Abstract
Since serial studies of patients are limited, researchers interested in Chagas disease have relied on animal models of Trypanosoma cruzi infection to explore many aspects of this important human disease. These studies have been important for evaluation of the immunology, pathology, physiology and other aspects of pathogenesis. While larger animals have been employed, mice have remained the most favoured animal model, as they recapitulate many aspects of the human disease, are easy to manipulate genetically and are amenable to study by small animal imaging technologies. Further, developments in non-invasive imaging technologies have permitted the study of the same animal over an extended period of time by multiple imaging modalities, thus permitting the study of the transition from acute infection through the chronic stage and during therapeutic regimens.
9.1. INTRODUCTION
For several decades, researchers interested in Chagas disease have relied on animal models of Trypanosoma cruzi infection to explore many aspects of this important human disease. Since serial studies on humans are limited, researchers have relied on animal models to evaluate the immunology, pathology, physiology and other aspects of pathogenesis. In addition, animal models have been routinely employed to screen for novel anti-T. cruzi drugs (Buckner and Navabi, 2010) and for vaccine development (Gupta and Garg, 2010).
The primary animal model employed to date has been the mouse because it has been demonstrated to recapitulate many of the immunological, pathological and physiological features of human Chagas disease. However, some have argued that the mouse model is not a credible model. Interestingly, similar arguments have recently appeared in the literature questioning the relevance of the mouse model of cerebral malaria to human cerebral malaria (Taylor-Robinson, 2010). Thus, several studies have employed T. cruzi-infected larger animals such as rabbits, rats, guinea pigs, dogs and subhuman primates (Barbabosa-Pliego et al., 2009; Bonecini-Almeida Mda et al., 1990; Chandrasekar et al., 1998; Chen et al., 1996; Cruz-Chan et al., 2009; da Silva et al., 1996; de Almeida et al., 1992; de Meirelles Mde et al., 1990; Figueiredo et al., 1986; Guedes et al., 2002, 2009; Junqueira Junior et al., 1992; Labrador-Hernandez et al., 2008; Milei et al., 1982; Morris et al., 1991; Perez et al., 2009; Ramirez and Brener, 1987; Teixeira et al., 1983; Zabalgoitia et al., 2004).
Mice are a good model because of ease of handling, housing and cost. In addition, the use of mice allows for acquiring greater numbers of samples, whereas with larger animals, the number of animals in the study is limited by cost and ethical considerations. However, even studies involving mice have been hampered by the inability to compare studies because of variability in parasite and mouse strains employed. For example, among the strains of T. cruzi often used in mice experiments are CL Brener (the strain used in the T. cruzi genome project), the Y, SylvioX10, Colombian, Brazil and Tulahuen strains. Each strain is a particular laboratory or country “favourite.” Thus, the combination of a particular T. cruzi strain and mouse strain results in early death with high parasitemia and high tissue parasitism, while other combinations result in a transient parasitemia, no death in the acute phase and the development of chronic disease often manifested by a cardiomyopathy. Similarly, infection of cultured myoblasts with different strains of T. cruzi may result in the up- or down-regulation of different genes in the host cell (Adesse et al., 2010).
Once having selected a parasite strain, another challenge is the choice of an appropriate mouse model. Among the important mouse strains that have been used are outbred strains such as CD-1 and inbred strains such as A/J, Balb/C, C57BL/6, C3H and others. Additionally, experiments may use various null and transgenic mice. Then there are the issues involving the size of the original infecting inoculum as well as the sex (de Souza et al., 2001; McHardy, 1978) and age (Maletto et al., 1996) of the mice. Although most investigators infect mice within 6–10 weeks of age, infection of mice at other ages may result in variability in mortality, parasitemia and pathology. The temperature of the environment may also be a confounding factor (Amrein, 1967; Anderson and Kuhn, 1989; Dimock et al., 1992). All of these factors have been demonstrated to be important, and one can achieve different results depending on the combination of many of these factors.
As far back as 1978, we demonstrated that when various inbred mice available at that time were infected with a standard inocula of trypomastigotes of the Brazil strain and observed for mortality, certain mouse strains were resistant and others highly susceptible (Trischmann et al., 1978). We further observed that infection of C57BL/6 mice with 1 × 104 trypomastigotes of the Brazil strain resulted in a transient parasitemia, no mortality and the appearance of a dilated cardiomyopathy by day 90 post-infection (Jelicks et al., 1999). However, infection of the same mouse strain with 1 × 103 trypomastigotes of the Tulahuen strain resulted in high parasitemia and death by day 20–25 post-infection (Chandra et al., 2002b).
In the use of various null mice, there are variations as well. For example, when nitric oxide synthase 2 (NOS2) null mice were infected with 104 trypomastigotes of the Brazil strain, none of the mice died, but they displayed less right ventricular dilation than displayed by Brazil strain-infected wild-type (WT) mice. Presumably, the reason was that there was less nitric oxide (NO) to contributing to development of cardiomyopathy. Prior to these experiments, it was thought that the mice would die, as NO is required for intracellular parasite killing. The interpretation at that time was that the genetic background of the host (C57BL/6) was more important than whether the mice could produce NO. However, when NOS2 null mice were infected with the Tulahuen strain, the mortality rate was 100%. Athymic nude mice lacking T-cells have a 100% mortality that was not dependant of parasite strain used (Kierszenbaum, 1980; Trischmann et al., 1978).
9.2. LARGER ANIMAL MODELS
9.2.1. Rabbit studies
Rabbits have been evaluated as a model for Chagas disease by several laboratories (da Silva et al., 1996; Figueiredo et al., 1986; Ramirez and Brener, 1987; Teixeira et al., 1983). In 1983, Teixeira et al. reported studies of the electrocardiogram (ECG) of rabbits infected with the Ernestina or Albuquerque strains of T. cruzi (Teixeira et al., 1983). During latent infection, there were no detectable alterations in the ECG of the infected rabbits. However, in the chronic stage of the disease, ECG changes consistent with enlargement and overload of cardiac chambers, alterations of ventricular repolarization, S–T changes and bundle-branch blocks were frequently recorded. Autopsy substantiated these findings. Megacolon was seen in two rabbits inoculated with the Ernestina strain of the parasite. In 1986, Figueiredo et al. reported findings in young rabbits infected with the Colombia strain of T. cruzi. Cineventriculography in the left ventricle of the rabbits during the chronic phase disclosed regional myocardial dysfunction, with typical apical systolic bulging.
