Pre-op CRT |
Post-op CRT |
Pre-op CRT |
Post-op CRT |
| Patient role in decision to have ____* | p=0.35 | p=0.12 | p=0.42 | |||
| Made decision with little or no input from doctors | 0% | 0% | 0% | 3% | 3% | 3% |
| Made decision after considering doctors' opinions | 32% | 45% | 31% | 40% | 33% | 44% |
| Made decision together with doctor | 53% | 47% | 52% | 44% | 52% | 48% |
| Doctors made decision after considering patient’s opinion | 5% | 3% | 5% | 8% | 7% | 5% |
| Doctors made the decision with little or no input from patient | 10% | 5% | 13% | 5% | 5% | 0% |
| Patient's belief that ____ will help him/her live longer† | p=0.30 | p=0.33 | p=1.00 | |||
| Very likely/ Somewhat likely | 96% | 91% | 95% | 90% | 98% | 98% |
| A little likely/ Not at all likely | 4% | 9% | 5% | 10% | 3% | 2% |
| Patient's belief ___ would help cure his/her cancer† | p<0.01 | p=0.08 | p=0.77 | |||
| Very likely/ Somewhat likely | 61% | 86% | 72% | 85% | 90% | 91% |
| A little likely/ Not at all likely | 39% | 14% | 28% | 15% | 10% | 9% |
| Patient's belief ___ would help with symptoms of cancer† | p=0.04 | p=0.26 | p=0.16 | |||
| Very likely/ Somewhat likely | 88% | 73% | 88% | 80% | 93% | 100% |
| A little likely/ Not at all likely | 12% | 27% | 12% | 20% | 7% | 0% |
| Patient's belief ___ would have side effects/complications† | p=0.48 | p=0.36 | p=0.99 | |||
| Very likely/ Somewhat likely | 79% | 84% | 89% | 93% | 54% | 54% |
| A little likely/ Not at all likely | 21% | 16% | 11% | 7% | 46% | 46% |
| Patient's rating of the quality health care received for ___* | p=0.17 | p=0.27 | p=1.00 | |||
| Excellent/ Very Good/ Good | 94% | 100% | 97% | 100% | 98% | 98% |
| Fair/ Poor | 6% | 0% | 3% | 0% | 2% | 2% |
| Patient Preferences‡ | Pre-op CRT |
Post-op CRT |
||||
| Preferences for treatment that extends life as much as possible vs. treatment that focuses on relieving pain and discomfort¥ | 61% | 60% | ||||
| Preferences for treatment that extends life as much as possible, even if it means using up financial resources vs. treatment that costs less¥ | 68% | 78% | ||||
Decision making questions and rating of health care quality asked on the full, brief, surrogate live and surrogate dead surveys (N=165).
Beliefs on therapies were asked on the full, brief and surrogate live surveys (N=163).
Patient preference questions were asked on the full survey only (N=128). P-values were non-significant.
The adjusted probabilities of receiving pre-op CRT based on the characteristics of 201 patients in the final logistic model are displayed in Table 3. Subjects visiting a medical or radiation oncologist prior to a surgeon had a 96% (95% CI, 92% to 100%) adjusted probability of receiving pre-op CRT, whereas those visiting a surgeon first had a 48% (95% CI, 41% to 55%) adjusted probability of receiving CRT. In addition, those who visited a surgeon and a medical or radiation oncologist prior to any treatment had a 92% (95% CI, 87% to 97%) adjusted probability of receiving pre-op CRT, whereas those visiting only a surgeon or a medical or radiation oncologist had a 32% (95% CI, 25% to 39%) adjusted probability. No other patient characteristics remained significant in this model. Sensitivity analyses limited to those with documented visits prior to treatment yielded very similar results.
Table 3.
