A detailed pharmacoeconomic analysis was conducted to estimate the incremental cost-effectiveness of bortezomib, melphalan, and prednisone versus thalidomide, melphalan, and prednisone versus lenalidomide, melphalan, and prednisone with lenalidomide maintenance as therapy for previously untreated transplant-ineligible multiple myeloma patients. Bortezomib, melphalan, and prednisone was found to be the cost-effective option in the U.S. setting.
Keywords: Bortezomib, Lenalidomide, Thalidomide, Multiple myeloma, Cost-effectiveness
Abstract
The outlook for transplant-ineligible multiple myeloma patients has improved enormously over recent years with the incorporation of new agents into standard regimens. Novel regimens combine melphalan and prednisone (MP) with bortezomib (VMP), with thalidomide (MPT), and with lenalidomide with (MPR-R) and without (MPR) lenalidomide maintenance. The efficacy, safety, and cost-effectiveness of these regimens have not yet been compared; therefore, we conducted a pharmacoeconomic analysis using data from randomized controlled trials versus MP.
Using a Markov model developed from a U.S. payer's perspective, we compared VMP with MPT and MPR-R over a lifetime horizon. MPT and MPR-R were chosen because, like VMP, they are superior to MP in response and outcomes. Data from the Velcade as Initial Standard Therapy in Multiple Myeloma (VISTA; VMP), Intergroupe Francophone du Myelome (IFM) 99–06 (MPT), and MM-015 (MPR-R) trials were used. The IFM 99–06 study was selected because of the superior activity in this study compared with other MPT studies. Using patient-level (VMP) and published (MPT, MPR-R) data, we estimated the health-state transition and adverse event probabilities for each regimen, related costs, and state-specific utility estimates. Costs (in 2010 U.S. dollars) and health outcomes were discounted at 3%.
Discounted lifetime direct medical costs were lowest with VMP at $119,102. MPT cost $142,452 whereas MPR-R cost $248,358. Incremental cost-effectiveness ratio calculations projected that VMP would confer cost savings and better health outcomes relative to MPT and MPR-R. We conclude that VMP is highly likely to be cost-effective compared with MP, MPT, and MPR-R.
Implications for Practice:
There is a growing number of treatment options available for previously untreated, transplant-ineligible multiple myeloma patients, based on the combination of melphalan and prednisone (MP) with bortezomib (VMP), thalidomide (MPT), or lenalidomide plus lenalidomide maintenance (MPR-R). These regimens confer substantial improvements in patient health outcomes compared with MP. However, they are also associated with higher costs than MP. With limited healthcare budgets, it is important to determine the most cost-effective treatment option. This paper presents the first published analysis of the potential cost-effectiveness of these regimens based on efficacy and safety data from randomized clinical trials. The findings show that VMP is projected to provide cost savings compared to MPT and MPR-R, and to be a cost-effective treatment option compared to MP, MPT, or MPR-R in previously untreated, transplant-ineligible multiple myeloma patients when managed within the U.S. healthcare system. These findings support the recommendation to use VMP for this patient population.
Introduction
The therapeutic landscape of multiple myeloma (MM) has changed in recent years, with the introduction of bortezomib (Velcade; Millennium Pharmaceuticals, Inc., Cambridge, MA), thalidomide (Thalomid; Celgene Corp., Summit, NJ), and lenalidomide (Revlimid; Celgene Corp., Summit, NJ) into routine clinical use. These therapies have been associated with compelling improvements in patient outcomes [1, 2], and have resulted in the introduction of novel three- and four-drug combination regimens to treat patients with previously untreated or relapsed or refractory MM.
The novel-agent-based regimens, which are recognized in current treatment guidelines for previously untreated, transplant-ineligible MM patients in the U.S. [3] and Europe [4], combine melphalan and prednisone (MP) with one or more new agents; recommended regimens include MP plus bortezomib (VMP), thalidomide (MPT), and lenalidomide with (MPR-R) and without (MPR) lenalidomide maintenance. Lenalidomide, bortezomib, thalidomide, and thalidomide–prednisone are recommended for maintenance [3]. These recommendations are based on current evidence from several randomized phase III studies comparing MP with VMP, MPT, MPR, and MPR-R in patients with previously untreated MM [5–15].
The U.S. National Comprehensive Cancer Network currently identifies VMP, MPT, and MPR as category 1 recommendations for previously untreated, transplant-ineligible patients with MM [3]. Similarly, VMP and MPT were given grade A recommendations for elderly MM patients in a recent European consensus statement, whereas MPR received a grade B recommendation on the basis of level IIa evidence [4]. A recent position paper from the European Myeloma Network also recommended VMP and MPT for first-line treatment of transplant-ineligible patients [16]. However, despite essentially similar recommendations in Europe and the U.S., the use of these regimens varies by region.