9.2.2. Dog studies
Dogs are both an important reservoir of the parasite and a good model to study the pathogenesis of T. cruzi infection. Cruz-Chan et al. (2009) found that three of nine T. cruzi seropositive stray dogs presented electrocardiographic alterations including right bundle-branch block, sinusal block and QRS complex alterations and some right-ventricle enlargement was noted. Dogs infected with a T. cruzi isolate from Mexico exhibited electrocardiographic alterations, left- and right-ventricle dilation and hydropericardium (Barbabosa-Pliego et al., 2009). Guedes et al. (2009) studied dogs infected with T. cruzi as a model to understand the immunopathogenic mechanisms involved in chronic chagasic infection. They have also used the dog model to evaluate chemotherapy (Guedes et al., 2002). Studies of the myocardial beta-adrenergic adenylate cyclase complex have also been performed in dogs (Chen et al., 1996; Morris et al., 1991).
9.2.3. Rat studies
Rats have been used to study potential therapeutic strategies (Perez et al., 2009) and to investigate the inflammatory response (Chandrasekar et al., 1998), cardiac autonomic dysfunction (Junqueira Junior et al., 1992) and mechanisms that lead to chronic chagasic cardiomyopathy (Labrador-Hernandez et al., 2008). These studies helped establish the role of immune system and involvement of the parasympathetic nervous system in the pathogenesis of chagasic cardiomyopathy.
9.2.4. Non-human primates
Non-human primates have also been explored as models for Chagas disease (Bonecini-Almeida Mda et al., 1990; de Almeida et al., 1992; de Meirelles Mde et al., 1990; Milei et al., 1982). Early studies revealed that ECG changes in infected monkeys were correlated with specific anatomic lesions (Milei et al., 1982). In later studies, Cebus apella sp. monkeys were infected with different strains of T. cruzi and were submitted to xenodiagnosis, serological testing clinical examination and electrocardiography (de Almeida et al., 1992). The ECGs were always normal for the 12 infected monkeys in the study, and at autopsy, only three of the monkeys exhibited chronic myocarditis. High parasitemia and positive serology, despite the normal ECG, lead the authors to conclude that Cebus monkeys were not susceptible to the development of the disease but they could be used to maintain strains and study serology in long-term infections. In another study, seropositive baboons were studied using myocardial contrast echocardiography (Zabalgoitia et al., 2004). Chagasic heart disease was present in 24% of seropositive baboons as evidenced by decreased fractional shortening, although there was no significant difference in the coronary microcirculation pattern in those animals.
9.3. MOUSE MODELS
The studies of Chagas disease in animals provide a good framework for the evaluation of novel drugs and vaccines. Here, we focus on the mouse model which has been most extensively studied.
9.3.1. Chagasic cardiomyopathy in mice
9.3.1.1. ECG studies
Although ECG studies are electrophysiological studies, they give insight into the morphological alterations in the heart. The first well-designed comprehensive ECG-based study in mice over a prolonged period after infection was that of Postan et al. (1987). These investigators infected C3H and C57Bl/6 mice with various T. cruzi strains. They found that there were differences in the degree of myocardial fibrosis and in ECG abnormalities. They also reported that prolongation in the P–R intervals and complete atrial–ventricular dissociation was mouse strain and parasite strain dependent. More recently, Eickhoff et al. reported an ECG study of chagasic cardiomyopathy in BALB/c, SCID, C57BL/6 and CH3 mice infected with the Tulahuen, Brazil and Sylvio-X10/4 strains of T. cruzi (Eickhoff et al., 2010). In this study, a prolongation of the QRS and QT intervals was consistently detected in BALB/c mice infected with the Brazil strain, and these alterations were correlated with the histopathological changes associated with chagasic cardiomyopathy. These studies over 20 years apart underscore the fact that the combination of mouse and parasite strain yields varying results.
9.3.1.2. Cardiac magnetic resonance imaging, echocardiography and positron emission tomography
In 1999, we reported the first magnetic resonance imaging (MRI) studies of mice infected with T. cruzi (Brazil strain; Huang et al., 1999; Jelicks et al., 1999). MRI is a non-invasive imaging modality with high resolution (~50–100 µm for small animal studies) and excellent soft tissue contrast. MRI can be used to image anatomic structures, blood flow and diffusion. For our studies, mice were anaesthetized with 1.5% of isoflurane; a set of standard, shielded, nonmagnetic electrocardiographic leads ending in silver wires were attached to the four limbs for monitoring the ECG and using the R wave to provide a cardiac gating signal to the MRI system. Infection of CD1 mice was associated with a significant increase in the right ventricular inner diameter (RVID) that was reversed in some mice by the calcium-channel blocker verapamil. We also evaluated chagasic cardiomyopathy in NOS2 null and syngeneic WT mice. Infected WT mice exhibited an increase in RVID in the acute phase that was more marked during chronic infection. Chronically infected NOS2 null mice also exhibited increased RVID; however, the increase was less than that in WT mice. The data supported the notion that the NOS2/NO pathway contributes to the pathogenesis of murine chagasic cardiomyopathy. Subsequently, we examined the cardiac structural and functional correlates of verapamil treatment in CD1 mice infected with the Brazil strain of T. cruzi using serial transthoracic echocardiography (Chandra et al., 2002a). That study was the first demonstration of the utility of echocardiography to study the functional and structural abnormalities in chronic murine chagasic heart disease. Ultrasound imaging has high spatial resolution (~50 µm) and contrast in soft tissue. In additional to being portable, it is a fast and economical technique and has been extensively used for echocardiography studies of small animals. Mice in the untreated-infected group compared with the mice in the infected-verapamil-treated group showed thinning of the left ventricular wall, increase in the left ventricular end-diastolic diameter and a reduction in percent fractional shortening. Verapamil is known to increase coronary blood flow and inhibit platelet aggregation. These properties of verapamil may be important in the amelioration of T. cruzi-induced cardiomyopathy. Other compounds, such as risedronate, a bisphosphonate, reduce mortality but have no effect on development cardiomyopathy in Brazil strain-infected CD1 mice (Bouzahzah et al., 2005). We also reported echocardiography studies of mice infected with the Tulahuen strain of T. cruzi, which results in an acutely fatal myocarditis with a toxic shock-like syndrome (Chandra et al., 2002b).