Observed and adjusted probability of receiving pre-op CRT for characteristics of patients with rectal cancer
| Population | Characteristics of population (p value) |
Observed |
Adjusted %* |
95% CI | |
|---|---|---|---|---|---|
| No. | % | ||||
| Model I: Subjects met inclusion criteria (n=201) |
Stage (p=0.863) | ||||
| Stage II | 77 | 73% | 68% | 61–75 | |
| Stage III | 56 | 59% | 64% | 56–72 | |
| Age (p=0.218) | |||||
| C-Statistic=0.039 | <55 | 48 | 66% | 66% | 58–73 |
| 55–64 | 44 | 70% | 68% | 60–75 | |
| >=65 | 41 | 63% | 65% | 57–74 | |
| Visit Sequence (p<0.001) | |||||
| Visited surgeon first | 75 | 54% | 48% | 41–55 | |
| Visited Med Onc/Rad Onc first | 58 | 95% | 96% | 92–100 | |
| Visited surgeon & Med Onc/Rad Onc† prior to any treatment (p<0.001) |
|||||
| No | 39 | 40% | 32% | 25–39 | |
| Yes | 94 | 91% | 92% | 87–97 | |
| Model II: Subjects who visited a surgeon first (n=140) |
Stage (p=0.736) | ||||
| Stage II | 43 | 61% | 58% | 47–70 | |
| Stage III | 32 | 46% | 48% | 38–60 | |
| Age (p=0.237) | |||||
| <55 | 28 | 54% | 51% | 38–63 | |
| C-Statistic=0.729 | 55–64 | 21 | 54% | 52% | 37–68 |
| >=65 | 26 | 53% | 58% | 45–71 | |
| CT-chest documented (p=0.014) | |||||
| No | 52 | 48% | 48% | 39–57 | |
| Yes | 23 | 72% | 72% | 57–87 | |
| MRI or EUS documented (p<0.001) | |||||
| No | 34 | 40% | 40% | 30–50 | |
| Yes | 41 | 73% | 74% | 62–85 | |
Adjusted percentages derived from a logistic regression model with receipt of pre-op CRT as dependent variable and those listed above as the independent variables.
Med Onc=Medical Oncologist/Rad Onc=Radiation oncologist
Since there was virtually no variation in CRT-surgery sequence among those who visited a medical or radiation oncologist before a surgeon, but there was substantial variation among those who visited a surgeon first, we created a separate logistic model for those 140 subjects (Table 3). Among patients who saw a surgeon first, those who had documentation of a pelvic MRI or EUS had a 74% (95% CI, 62% to 85%) adjusted probability of receiving pre-op CRT, whereas the others had a 40% (95% CI, 30% to 50%) adjusted probability. Also, those who had a documentation of a chest CT were more likely to have pre-op CRT.
We reported survey results from surgeons, radiation oncologists and medical oncologists who had cared for at least one CanCORS colorectal cancer (CRC) patient in the previous 12 months in Table 4. The most notable differences among these provider types were related to the volume of CRC patients treated in the last month and the percentage reporting weekly attendance at multidisciplinary meetings (e.g. tumor boards). Medical oncologists, radiation oncologists and surgeons reported seeing a median of 10, 3 and 3 CRC patients in the last month respectively, and 67%, 82% and 36% reported attending weekly multidisciplinary meetings, respectively.
Table 4.