Overall response rates and outcomes with novel-agent-based regimens that have a demonstrated survival benefit over MP are shown in Table 1. The Velcade as Initial Standard Therapy in Multiple Myeloma (VISTA), Intergroupe Francophone du Myelome (IFM) 99–06, and MM-015 trials were chosen for inclusion in this analysis based on the survival benefit conferred over MP as described below. Long-term survival data were recently presented for the VISTA trial in transplant-ineligible patients and indicate that VMP is associated with a 13.3-month benefit in the median survival time, compared with MP (56.4 months versus 43.1 months; p = .0004) [17]. Six phase III trials have compared MPT with MP in patients with previously untreated MM [5–7, 9, 10, 14, 15]; however, only three demonstrated a significant survival advantage for MPT over MP: the IFM 99–06 trial, involving patients aged <75 years [6]; the IFM 01/01 trial, involving patients aged >75 years [7]; and the Dutch-Belgian Hemato-Oncology Cooperative Group 49 study in patients aged >65 years [15]. It should be noted that crossover from the MP arm to a novel-agent-based regimen at relapse may explain the lack of survival benefit versus MP in three of the MPT studies. Other factors that could have affected the outcome include the potential for a more resistant relapse with thalidomide and its toxic effects, which contribute to toxic deaths, patient withdrawals, and dose reductions. Two recent large meta-analyses, which included the IFM 99–06 and IFM 01/01 trials, demonstrated better progression-free survival (PFS) and overall survival (OS) outcomes with MPT than with MP [18, 19]. The IFM 99–06 trial was chosen because that study produced the most favorable outcomes of all available MPT versus MP studies, and therefore provided the most conservative comparison with VMP and MPT.
Table 1.
Response rates and outcomes in phase III trials of VMP, MPT, and MPR-R versus MP
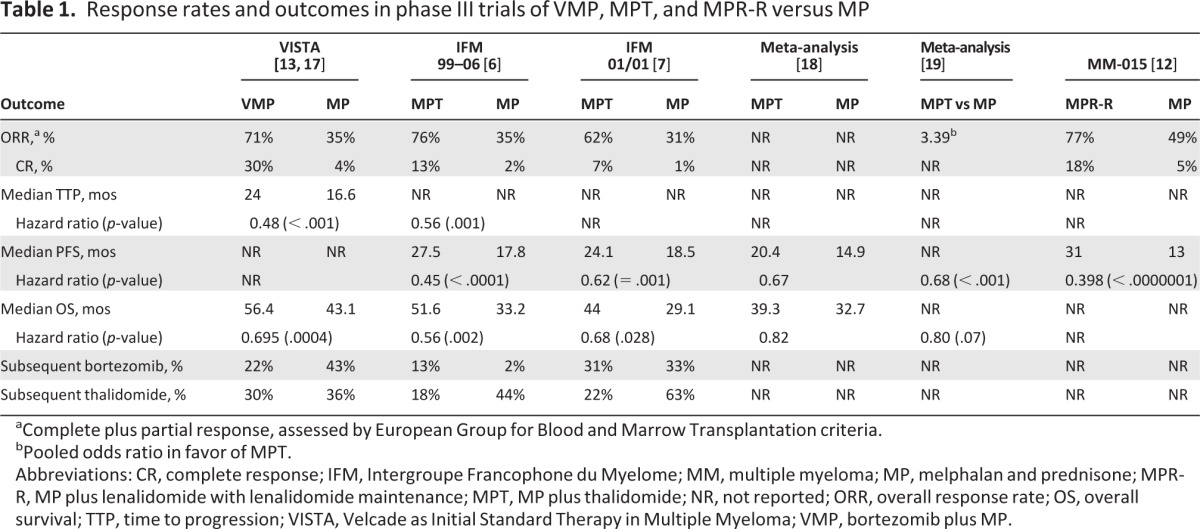
aComplete plus partial response, assessed by European Group for Blood and Marrow Transplantation criteria.
bPooled odds ratio in favor of MPT.
Abbreviations: CR, complete response; IFM, Intergroupe Francophone du Myelome; MM, multiple myeloma; MP, melphalan and prednisone; MPR-R, MP plus lenalidomide with lenalidomide maintenance; MPT, MP plus thalidomide; NR, not reported; ORR, overall response rate; OS, overall survival; TTP, time to progression; VISTA, Velcade as Initial Standard Therapy in Multiple Myeloma; VMP, bortezomib plus MP.
The international MM-015 trial reported results for the primary comparison of MP with MPR-R as well as the secondary comparison of MPR with MPR-R [11, 12, 20]. Those results showed that the PFS interval was significantly longer with MPR-R than with MP (p < .001) [11]; however, an OS advantage for MPR-R has not been established [20]. Because there have been no data indicating that MPR without maintenance provides any survival benefit over MP, MPR was excluded from our analysis and MPR-R was chosen for comparison with MP.