Endothelin-1, a 21-amino acid peptide, is a powerful vasoconstrictor synthesized by many cell types in the cardiovascular system including endothelial cells, fibroblasts and cardiac myocytes. Endothelin has been implicated in the pathogenesis of chagasic cardiomyopathy in mice (Huang et al., 2002; Petkova et al., 2001; Tanowitz et al., 2005). Employing MRI and echocardiography, we demonstrated that early treatment of T. cruzi infected mice with phosphoramidon, a compound that inhibits endothelin-converting enzyme at neutral endopeptidases, or verapamil, which inhibits the actions of endothelin at a post-receptor site are effective for reducing mortality and development of cardiac pathology (De Souza et al., 2004; Jelicks et al., 2002a,b; Tanowitz et al., 1999). These imaging methods were also valuable for studying the roles of interferon-gamma-inducible gene IGTP (de Souza et al., 2003), and in other studies, we validated the use of the MRI methods for measuring the right ventricular dimensions and for monitoring wall motion abnormalities (de Souza et al., 2005; Durand et al., 2006).
Recently, we used MRI to evaluate changes in the cardiac morphology of T. cruzi-infected mice following administration of bone marrow-derived cell therapy and selenium supplementation (Goldenberg et al., 2008; Souza et al., 2010). These studies revealed regression of the right ventricular dilatation typically observed in the chagasic mouse model with bone marrow cell therapy and prevention of right ventricular dilatation with selenium supplementation. Selenium supplementation also resulted in regression of ECG abnormalities (prolonged P wave duration) in mice with pre-existing cardiac pathology (Souza et al., 2010).
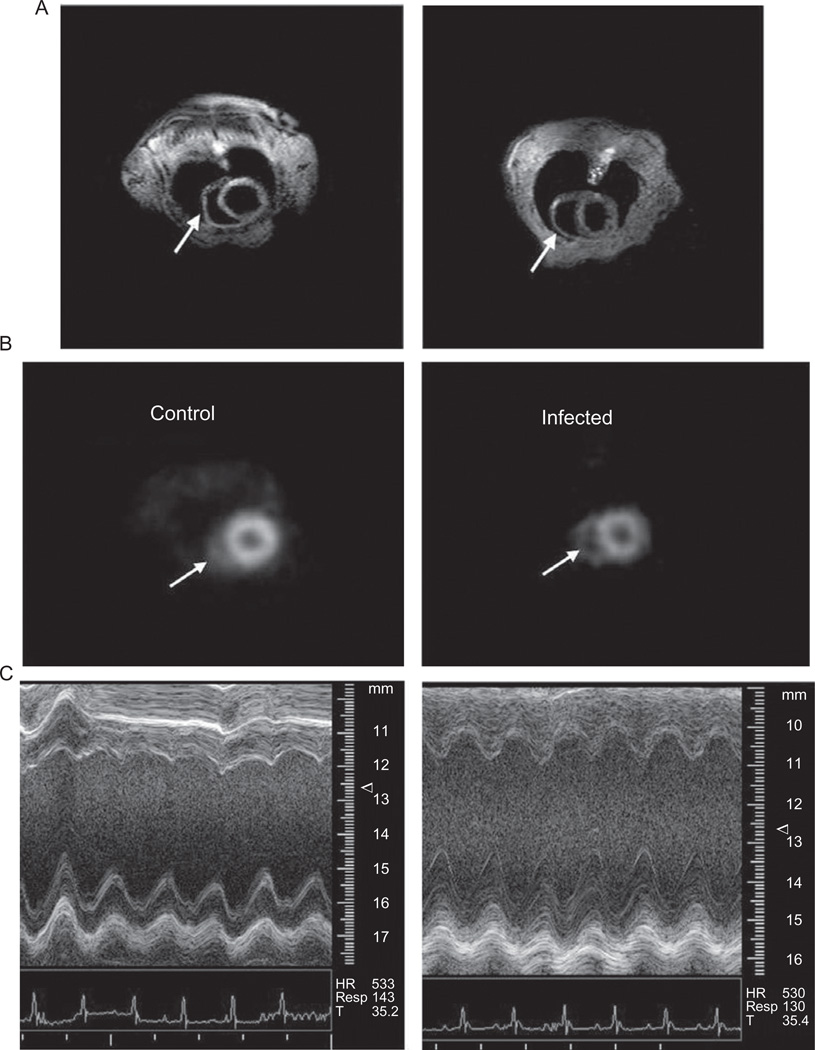
Our group performed multimodality imaging employing MRI, echocardiography and microPET imaging in a longitudinal study of Brazil strain-infected CD1 mice (Prado et al., 2009). Positron emission tomography (PET) is a highly sensitive (pM) molecular imaging technique that can be used to visualize a variety of in vivo biological processes. The resolution of microPET (1–2 mm) is not as high as CT or MRI but is adequate for small animal imaging. We used the molecule 2-deoxy-2-[18F]-fluoro-d-glucose, which is routinely used in the clinical setting. Cardiac gating of the PET acquisition was accomplished using standard ECG leads interfaced to the microPET scanner. Figure 9.1 shows representative MRI, microPET and echocardiography data from that study. Using these three complementary imaging technologies, it was possible to non-invasively quantify cardiovascular function, morphology and metabolism from the earliest days of infection through the chronic phase.
FIGURE 9.1.
Representative MRI (A), microPET (B) and echocardiography M-mode data (C) for uninfected control mice (left) and CD1 mice infected with the Brazil strain of T. cruzi (right). Arrows indicate the right ventricle in the MRI and microPET images.
9.3.2. Megasyndromes of the gastrointestinal tract and other organs in mice
For many years, it was difficult to establish that T. cruzi-infected mice could display alterations in gastrointestinal tract (GI) function. Mori et al. (1995) used X-ray methods to investigate GI tract abnormalities in T. cruzi-infected mice (Mori et al., 1995). In those mice, intestinal transit time was normal during acute infection, although delayed evacuation time was observed in the chronic phase. Another X-ray study of infected mice demonstrated swelling of the stomach and colon (Guillen-Pernia et al., 2001). Histological examination revealed extensive changes of the intestinal muscle layer and the loss of colonic folds and myenteric plexus. Recently, de Oliveira et al. (2008) demonstrated decreased intestinal motility in T. cruzi (Y strain)-infected Swiss Webster mice using charcoal.