Characteristics of physicians practicing in participating CanCORS hospitals/facilities who cared for patients with colorectal cancer (CRC) in the last 12 months*
| Variable | Radiation oncologists N = 1,436 |
Medical oncologists N = 3,281 |
Surgeons N = 4,366 |
|---|---|---|---|
| Number of CRC patients treated/evaluated in past month (median) | 3 | 10 | 3 |
| Hospital/practice is part of a Community Clinical Oncology Program | 40% | 32% | 31% |
| Practice at a National Cancer Institute-designated cancer center | 23% | 22% | 29% |
| In practice affiliated with a National Comprehensive Cancer Network | 22% | 22% | 25% |
| Board certified | 94% | 84% | 90% |
| Involved in teaching medical students/residents | 38% | 53% | 53% |
| Attend multidisciplinary meeting to discuss cancer care (e.g. tumor boards) at least weekly | 82% | 67% | 36% |
| Do not attend multidisciplinary meeting to discuss cancer care | 0% | 2% | 5% |
| Multidisciplinary meetings involve: | |||
| A pretreatment planning function | 86% | 77% | 73% |
| Includes evaluation/review of treatment decisions already made | 92% | 86% | 85% |
| Reviews all participants’ cases | 38% | 41% | 41% |
| Reviews only challenging, unusual or controversial cases | 58% | 61% | 50% |
| Reviews a variety of cancer cases | 91% | 83% | 82% |
| Serves as a teaching session only, without review of cases | 11% | 14% | 9% |
These are not necessarily the physicians who cared for the rectal cancer population in this study. Rather, the sampling frame included providers who cared for patients in the overall CanCORS initiative.
A description of treatment characteristics and their association with the sequence of CRT and surgery is provided in Table 5. Those who received pre-op CRT were more likely to have been given the standard total radiation therapy dose of 4500–5040 cGy and to have had an APR approach, whereas the post-op CRT patients were more likely to have been given a dose greater than 5040 cGy and have had a LAR approach. There were no significant differences in chemotherapy regimens or number of days between initiation of CRT and surgery.
Table 5.
Treatment characteristics of patients with stages II or III rectal cancer by CRT--surgery sequence status
| Variable | Pre-op CRT (N=133) |
Post-op CRT (N=68) |
p |
|---|---|---|---|
| Surgical approach | |||
| Abdominal perineal resection | 41% | 25% | 0.03 |
| Lower anterior resection | 58% | 72% | |
| Other | 1% | 3% | |
| Positive margins documented | 4% | 3% | 1.00 |
| Median number of days (interquartile range) between initiation of radiation & surgery |
88 (78,101) | 82 (49,108) | 0.14 |
| Chemotherapy regimen | |||
| 5FU/leucovorin or capecitabine | 73% | 75% | 0.20 |
| Regimens including other agents* | 23% | 25% | |
| Unspecified | 5% | 0% | |
| Radiation Therapy total dose (cGy) category (N=185) | |||
| <4500 | 6% | 6% | <0.01 |
| ≥4500 & ≤ 5040 | 87% | 59% | |
| >5040 | 7% | 35% | |
| Missing dose information | N=11 | N=5 | |
Other agents predominantly include Irinotecan, Oxaliplatin and Bevacizumab
Discussion
Consistent with previous evaluations of SEER data, we found 34% of patients with stage II/III rectal cancer received post-op CRT. Unlike those studies, we did not find significant differences in use of pre- vs. post-op CRT related to patient gender or age.10–11 Rather, our findings suggest provider characteristics play a larger role in determining sequencing of rectal cancer therapies than patient characteristics. After accounting for multiple factors, nearly all subjects visiting a medical and/or radiation oncologist prior to visiting a surgeon received pre-op CRT, whereas only 48% of those who visited a surgeon first received pre-op CRT. Similar to our study, Luo (2006) reported that referral to a medical oncologist was the most important factor associated with receipt of chemotherapy among older patients with stage III colon cancer.27 However, no other studies have reported on the significance of the visit sequence of surgeons and oncologists. It is possible that the visit sequence variables available in CanCORS, which are not available in SEER, are far stronger predictor variables than age and gender and explain the difference in findings among the studies.