The substantial improvement in patient health outcomes conferred by VMP, MPT, and MPR-R is, however, associated with a higher cost than with MP. Because these regimens are increasingly being used in clinical practice, comparing the cost-effectiveness of each combination to determine the best partner for MP becomes even more important. Thus, a detailed pharmacoeconomic analysis was conducted to estimate the incremental cost-effectiveness of VMP versus MPT and VMP versus MPR-R as therapy for previously untreated transplant-ineligible MM patients using outcome data from the VISTA, IFM 99–06, and MM-015 trials. The available data from the VISTA study allowed a direct comparison between VMP and MP, whereas the IFM 99–06 and MM-015 studies facilitated indirect comparisons between VMP and MPT and between VMP and MPR-R. Patient-level data from the VISTA trial database were used for the VMP regimen, whereas published trial data were used for the MPT and MPR-R regimens.
Materials and Methods
Cost-Effectiveness Model
An Excel-based Markov model from the U.S. payer perspective was developed using available clinical trial and published data to compare VMP with MPT and with MPR-R using data from the VISTA, IFM 99–06, and MM-015 studies. Results are presented for a 20-year patient time horizon as the base case, aiming to reflect a maximal remaining lifetime. A review of good practice guidelines for decision-analytic modeling indicates that lifetime horizons are appropriate for most models, particularly Markov models [21].
The VISTA trial was a randomized, multicenter, international, open-label, phase III study involving previously untreated MM patients ineligible for stem cell transplantation who received either MP (n = 338) or VMP (n = 344) [13]. Patients received nine 6-week cycles of MP (9 mg/m2 melphalan and 60 mg/m2 prednisone on days 1–4) with or without bortezomib at a dose of 1.3 mg/m2 twice weekly (days 1, 4, 8, 11, 22, 25, 29, and 32 in cycles 1–4) then weekly (days 1, 8, 22, and 29 in cycles 5–9). The primary endpoint was time to disease progression; secondary endpoints included the complete response (CR) rate, duration of response, time to next MM therapy, and OS time. Patient-level data from the trial prior to June 15, 2007 were analyzed for the study.
The IFM 99–06 trial was a randomized, multicenter, phase III study comparing MPT (n = 125) with MP (n = 196) in previously untreated patients aged 65–75 years and younger patients who were ineligible for high-dose treatment [6]. Patients received 12 6-week cycles of MP (0.25 mg/kg melphalan and 2 mg/kg prednisone on days 1–4) with or without thalidomide. Thalidomide was given continuously, initially at a dose of 200 mg/day; in the absence of severe adverse effects, the dose was increased to 400 mg/day after 2 to 4 weeks. The primary endpoint was the OS time, whereas secondary endpoints included the response rate, PFS interval, survival duration after progression, and toxicity. Data were taken from the published results of this study.
In the randomized, double-blind, multicenter, international MM-015 study, previously untreated patients aged ≥65 years received MPR (n = 153), MPR-R (n = 152), or MP (n = 154) [11]. As previously mentioned, VMP was only compared with MPR-R in this analysis as a result of the lack of evidence of superiority of MPR over MP. Patients received nine 4-week cycles of MP (0.18 mg/kg melphalan and 2 mg/kg prednisone on days 1–4) plus lenalidomide (10 mg on days 1–21) followed by lenalidomide maintenance (10 mg/day on days 1–21 of 4-week cycles) that continued until disease progression. Patients progressing in any arm were subsequently treated with lenalidomide at a dose of 25 mg/day (with or without dexamethasone) in an open-label extension or follow-up phase. The primary endpoint was the PFS interval; secondary endpoints included the OS time, time to progression, response rate, time to response, response duration, and time to next MM therapy. Data were taken from an interim analysis presented at the 2009 Annual Meeting of the American Society of Hematology [11].