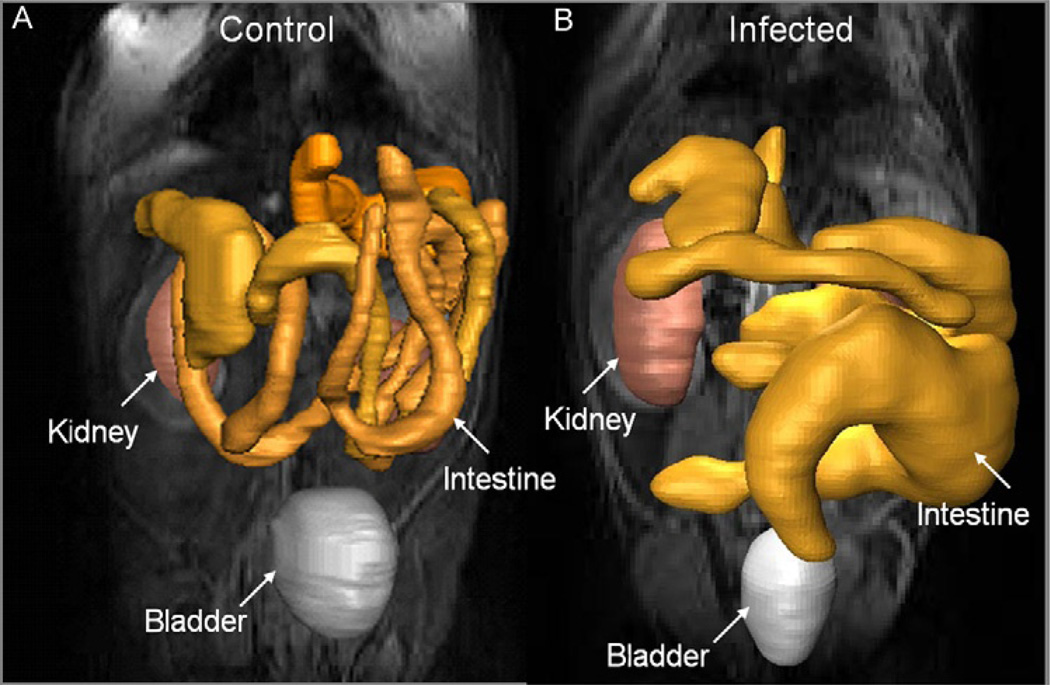
MRI was employed to monitor alterations in the GI tract of Brazil strain-infected mice and to assess the role of NO in the development of intestinal dilation (Ny et al., 2008). In that study, infected C57BL/6 WT mice exhibited dilatation of the intestines by 30 days post-infection with the average intestine lumen diameter increased by 72%. Levels of intestinal NO synthase (NOS) isoforms, NOS2 and NOS3, were elevated in infected WT mice. Inflammation and ganglionitis were observed in all infected mice. Intestinal dilation was observed in infected WT, NOS1, NOS2 and NOS3 null mice. The data strongly suggested that NO is not the sole contributor to intestinal dysfunction resulting from this infection. In a more recent study, we employed MRI to non-invasively monitor the effect of selenium supplementation on alterations in the GI tract of T. cruzi-infected mice (de Souza et al., 2010). CD1 mice infected with the Brazil strain exhibited dilatation of the intestines similar to what we observed in infected C57BL/6 mice. The average intestine lumen diameter increased by 65%, and the increase was reduced to 29% in mice supplemented with 2-ppm selenium in the drinking water. When supplemented with 3-ppm selenium in chow, the lumen diameter was also significantly reduced, although the difference between the infected and infected supplemented mice was smaller. Intestinal motility in infected mice fed with selenium-enriched chow was increased compared with infected mice fed with normal unsupplemented chow and was not significantly different from intestinal motility in uninfected mice. Figure 9.2 shows representative MRI data of mice showing intestinal dilation.
FIGURE 9.2.
Representative MRI of an uninfected CD1 mouse (A) and a CD1 mouse infected with the Brazil strain of T. cruzi (B). One of the kidneys, the bladder and the GI tract are indicated in the 3D greyscale overlay. Note the enlargement of the intestine in the infected mouse.
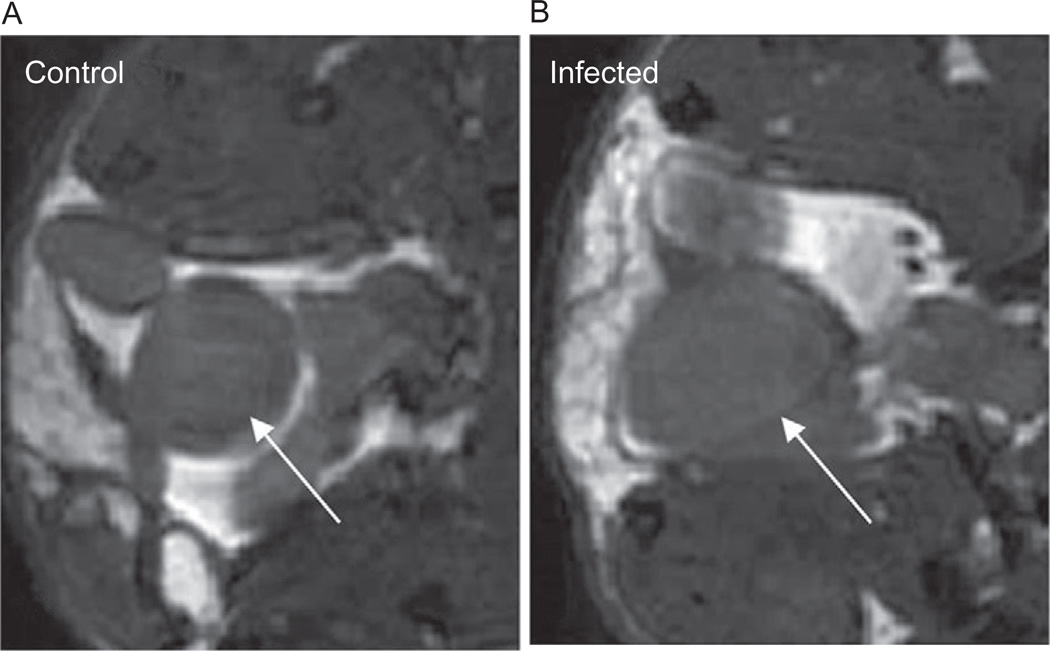
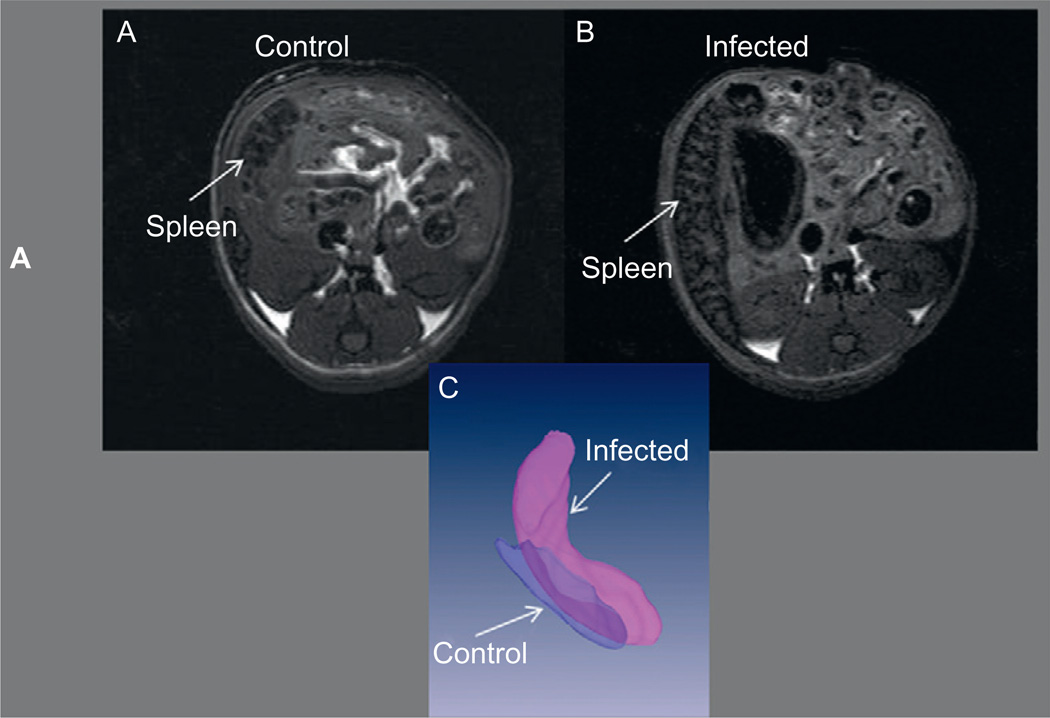
T. cruzi infection has also been shown to induce megasyndromes in other organs. We reported megabladder accompanied by fibrosis and decreased bladder compliance (Boczko et al., 2005) in mice infected with the Brazil strain. Figure 9.3 shows a comparison of the MRI of the normal bladder of a control mouse and the enlarged flaccid bladder of an infected mouse. Although there had been only rare descriptions of megaureter in humans (Koeberle, 1968), megabladder had been reported before in experimental T. cruzi infection in mice and dogs (Barr et al., 1991; Scremin et al., 1999). Hepatosplenomegaly has also been reported in patients with Chagas disease, and we have observed enlargement of both the spleen and the liver in mice infected with the Brazil strain of T. cruzi. Figure 9.4 shows representative MRI of the abdominal region of control and infected mice highlighting the enlarged spleen in the infected mouse.
FIGURE 9.3.
Zoomed region of the transverse MRI of an uninfected control CD1 mouse (A) and a CD1 mouse infected with the Brazil strain of T. cruzi (B) at the region of the bladder (indicated by the white arrow). Note the large irregular shape of the flaccid bladder in the infected mouse.