Among those who visited a surgeon first, we found patients who had a pelvic MRI or EUS and a chest CT were more likely to receive pre-op CRT. It is possible that access to EUS was an issue for some patients, and that pelvic MRI had not been adopted by some providers as an acceptable alternative, so the decision was made to perform surgery first. In addition, receipt of these recommended staging procedures may be a proxy measure capturing quality care provided by physicians experienced in treating patients with rectal cancer. While characteristics of specific providers treating patients in our study were not available, we evaluated the overall CanCORS colorectal cancer provider population. Surgeon respondents were less likely than medical or radiation oncologists to report attendance at multidisciplinary meetings to discuss cancer care (e.g. tumor boards) at least weekly. This may indicate that some surgeons do not treat a high enough volume of rectal cancer patients to be familiar with changing guidelines for care. We found the median number of CRC patients evaluated in the previous month by the surgeon respondents was three, and the median number of colon or rectal resections per month was two; fewer than half of those are likely to be rectal resections.
Another analysis of CanCORS data found those surgeons identifying themselves as colorectal surgeons or surgical oncologists (16%) were more likely to report high volumes of colorectal cancer resections, and high volume surgeons were more likely to report collaborative decision-making with other physicians on adjuvant therapies.28 Several studies have previously shown that surgeon specialty influences patient management in colorectal cancer care. One study showed patients treated by colorectal surgeons were far more likely to receive pre-op CRT than those treated by general surgeons (91% vs. 17%).29 A survey of surgeons in Florida also found that general surgeons were significantly less likely than colorectal surgeons or surgical oncologists to refer their patients for pre-op CRT, and less likely to report adherence to NCCN recommended staging guidelines.30 A survey of Canadian surgeons yielded similar results regarding recommended staging procedures.31 Other studies have linked specialty training and/or surgeon volume of colorectal cancer patients treated annually with receipt of recommended treatment and better outcomes.32–34 Interestingly, in contrast to other GI malignancies, there has been no evidence of shift toward high volume centers for rectal cancer cases.35–36
Our findings suggest that patients’ beliefs about the effects of radiation treatment are influenced by the order in which treatments are recommended to them. In unadjusted analyses, a higher proportion of people who received post-op CRT reported belief that RT would very likely or somewhat likely cure their cancer whereas a higher proportion of patients who received post-op CRT believed RT would not be likely to help with their symptoms. That seeing a surgeon first removed this association in multivariate models suggests that it was the sequence of visits that mediated the difference between pre-op and post-op patient beliefs. The phrasing of the survey items to elicit beliefs after discussing treatment with their physicians (i.e. “after talking with your doctors about radiation therapy, how likely did you think it was that….”) further supports a direct influence of physician visit sequence and not merely selection of patients who innately believe in the curability of cancer to seek out surgeons first. It is noteworthy that nearly all subjects, regardless of treatment sequence, rated their quality of care good to excellent for surgery, RT and chemotherapy.
With respect to subsequent treatment regimen characteristics, a higher proportion of those who underwent pre-op CRT received standard doses of radiation (4500 to 5040 cGy in 25 to 28 fractions regardless of the sequence of RT and surgery)9, whereas a higher proportion of those who underwent post-op CRT received a higher dose of radiation (>5040 cGy). We hypothesized that among patients who underwent surgery as first line treatment, those who had positive surgical margins may have been given a higher radiation dose post-operatively. However, there were only two post-op CRT subjects with documented positive margins, and only one of those had a higher than normal dose of radiation. Since the post-op surgical bed is associated with more damage to blood vessels and diminished blood flow and oxygen supply, some radiation oncologists may use a higher dose to achieve better local control.37
Patients who received pre-op CRT were more likely to have open APR approach, whereas those who received post-op CRT were more likely to have a LAR approach, which is not consistent with results of other studies of pre-op and post-op CRT.7, 38–39 It is possible that surgeons are more likely to refer patients with low rectal tumors for pre-op CRT in attempts to reduce the tumor size sufficiently enough to allow for the sphincter-sparing LAR approach. Pre-op CRT may not be able to always achieve this objective in many instances and the APR approach may still be necessary.