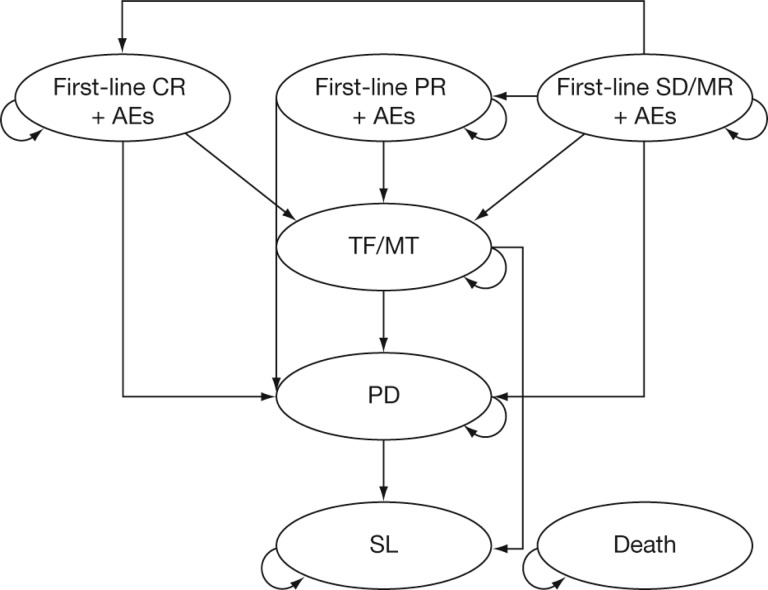
Simulations were performed for hypothetical cohorts of previously untreated transplant-ineligible MM patients with an average age of 70 years at treatment initiation. The model included seven mutually exclusive Markov health states, including: stable disease/minimal response (SD/MR), partial response (PR), CR, treatment-free (TF) interval for VMP and MPT or lenalidomide maintenance treatment with MPR (TF/MT), progressive disease (PD), second-line therapy (SL), and death (Fig. 1). Patient cohorts entered the model in the SD/MR state. In subsequent monthly cycles, patients moved to their best response state (PR or CR) or remained in SD/MR until they discontinued treatment, progressed, or died. Patients in SL received at most 6 months of second-line MM treatment.
Figure 1.
Markov model schema. Transition to the absorbing state “death” is possible from all health states.
Abbreviations: AE, adverse event; CR, complete response; MR, minimal response; MT, maintenance treatment; PD, progressive disease; PR, partial response; SD, stable disease; SL, second-line therapy; TF, treatment-free interval.
Health outcomes, as indicators of the effectiveness of therapy, were expressed in life years (LYs) and quality-adjusted LYs (QALYs). LYs represent the difference in survival time gained by undergoing the specified treatment, whereas the QALY combines both the quantity and quality of the additional LYs conferred by the intervention. Quality of life assessments over time were used to estimate a utility value for each health state. Utilities are measured on an interval scale with zero reflecting states of health equivalent to death and one reflecting perfect health. Incremental cost-effectiveness ratios (ICERs) were calculated for VMP compared with MPT and with MPR-R over a patient lifetime horizon of 20 years.
Model Inputs
Model inputs included treatment efficacies (or transition probabilities of moving from one health state to the next), treatment-related adverse event (AE) probabilities, costs, and state-specific utilities.
Transition Probabilities
For VMP and MP, health state transition probabilities were estimated from patient-level VISTA trial data [8, 13] using survival analysis methods. For MPT, the transition probabilities were estimated by adjusting the hazard ratios (HRs) for MP from the VISTA trial by the HRs for MPT versus MP from the IFM 99–06 trial [6]. For MPR-R, transition probabilities were estimated from presented results from the MM-015 trial [11].
Based on published data, the MPT versus MP HR for the PFS outcome was set to 0.51 and that for the OS outcome was set to 0.59 [6]. For MPR-R, the HR for the PFS outcome was set to 0.499 and that for the OS outcome was set to 1 as a result of the lack of survival benefit with MPR-R over MP observed in the MM-015 trial [11].
Treatment-Related AEs
For VMP and MP, AE probabilities were estimated from patient-level VISTA trial data [8, 13] using Poisson regressions. For MPT, AE probabilities were estimated by adjusting the probabilities for MP from the VISTA trial [8, 13] using cumulative incidence ratios for MPT versus MP from the IFM 99–06 trial [6], whereas AE probabilities for MPR-R were estimated from presented results from the MM-015 trial [11].
Resource Use and Costs
Costs included per-protocol drug and medical costs, treatment-related AE management costs, resource use, and second-line treatment. Unit costs were obtained from the published literature.
Drug, medical, and treatment-related AE management costs for each regimen are shown in Table 2. For VMP, costs included i.v. drug administration and hydration for bortezomib and prophylaxis for herpes zoster (valacyclovir). Because MPT and MPR-R are oral regimens, no drug administration costs were necessary. Medical costs for MPT included deep vein thrombosis or pulmonary embolism prophylaxis (low-dose aspirin, low-molecular-weight heparin, or warfarin), whereas neutropenia prophylaxis (G-CSF) was included for MPR-R.
Table 2.