FIGURE 9.4.
Representative MRI of an uninfected CD1 mouse (A) and a CD1 mouse infected with the Brazil strain of T. cruzi (B). The 3D reconstructions of the normal (darker grey translucent) enlarged (lighter opaque grey) spleens are shown in (C).
9.3.3. Adipose tissue
In 2005, we reported the first detailed analysis of the consequences of T. cruzi (Brazil strain) infection on adipose tissue (Combs et al., 2005). Infected mice gained more weight than controls, and MRI studies revealed that total fat and abdominal fat depots were reduced in infected mice, consistent with reduced levels of leptin. Infected mice exhibiting dilated cardiomyopathy had even further reduced fat stores and weight gain in those infected animals appeared to be related to oedema, a consequence of cardiac dysfunction in these animals.
Recently, we measured body composition in T. cruzi-infected mice. This was determined by magnetic relaxometry with the EchoMRI-100 System (Echo Medical Systems), which provides quantitative measurements of fat mass and fat-free mass in live mice weighing up to 130 g without anaesthesia or sedation. Daily tuning occurs through automated calibration and testing procedures with self-correcting adjustments via custom software. Saturated and unsaturated fat standards are used to calibrate the instrument immediately prior to each set of MRI scans that were performed, and at the end of the study, these were validated by analysis of the carcasses (Nagajyothi et al., 2010). Using this technique, we determined that infection resulted in reduction in fat mass, which correlates with the infection-associated decrease in leptin. This decrease in fat may be a consequence of both decreased food intake and infection-associated lipolysis.
9.4. FUTURE DIRECTIONS: MULTIMODALITY IMAGING APPROACHES
As we have shown in our studies employing MRI, echocardiography and microPET, the imaging techniques appropriate for study of small animal models of Chagas disease are complementary and may be applied in a multiple modality manner. With the advent of new integrated technologies, such as PET/CT (computed tomography), and molecular imaging probes, the co-registration of functional images from PET with CT will be easier and will permit more detailed and accurate analysis of function/metabolism (Brewer et al., 2008). Similar integrated technologies are under development for PET/MRI that will permit anatomic co-registration without the additional exposure to radiation inherent in the PET/CT systems (Judenhofer et al., 2008).
Serial imaging of animals infected with T. cruzi allows for detailed study of the pathogenesis of Chagas disease that cannot be obtained solely by examination of the pathology which requires the sacrifice of animals. Serial imaging allows for assessment of the efficacy of chemotherapeutic agents. Many drugs are capable of lowering or eliminating parasites from peripheral blood or tissue, but imaging of the heart or GI systems is also required to assess whether these agents also ameliorate the progression to chronic disease.
ACKNOWLEDGEMENTS
This study was supported by grants from the United States National Institutes of Health AI-062730 and CA-123334 (L. A. J.) and AI-076248 (H. B. T.).