This study has some potential limitations. Staging was based upon the highest level of pathologic and clinical information available in the medical record. Thus, it is possible that some of the patients receiving post-op CRT could have been clinically staged as stage I and consequently received first line surgery, and were then found to have stage II/III rectal cancer during surgery and received post-op CRT. However, those who received post-op CRT were less likely to have documentation of a chest CT or pelvic MRI/EUS in their medical record as part of clinical staging. NCCN guidelines indicate that all rectal patients should have pelvic MRI or EUS, and a CT of the chest, abdomen and pelvis to rule out metastases to the liver and lung.9, 40 Thus, some patients may have failed to receive the recommended treatment for stage II/III rectal cancer due to inaccurate staging.
Some patients with rectal cancer present emergently with bleeding or obstructing tumors. As a result, it is reasonable to expect that a small portion of patients may have initial surgery for symptom control. Still, even among cases of bleeding or obstruction, symptoms can often be controlled with a diverting ostomy and CRT, so this does not justify the large differences in rates of pre-op CRT.
In conclusion, a substantial proportion of patients with stages II/III rectal cancer received post-op CRT despite compelling evidence supporting pre-op CRT. Receipt of pre-op CRT was associated with visits to medical or radiation oncologists prior to surgeons and visits to multiple provider types prior to initiation of treatment. The increased participation of oncologists (compared to surgeons) in multidisciplinary team conferences, which have been shown to improve processes and outcomes of care for colorectal cancer patients,41 is a potential driver of the observed association and an ideal target for national quality improvement initiatives. Pre-op CRT was also associated with receipt of recommended staging procedures among subjects visiting a surgeon first. It is also possible that the decision to perform post-op CRT may have been related to patient preference, lack of access to recommended staging procedures or other logistic factors that created justifiable circumstances. Consequently, further evaluation of patient treatment preferences, provider characteristics, presence of multi-disciplinary treatment planning, access/availability of staging procedures, referral patterns and health system processes that determine treatment should be undertaken to understand variation from recommended care and design targeted interventions to improve quality of care.
Acknowledgments
Acknowledgments and Financial Disclosure: The work of the CanCORS Consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (U01 CA093344) and the NCI supported Primary Data Collection and Research Centers (Dana-Farber Cancer Institute/Cancer Research Network U01 CA093332, Harvard Medical School/Northern California Cancer Center U01 CA093324, RAND/UCLA U01 CA093348, University of Alabama at Birmingham U01 CA093329, University of Iowa U01 CA093339, University of North Carolina U01 CA093326) and by a Department of Veterans Affairs grant to the Durham VA Medical Center CRS 02-164.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: This manuscript is not under review elsewhere and there is no prior publication of manuscript contents. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
The authors report no conflict of interest in regards to this study.
References
- 1.American Cancer Society. Colorectal Cancer Early Detection. 2011 http://www.cancer.org/Cancer/ColonandRectumCancer/MoreInformation/ColonandRectumCancerEarlyDetection/colorectal-cancer-early-detection-importance-of-c-r-c-screening.