Monthly drug, medical, and AE management costs for on-treatment states (SD/MR, PR, CR)
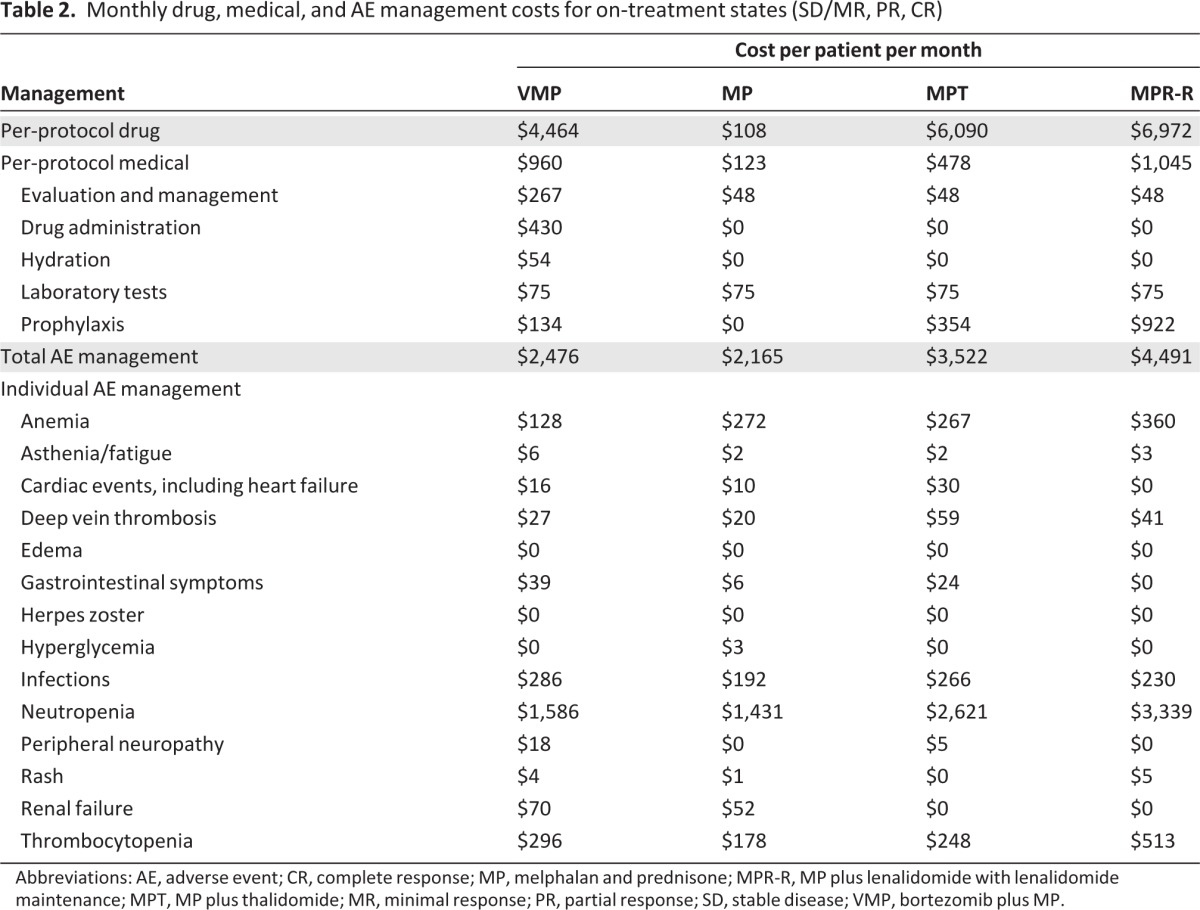
Abbreviations: AE, adverse event; CR, complete response; MP, melphalan and prednisone; MPR-R, MP plus lenalidomide with lenalidomide maintenance; MPT, MP plus thalidomide; MR, minimal response; PR, partial response; SD, stable disease; VMP, bortezomib plus MP.
The costs of resource use during the off-treatment states (TF and PD) are shown in Table 3. Eight types of resources were considered: emergency room, hospice care, inpatient care, inpatient care plus chemotherapy, intensive care unit, laboratory department, palliative care unit, and physician or clinic visit.
Table 3.
Monthly cost of resource use for off-treatment states (TF, PD)

aIncludes costs for CBC, M-protein quantification, and comprehensive metabolic panel.
Abbreviations: PD, progressive disease; TF, treatment-free interval.
The monthly drug and medical costs of second-line treatment were estimated from the results of a budget impact model that compared the resource use of four approved therapies for relapsed MM (bortezomib, bortezomib plus pegylated liposomal doxorubicin, lenalidomide plus dexamethasone, and thalidomide plus dexamethasone) (Millennium Pharmaceuticals, Inc. data on file). Per-patient per-month drug and medical costs were $6,881 and $2,795, respectively.
Both cost and health outcomes were discounted at 3%, and all costs were adjusted to represent 2010 U.S. dollars.