REFERENCES
- Adesse D, Iacobas DA, Iacobas S, Garzoni LR, Meirelles Mde N, Tanowitz HB, et al. Transcriptomic signatures of alterations in a myoblast cell line infected with four distinct strains of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2010;82:846–854. doi: 10.4269/ajtmh.2010.09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein YU. Effects of environmental temperature on Trypanosoma cruzi infection in mice. J Parasitol. 1967;53:1160. [PubMed] [Google Scholar]
- Anderson KJ, Kuhn RE. Elevated environmental temperature enhances immunity in experimental Chagas’ disease. Infect. Immun. 1989;57:13–17. doi: 10.1128/iai.57.1.13-17.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbabosa-Pliego A, Diaz-Albiter HM, Ochoa-Garcia L, Aparicio-Burgos E, Lopez-Heydeck SM, Velasquez-Ordonez V, et al. Trypanosoma cruzi circulating in the southern region of the State of Mexico (Zumpahuacan) are pathogenic: a dog model. Am. J Trop. Med. Hyg. 2009;81:390–395. [PMC free article] [PubMed] [Google Scholar]
- Barr SC, Schmidt SP, Brown CC, Klei TR. Pathologic features of dogs inoculated with North American Trypanosoma cruzi isolates. Am. J. Vet. Res. 1991;52:2033–2039. [PubMed] [Google Scholar]
- Boczko J, Tar M, Melman A, Jelicks LA, Wittner M, Factor SM, et al. Trypanosoma cruzi infection induced changes in the innervation, structure and function of the murine bladder. J. Urol. 2005;173:1784–1788. doi: 10.1097/01.ju.0000154170.87947.b8. [DOI] [PubMed] [Google Scholar]
- Bonecini-Almeida Mda G, Galvao-Castro B, Pessoa MH, Pirmez C, Laranja F. Experimental Chagas’ disease in rhesus monkeys. I. Clinical, parasitological, hematological and anatomo-pathological studies in the acute and indeterminate phase of the disease. Mem. Inst. Oswaldo Cruz. 1990;85:163–171. doi: 10.1590/s0074-02761990000200004. [DOI] [PubMed] [Google Scholar]
- Bouzahzah B, Jelicks LA, Morris SA, Weiss LM, Tanowitz HB. Risedronate in the treatment of Murine Chagas’ disease. Parasitol. Res. 2005;96:184–187. doi: 10.1007/s00436-005-1331-9. [DOI] [PubMed] [Google Scholar]
- Brewer S, McPherson M, Fujiwara D, Turovskaya O, Ziring D, Chen L, et al. Molecular imaging of murine intestinal inflammation with 2-deoxy-2-[18F]fluoro-d-glucose and positron emission tomography. Gastroenterology. 2008;135:744–755. doi: 10.1053/j.gastro.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner FS, Navabi N. Advances in Chagas disease drug development: 2009–2010. Curr. Opin. Infect. Dis. 2010;23:609–616. doi: 10.1097/QCO.0b013e3283402956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra M, Shirani J, Shtutin V, Weiss LM, Factor SM, Petkova SB, et al. Cardioprotective effects of verapamil on myocardial structure and function in a murine model of chronic Trypanosoma cruzi infection (Brazil Strain): an echocardiographic study. Int. J. Parasitol. 2002a;32:207–215. doi: 10.1016/s0020-7519(01)00320-4. [DOI] [PubMed] [Google Scholar]
- Chandra M, Tanowitz HB, Petkova SB, Huang H, Weiss LM, Wittner M, et al. Significance of inducible nitric oxide synthase in acute myocarditis caused by Trypanosoma cruzi (Tulahuen strain) Int. J. Parasitol. 2002b;32:897–905. doi: 10.1016/s0020-7519(02)00028-0. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Melby PC, Troyer DA, Colston JT, Freeman GL. Temporal expression of pro-inflammatory cytokines and inducible nitric oxide synthase in experimental acute Chagasic cardiomyopathy. Am. J. Pathol. 1998;152:925–934. [PMC free article] [PubMed] [Google Scholar]
- Chen G, Barr S, Walsh D, Rohde S, Brewer A, Bilezikian JP, et al. Cardioprotective actions of verapamil on the beta-adrenergic receptor complex in acute canine Chagas’ disease. J Mol. Cell. Cardiol. 1996;28:931–941. doi: 10.1006/jmcc.1996.0087. [DOI] [PubMed] [Google Scholar]
- Combs TP, Nagajyothi, Mukherjee S, de Almeida CJ, Jelicks LA, Schubert W, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol. Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- Cruz-Chan JV, Bolio-Gonzalez M, Colin-Flores R, Ramirez-Sierra MJ, Quijano-Hernandez I, Dumonteil E. Immunopathology of natural infection with Trypanosoma cruzi in dogs. Vet. Parasitol. 2009;162:151–155. doi: 10.1016/j.vetpar.2009.02.024. [DOI] [PubMed] [Google Scholar]
- da Silva AM, Eduardo Ramirez L, Vargas M, Chapadeiro E, Brener Z. Evaluation of the rabbit as a model for Chagas disease. II. Histopathologic studies of the heart, digestive tract and skeletal muscle. Mem. Inst. Oswaldo Cruz. 1996;91:199–206. doi: 10.1590/s0074-02761996000200015. [DOI] [PubMed] [Google Scholar]
- de Almeida EA, Navarro MR, Guariento ME, Carvalhal Sdos S. The experimental infection of Cebus apella sp. monkeys with Trypanosoma cruzi. Its clinical, electrocardiographic and anatomicopathological assessment. Rev. Soc. Bras. Med. Trop. 1992;25:7–12. doi: 10.1590/s0037-86821992000100002. [DOI] [PubMed] [Google Scholar]
- de Meirelles Mde N, Bonecini-Almeida Mda G, Pessoa MH, Galvao-Castro B. Trypanosoma cruzi: experimental Chagas’ disease in rhesus monkeys. II. Ultrastructural and cytochemical studies of peroxidase and acid phosphatase activities. Mem. Inst. Oswaldo Cruz. 1990;85:173–181. doi: 10.1590/s0074-02761990000200005. [DOI] [PubMed] [Google Scholar]
- de Oliveira GM, de Melo Medeiros M, da Silva Batista W, Santana R, Araujo-Jorge TC, de Souza AP. Applicability of the use of charcoal for the evaluation of intestinal motility in a murine model of Trypanosoma cruzi infection. Parasitol. Res. 2008;102:747–750. doi: 10.1007/s00436-007-0829-8. [DOI] [PubMed] [Google Scholar]