- 2.O'Connell JB, Maggard MA, Ko CY. Re: Colon cancer survival rates with the new American Joint Committee on Cancer - Sixth edition staging - Responses. J. Natl. Cancer Inst. 2005 Nov;97(22):1706–1707. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 3.Rajput A, Bullard Dunn K. Surgical Management of Rectal Cancer. Semin. Oncol. 2007;34(3):241–249. doi: 10.1053/j.seminoncol.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Weiser MR, Landmann RG, Wong WD, et al. Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum. 2005 Jun;48(6):1169–1175. doi: 10.1007/s10350-004-0930-3. [DOI] [PubMed] [Google Scholar]
- 5.Wiig JN, Larsen SG, Giercksky KE. Operative treatment of locally recurrent rectal cancer. Recent Results Cancer Res. 2005;165:136–147. doi: 10.1007/3-540-27449-9_15. [DOI] [PubMed] [Google Scholar]
- 6.Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative Multimodality Therapy Improves Disease-Free Survival in Patients With Carcinoma of the Rectum: NSABP R-03. Journal of Clinical Oncology. 2009 Nov;27(31):5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004 Oct 21;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 8.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer (vol 355, pg 1114, 2006) New England Journal of Medicine. 2006 Aug;357(7):728–728. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ™) Rectal Cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2011. V 4.2011. [Google Scholar]
- 10.Lin C, Charlton ME, Meza JL, Enke CA, Loberiza FRJ. Temporal and Regional Variations in the Use of Preoperative Radiation Therapy for Rectal Cancer. American Journal of Clinical Oncology. 2010;33(5):443–447. doi: 10.1097/COC.0b013e3181b4b175. 410.1097/COC.1090b1013e3181b1094b1175. [DOI] [PubMed] [Google Scholar]
- 11.Mak RH, McCarthy EP, Das P, Hong TS, Mamon HJ, Hoffman KE. Adoption of Preoperative Radiation Therapy for Rectal Cancer From 2000 to 2006: A Surveillance, Epidemiology, and End Results Patterns-of-Care Study. International Journal of Radiation Oncology*Biology*Physics. 2010 doi: 10.1016/j.ijrobp.2010.03.056. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 12.Stitzenberg KB, Sigurdson ER. Changing trends in the timing of radiotherapy for rectal cancer. Annals of Surgical Oncology. 2009 Feb;16:71–71. [Google Scholar]
- 13.National Cancer Institute D, Surveillance Research Program, Cancer Statistics Branch, released January 2011. Cancer Statistics Branch. Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2009) http://www.seer.cancer.gov/popdata/. [Google Scholar]
- 14.NCCN. Rectal Cancer: Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. 2003;1:54–63. doi: 10.6004/jnccn.2003.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh MS, Petrelli NWH. Phase III randomized trial of preoperative versus postoperative multimodality therapy in patients with carcinoma of the rectum (NSABP R-03) Proc Am Soc Clin Oncol. 2001;(20) [Google Scholar]
- 16.Sauer R, Fietkau R, Wittekind C, et al. Adjuvant versus neoadjuvant radiochemotherapy for locally advanced rectal cancer. A progress report of a phase-III randomized trial (protocol CAO/ARO/AIO-94) Strahlenther Onkol. 2001 Apr;177(4):173–181. doi: 10.1007/pl00002396. [DOI] [PubMed] [Google Scholar]
- 17.Bosset JF, Calais G, LM Does the addition of chemotherapy to preoperative radiation increase acute toxicity in patients with rectal cancer Report of 22921 EORTC phase III trial. J Clin Oncol. 2003;22 Proc SACO abstract 1179. [Google Scholar]
- 18.Roh MS, Petrelli N, S W. Phase II randomized trial of preoperative versus postoperative multi modality therapy in patients with carcinoma of the rectum (NSABP R-03) J Clin Oncol Proc. ASCO. 2001;20 Abstract 450. [Google Scholar]
- 19.Sauer R. Adjuvant versus Neoadjuvant Combined Modality Treatment for Locally Advanced Rectal Cancer: First Results of the German Rectal Cancer Study (CAO/ARO/AIO-94) ASTRO. 2003 [Google Scholar]
- 20.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium (vol 22, pg 2992, 2004) Journal of Clinical Oncology. 2004 Dec;22(24):5026–5026. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients' experience and outcomes: development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Supportive Care in Cancer. 2006 Aug;14(8):837–848. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 22.Catalano PJ, Ayanian JZ, JC W, et al. Representativeness of Participants in the Cancer Care Outcomes Research and Surveillance Consortium Relative to the Surveillance, Epidemiology, and End Results Program. MedCare. doi: 10.1097/MLR.0b013e318222a711. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, A T. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 24.Piccirillo JF, Costas I, P C, et al. The measurement of comorbidity by cancer registries. J Registry Manage. 2003;30:8–14. [Google Scholar]