Utilities
State-specific utility estimates were derived from patient-level EQ-5D (EuroQol Group, Rotterdam, The Netherlands) data from the VISTA trial using U.S.-specific weights. On-treatment state (SD/MR, PR, CR) utilities were also adjusted by the utility impact of experiencing treatment-related AEs.
Sensitivity Analyses
One-way sensitivity analyses were performed for the VMP versus MPT study and for the VMP versus MPR-R study by varying key model inputs from their 95% confidence interval, if available, or ±50%. These analyses assessed the general robustness of model findings and identified key drivers. Probabilistic sensitivity analyses were also conducted.
Results
Patients
Table 4 shows the patient baseline demographic and disease characteristics and the response rates to the study regimens. The median ages were 71 years in both treatment groups of the VISTA study and 71 years in the MPR-R and 72 years in the MP groups of the MM-015 study. Median age was not reported for the IFM 99–06 study, although 40% and 43% of patients were aged ≥70 years in the MPT and MP treatment groups, respectively. The MM-015 and IFM 99–06 trials had fewer patients aged ≥75 years (MPR-R/MP, 24%/25%) and ≥70 years (MPT/MP, 40%/43%) than did the VISTA study (VMP/MP, 31%/30% and 63%/62%, respectively). In addition, the MM-015 population had more patients with stage III disease (MPR-R/MP, 49%/51%) than did the VISTA (VMP/MP, 35%/34%) or IFM 99–06 (MPT/MP, 29%/30%) populations. Within each study, the patient populations were well balanced with respect to the MP control.
Table 4.
Baseline demographic and disease characteristics of patients in the VISTA, IFM 99–06, and MM-015 trials
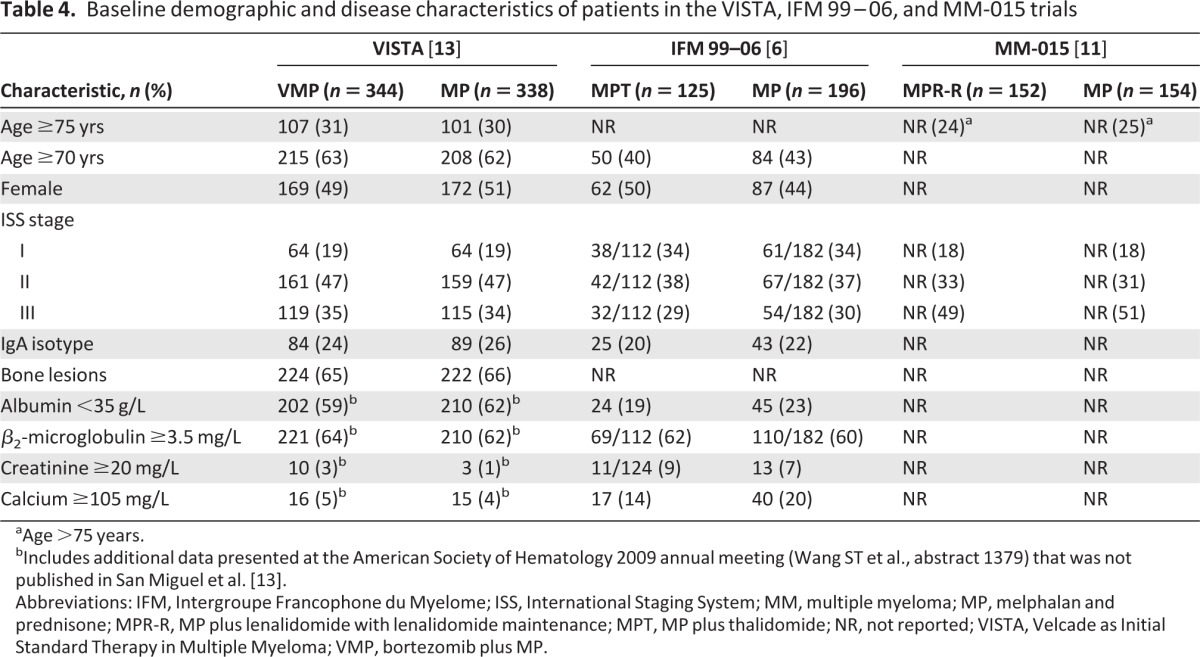
aAge >75 years.
bIncludes additional data presented at the American Society of Hematology 2009 annual meeting (Wang ST et al., abstract 1379) that was not published in San Miguel et al. [13].
Abbreviations: IFM, Intergroupe Francophone du Myelome; ISS, International Staging System; MM, multiple myeloma; MP, melphalan and prednisone; MPR-R, MP plus lenalidomide with lenalidomide maintenance; MPT, MP plus thalidomide; NR, not reported; VISTA, Velcade as Initial Standard Therapy in Multiple Myeloma; VMP, bortezomib plus MP.