- de Souza EM, Rivera MT, Araujo-Jorge TC, de Castro SL. Modulation induced by estradiol in the acute phase of Trypanosoma cruzi infection in mice. Parasitol. Res. 2001;87:513–520. doi: 10.1007/s004360100376. [DOI] [PubMed] [Google Scholar]
- de Souza AP, Tang B, Tanowitz HB, Factor SM, Shtutin V, Shirani J, et al. Absence of interferon-gamma-inducible gene IGTP does not significantly alter the development of chagasic cardiomyopathy in mice infected with Trypanosoma cruzi (Brazil strain) J. Parasitol. 2003;89:1237–1239. doi: 10.1645/GE-3185RN. [DOI] [PubMed] [Google Scholar]
- De Souza AP, Tanowitz HB, Chandra M, Shtutin V, Weiss LM, Morris SA, et al. Effects of early and late verapamil administration on the development of cardiomyopathy in experimental chronic Trypanosoma cruzi (Brazil strain) infection. Parasitol. Res. 2004;92:496–501. doi: 10.1007/s00436-004-1080-1. [DOI] [PubMed] [Google Scholar]
- de Souza AP, Tang B, Tanowitz HB, Araujo-Jorge TC, Jelicks EL. Magnetic resonance imaging in experimental Chagas disease: a brief review of the utility of the method for monitoring right ventricular chamber dilatation. Parasitol. Res. 2005;97:87–90. doi: 10.1007/s00436-005-1409-4. [DOI] [PubMed] [Google Scholar]
- de Souza AP, Sieberg R, Li H, Cahill HR, Zhao D, Araujo-Jorge TC, et al. The role of selenium in intestinal motility and morphology in a murine model of Trypanosoma cruzi infection. Parasitol. Res. 2010;106:1293–1298. doi: 10.1007/s00436-010-1794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimock KA, Davis CD, Kuhn RE. Changes in humoral responses to Trypanosoma cruzi during the course of infection in mice held at elevated temperature. J Parasitol. 1992;78:687–696. [PubMed] [Google Scholar]
- Durand JL, Tang B, Gutstein DE, Petkova S, Teixeira MM, Tanowitz HB, et al. Dyskinesis in Chagasic myocardium: centerline analysis of wall motion using cardiac-gated magnetic resonance images of mice. Magn. Reson. Imaging. 2006;24:1051–1057. doi: 10.1016/j.mri.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff CS, Lawrence CT, Sagartz JE, Bryant LA, Labovitz AJ, Gala SS, et al. ECG detection of murine chagasic cardiomyopathy. J Parasitol. 2010;96:758–764. doi: 10.1645/GE-2396.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo F, Marin-Neto JA, Rossi MA. The evolution of experimental Trypanosoma cruzi cardiomyopathy in rabbits: further parasitological, morphological and functional studies. Int. J. Cardiol. 1986;10:277–290. doi: 10.1016/0167-5273(86)90009-4. [DOI] [PubMed] [Google Scholar]
- Goldenberg RC, Jelicks LA, Fortes FS, Weiss LM, Rocha LL, Zhao D, et al. Bone marrow cell therapy ameliorates and reverses chagasic cardiomyopathy in a mouse model. J. Infect. Dis. 2008;197:544–547. doi: 10.1086/526793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes PM, Veloso VM, Tafuri WL, Galvao LM, Carneiro CM, Lana M, et al. The dog as model for chemotherapy of the Chagas’ disease. Acta Trop. 2002;84:9–17. doi: 10.1016/s0001-706x(02)00139-0. [DOI] [PubMed] [Google Scholar]
- Guedes PM, Veloso VM, Afonso LC, Caliari MV, Carneiro CM, Diniz LF, et al. Development of chronic cardiomyopathy in canine Chagas disease correlates with high IFN-gamma, TNF-alpha, and low IL-10 production during the acute infection phase. Vet. Immunol. Immunopathol. 2009;130:43–52. doi: 10.1016/j.vetimm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Guillen-Pernia B, Lugo-Yarbuh A, Moreno E. Digestive tract dilation in mice infected with Trypanosoma cruzi. Invest. Clin. 2001;42:195–209. [PubMed] [Google Scholar]
- Gupta S, Garg NJ. Prophylactic efficacy of TcVac2 against Trypanosoma cruzi in mice. PLoS Negl. Trop. Dis. 2010;4:e797. doi: 10.1371/journal.pntd.0000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Chan J, Wittner M, Jelicks LA, Morris SA, Factor SM, et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J. Mol. Cell. Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- Huang H, Yanagisawa M, Kisanuki YY, Jelicks LA, Chandra M, Factor SM, et al. Role of cardiac myocyte-derived endothelin-1 in chagasic cardiomyopathy: molecular genetic evidence. Clin. Sci. (Lond.) 2002;103(Suppl. 48):263S–266S. doi: 10.1042/CS103S263S. [DOI] [PubMed] [Google Scholar]
- Jelicks LA, Shirani J, Wittner M, Chandra M, Weiss LM, Factor SM, et al. Application of cardiac gated magnetic resonance imaging in murine Chagas’ disease. Am. J. Trop. Med. Hyg. 1999;61:207–214. doi: 10.4269/ajtmh.1999.61.207. [DOI] [PubMed] [Google Scholar]
- Jelicks LA, Chandra M, Shirani J, Shtutin V, Tang B, Christ GJ, et al. Cardioprotective effects of phosphoramidon on myocardial structure and function in murine Chagas’ disease. Int. J. Parasitol. 2002a;32:1497–1506. doi: 10.1016/s0020-7519(02)00136-4. [DOI] [PubMed] [Google Scholar]
- Jelicks LA, Chandra M, Shtutin V, Petkova SB, Tang B, Christ GJ, et al. Phosphoramidon treatment improves the consequences of chagasic heart disease in mice. Clin. Sci. (Lond.) 2002b;103(Suppl. 48):267S–271S. doi: 10.1042/CS103S267S. [DOI] [PubMed] [Google Scholar]
- Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat. Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- Junqueira Junior LF, Beraldo PS, Chapadeiro E, Jesus PC. Cardiac autonomic dysfunction and neuroganglionitis in a rat model of chronic Chagas’ disease. Cardiovasc. Res. 1992;26:324–329. doi: 10.1093/cvr/26.4.324. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F. Protection of congenitally athymic mice against Trypanosoma cruzi infection by passive antibody transfer. J. Parasitol. 1980;66:673–675. [PubMed] [Google Scholar]