- 25.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999 Jun;55(2):652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 26.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? Journal of Clinical Oncology. 2008 May;26(15):2532–2537. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 27.Luo RL, Giordano SH, Freeman JL, Zhang D, Goodwin JS. Referral to medical oncology: A crucial step in the treatment of older patients with stage III colon cancer. Oncologist. 2006;11(9):1025–1033. doi: 10.1634/theoncologist.11-9-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers SO, Ayanian JZ, Ko CY, et al. Surgeons' Volume of Colorectal Cancer Procedures and Collaborative Decision-Making About Adjuvant Therapies. Annals of Surgery. 2009 Dec;250(6):895–900. doi: 10.1097/SLA.0b013e3181afe0c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyman N, Healey C, Osler T, Cataldo P. Understanding variation in the management of rectal cancer: the potential of a surgeon-initiated database. Am J Surg. 2007 Oct;194(4):559–562. doi: 10.1016/j.amjsurg.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Emmett K, Mattingly-Wells L, Santiago L, Marcet J, Cromwell J. Do Surgeon Attitudes Contribute to the Underutilization of Neoadjuvant Chemoradiation Therapy for the Treatment of Rectal Cancer?. Paper presented at: Paper presented at the 2008 annual meeting of the American Society of Colon and Rectal Surgeons; June 7–11, 2008; Boston, MA. [Google Scholar]
- 31.McMullen TPW, Easson AM, Cohen Z, Swallow CJ. The investigation of primary rectal cancer by surgeons: current pattern of practice. Can. J. Surg. 2005 Feb;48(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 32.Chuah TK, Lee T, Wirtzfeld D, Pollett W. Management of primary rectal cancer by surgeons in Atlantic Canada: results of a regional survey. Can. J. Surg. 2010 Dec;53(6):396–402. [PMC free article] [PubMed] [Google Scholar]
- 33.Read TE, Myerson RJ, Fleshman JW, et al. Surgeon specialty is associated with outcome in rectal cancer treatment. Diseases of the Colon & Rectum. 2002 Jul;45(7):904–914. doi: 10.1007/s10350-004-6327-5. [DOI] [PubMed] [Google Scholar]
- 34.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Annals of Surgery. 2002 Nov;236(5):583–592. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stitzenberg K, Meropol N. Trends in Centralization of Cancer Surgery. Annals of Surgical Oncology. 2010;17(11):2824–2831. doi: 10.1245/s10434-010-1159-0. [DOI] [PubMed] [Google Scholar]
- 36.Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of Cancer Surgery: Implications for Patient Access to Optimal Care. Journal of Clinical Oncology. 2009 Oct 1;27(28):4671–4678. doi: 10.1200/JCO.2008.20.1715. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall EJ. Radiobiology for the radiologist [by] Eric J. Hall. Hagerstown, Md: Medical Dept., Harper & Row; 1973. [Google Scholar]
- 38.Bujko K, Kepka L, Michatski W, Nowacki MP. Does rectal cancer shrinkage induced by preoperative radio (chemo)therapy increase the likelihood of anterior resection? A systematic review of randomised trials. Radiotherapy and Oncology. 2006 Jul;80(1):4–12. doi: 10.1016/j.radonc.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Wong RK, Tandan V, De Silva S, Figueredo A. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev. 2007;(2):CD002102. doi: 10.1002/14651858.CD002102.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui A, Fayiga Y, Huerta S. The role of endoscopic ultrasound in the evaluation of rectal cancer. International Seminars in Surgical Oncology. 2006;3(1):36. doi: 10.1186/1477-7800-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris E, Haward RA, Gilthorpe MS, Craigs C, Forman D. The impact of the Calman-Hine report on the processes and outcomes of care for Yorkshire's colorectal cancer patients. Br J Cancer. 2006;95(8):979–985. doi: 10.1038/sj.bjc.6603372. [DOI] [PMC free article] [PubMed] [Google Scholar]