Costs
Discounted 20-year lifetime direct medical costs were lowest for MP at $63,294, were $119,102 for VMP, were $142,452 for MPT, and were highest for MPR-R at $248,358 (Table 5). Both drug and treatment-related AE costs were highest with MPR-R, at $132,215 and $63,980 (primarily because of neutropenia), respectively, whereas these costs were substantially lower with VMP ($39,754 and $22,055, respectively) and MPT ($56,435 and $32,638, respectively) and lowest with MP ($728 and $14,552, respectively). Of note, the proportions of total costs attributable to drug costs were ∼58%, ∼65%, and ∼83% for VMP, MPT, and MPR-R, respectively.
Table 5.
Discounted 20-year lifetime direct medical costs

aAEs considered were: anemia, asthenia or fatigue, cardiac events (including heart failure), deep vein thrombosis, edema, gastrointestinal symptoms, herpes zoster, hyperglycemia, infections, neutropenia, peripheral neuropathy, rash, renal failure, and thrombocytopenia.
Abbreviations: AEs, adverse events; MP, melphalan and prednisone; MPR-R, MP plus lenalidomide with lenalidomide maintenance; MPT, MP plus thalidomide; PD, progressive disease; SL, second-line therapy; TF, treatment-free interval; VMP, bortezomib plus MP.
Cost-Effectiveness
Model base case results for the incremental cost-effectiveness of VMP relative to MPT and MPR-R over a 20-year lifetime horizon are shown in Table 6. The estimated OS duration was 4.187 years with VMP, compared with 2.864 years with MP, 4.140 years with MPT, and 3.409 years with MPR-R. Model-simulated OS curves for VMP, MP, MPT, and MPR-R are shown in supplemental online Figure 1. VMP was cost-saving compared with both MPT and MPR-R: compared with MPT, VMP cost ∼16% less and provided 0.043 additional QALYs; compared with MPR-R, VMP cost ∼50% less and provided 0.566 more QALYs. The ICERs indicated that, relative to MP, VMP cost $42,169 per LY gained and $59,076 per QALY gained. VMP conferred cost savings relative to both MPT and MPR-R. Thus, VMP is projected to be cost-effective when compared with MP and to be cost-saving and have better health outcomes when compared with MPT and MPR-R. The incremental cost-effectiveness of VMP relative to MP, MPT, and MPR-R over a 10-year time horizon is shown in supplemental online Table 1 and is similar to the 20-year horizon results.
Table 6.
Cost-effectiveness of VMP versus MP, VMP versus MPT, and VMP versus MPR-R over a 20-year lifetime horizon
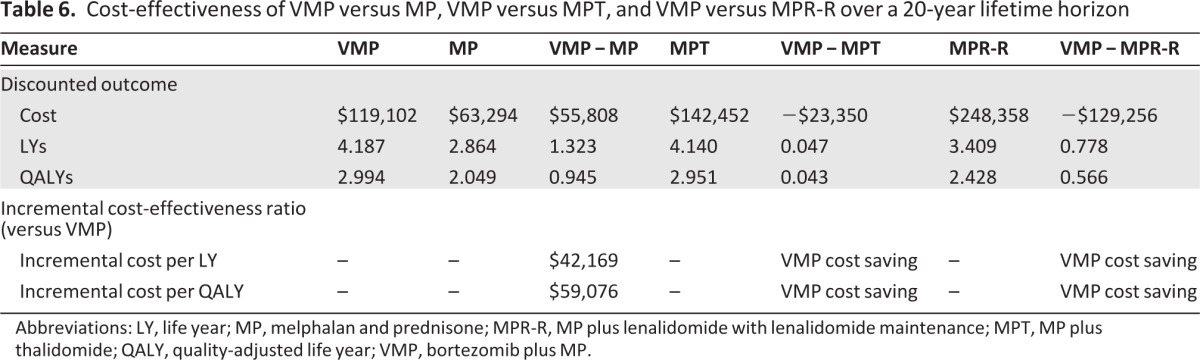
Abbreviations: LY, life year; MP, melphalan and prednisone; MPR-R, MP plus lenalidomide with lenalidomide maintenance; MPT, MP plus thalidomide; QALY, quality-adjusted life year; VMP, bortezomib plus MP.
Sensitivity Analyses
Table 7 shows the sensitivity analyses of the cost-effectiveness of VMP compared with MPT and MPR-R. These one-way sensitivity analyses supported the general robustness of the model findings. The key drivers in the one-way sensitivity analyses of VMP versus MPT were the MPT versus MP HRs for the OS outcome, treatment-free interval, progression rate, and survival time after progression. For the one-way sensitivity analyses of VMP versus MPR-R, the MPR-R versus MP HR for the OS outcome was identified as the key driver.
Table 7.