- Koeberle F. Chagas’ disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Adv. Parasitol. 1968;6:63–116. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- Labrador-Hernandez M, Suarez-Graterol O, Romero-Contreras U, Rumenoff L, Rodriguez-Bonfante C, Bonfante-Cabarcas R. The cholinergic system in cyclophosphamide-induced Chagas dilated myocardiopathy in Trypanosoma cruzi-infected rats: an electrocardiographic study. Invest. Clin. 2008;49:207–224. [PubMed] [Google Scholar]
- Maletto BA, Gruppi A, Moron G, Pistoresi-Palencia MC. Age-associated changes in lymphoid and antigen-presenting cell functions in mice immunized with Trypanosoma cruzi antigens. Mech. Ageing Dev. 1996;88:39–47. doi: 10.1016/0047-6374(96)01719-8. [DOI] [PubMed] [Google Scholar]
- McHardy N. Effect of sex of mice in relation to their response to immunization with vaccines prepared from Trypanosoma cruzi. Trans. R. Soc. Trop. Med. Hyg. 1978;72:201–202. doi: 10.1016/0035-9203(78)90064-0. [DOI] [PubMed] [Google Scholar]
- Milei J, Bolomo NJ, Vazquez A, Nagle CA. Normal and pathological electrocardiographic patterns in the Cebus monkey. J. Med. Primatol. 1982;11:10–19. doi: 10.1159/000460019. [DOI] [PubMed] [Google Scholar]
- Mori T, Yoon HS, Iizuka FH, Myung JM, Sato HR, Silva MF, et al. Intestinal transit and opaque enema study in chagasic mice. Rev. Hosp. Clin. Fac. Med. Sao Paulo. 1995;50:63–66. [PubMed] [Google Scholar]
- Morris SA, Barr S, Weiss L, Tanowitz H, Wittner M, Bilezikian JP. Myocardial beta-adrenergic adenylate cyclase complex in a canine model of chagasic cardiomyopathy. Circ. Res. 1991;69:185–195. doi: 10.1161/01.res.69.1.185. [DOI] [PubMed] [Google Scholar]
- Nagajyothi F, Zhao D, Machado FS, Weiss LM, Schwartz GJ, Desruisseaux MS, et al. Crucial role of the central leptin receptor in murine Trypanosoma cruzi (Brazil strain) infection. J. Infect. Dis. 2010;202:1104–1113. doi: 10.1086/656189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny L, Li H, Mukherjee S, Persson K, Holmqvist B, Zhao D, et al. A magnetic resonance imaging study of intestinal dilation in Trypanosoma cruzi-infected mice deficient in nitric oxide synthase. Am. J. Trop. Med. Hyg. 2008;79:760–767. [PMC free article] [PubMed] [Google Scholar]
- Perez AR, Fontanella GH, Nocito AL, Revelli S, Bottasso OA. Short treatment with the tumour necrosis factor-alpha blocker infliximab diminishes chronic chagasic myocarditis in ratswithout evidence of Trypanosoma cruzi reactivation. Clin. Exp. Immunol. 2009;157:291–299. doi: 10.1111/j.1365-2249.2009.03946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova SB, Huang H, Factor SM, Pestell RG, Bouzahzah B, Jelicks LA, et al. The role of endothelin in the pathogenesis of Chagas’ disease. Int. J. Parasitol. 2001;31:499–511. doi: 10.1016/s0020-7519(01)00168-0. [DOI] [PubMed] [Google Scholar]
- Postan M, Bailey JJ, Dvorak JA, McDaniel JP, Pottala EW. Studies of Trypanosoma cruzi clones in inbred mice. III. Histopathological and electrocardiographical responses to chronic infection. Am. J. Trop. Med. Hyg. 1987;37:541–549. doi: 10.4269/ajtmh.1987.37.541. [DOI] [PubMed] [Google Scholar]
- Prado CM, Fine EJ, Koba W, Zhao D, Rossi MA, Tanowitz HB, et al. Micro-positron emission tomography in the evaluation of Trypanosoma cruzi-induced heart disease: comparison with other modalities. Am. J. Trop. Med. Hyg. 2009;81:900–905. doi: 10.4269/ajtmh.2009.09-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez LE, Brener Z. Evaluation of the rabbit as a model for Chagas’ disease. I. Parasitological studies. Mem. Inst. Oswaldo Cruz. 1987;82:531–536. doi: 10.1590/s0074-02761987000400010. [DOI] [PubMed] [Google Scholar]
- Scremin LH, Corbett CE, Laurenti MD, Nunes EV, Gama-Rodrigues JJ, Okumura M. Megabladder in experimental Chagas disease: pathological features of the bladder wall. Rev. Hosp. Clin. Fac. Med. Sao Paulo. 1999;54:43–46. doi: 10.1590/s0041-87811999000200003. [DOI] [PubMed] [Google Scholar]
- Souza AP, Jelicks LA, Tanowitz HB, Olivieri BP, Medeiros MM, Oliveira GM, et al. The benefits of using selenium in the treatment of Chagas disease: prevention of right ventricle chamber dilatation and reversion of Trypanosoma cruzi-induced acute and chronic cardiomyopathy in mice. Mem. Inst. Oswaldo Cruz. 2010;105:746–751. doi: 10.1590/s0074-02762010000600003. [DOI] [PubMed] [Google Scholar]
- Tanowitz HB, Wittner M, Morris SA, Zhao W, Weiss LM, Hatcher VB, et al. The putative mechanistic basis for the modulatory role of endothelin-1 in the altered vascular tone induced by Trypanosoma cruzi. Endothelium. 1999;6:217–230. doi: 10.3109/10623329909053412. [DOI] [PubMed] [Google Scholar]
- Tanowitz HB, Huang H, Jelicks LA, Chandra M, Loredo ML, Weiss LM, et al. Role of endothelin 1 in the pathogenesis of chronic chagasic heart disease. Infect. Immun. 2005;73:2496–2503. doi: 10.1128/IAI.73.4.2496-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson A. Validity of modelling cerebral malaria in mice: argument and counter argument. J. Neuroparasitol. 2010;1 Article ID N100601. [Google Scholar]
- Teixeira AR, Figueiredo F, Rezende Filho J, Macedo V. Chagas’ disease: a clinical, parasitological, immunological, and pathological study in rabbits. Am. J. Trop. Med. Hyg. 1983;32:258–272. doi: 10.4269/ajtmh.1983.32.258. [DOI] [PubMed] [Google Scholar]
- Trischmann T, Tanowitz H, Wittner M, Bloom B. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp. Parasitol. 1978;45:160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]
- Zabalgoitia M, Ventura J, Lozano JL, Anderson L, Carey KD, Hubbard GB, et al. Myocardial contrast echocardiography in assessing microcirculation in baboons with Chagas disease. Microcirculation. 2004;11:271–278. doi: 10.1080/10739680490425976. [DOI] [PubMed] [Google Scholar]