Sensitivity analyses on cost-effectiveness of VMP versus MPT and MPR-R
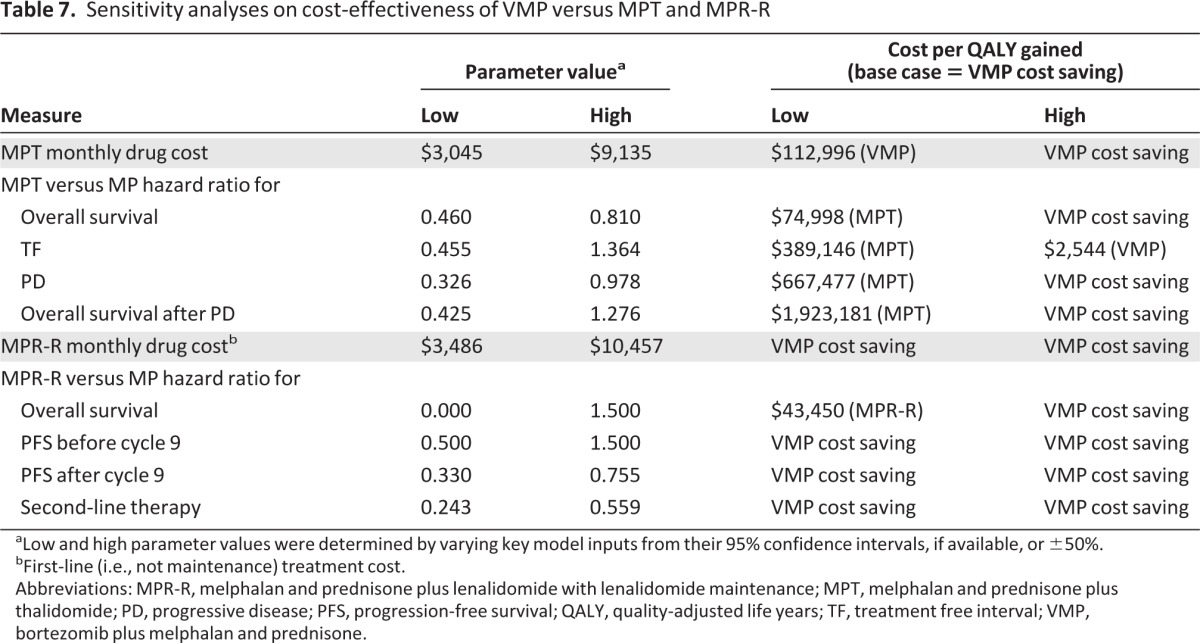
aLow and high parameter values were determined by varying key model inputs from their 95% confidence intervals, if available, or ±50%.
bFirst-line (i.e., not maintenance) treatment cost.
Abbreviations: MPR-R, melphalan and prednisone plus lenalidomide with lenalidomide maintenance; MPT, melphalan and prednisone plus thalidomide; PD, progressive disease; PFS, progression-free survival; QALY, quality-adjusted life years; TF, treatment free interval; VMP, bortezomib plus melphalan and prednisone.
In contrast to the data for VMP and MPT, which were mature with long follow-up at the time of this analysis (3 years for the VISTA study and 4 years for the IFM 99–06 study), the MPR-R data were immature, with only minimal follow-up included in the analysis (9 months), and the HR was not statistically different for the OS outcome. We found that, at a threshold of $100,000 per QALY, MPR-R only became cost-effective at an HR ≤0.24, when compared with MP.
Using the generally accepted cost-effectiveness range of $50,000–$100,000 per QALY, the probabilistic sensitivity analyses were consistent with the deterministic analyses. At a relatively low willingness-to-pay threshold of $50,000 per QALY, the probability of VMP being cost-effective compared with MP was 20.4%. But at a threshold of $100,000 per QALY, the probability was virtually 100%.
Discussion
The expansion of treatment options for MM since 2005 has yielded substantial improvement in patients' expected survival times and has created a much broader range of options for clinicians and patients. These multidrug regimens are, however, more costly, and this raises questions about their cost-effectiveness. This analysis, projecting outcomes over a 20-year horizon, confirms that these regimens are superior to the MP-based regimen in terms of response rates and OS times. Because no direct head-to-head trials are available, this analysis used indirect comparison methods. We found that the incremental cost of adding bortezomib to the MP base is lower than that of adding either thalidomide or lenalidomide. Hence, our projections indicate that the VMP regimen is not only cost-effective relative to MPT and MPR-R but it is cost-saving. From a pharmacoeconomic payer perspective, VMP would, therefore, be the preferred first-line treatment option for the typical elderly MM patient.
Although adding bortezomib to the MP regimen clearly raises costs, our results suggest that the additional LYs gained justify the greater drug costs. This analysis projects a gain of 1.32 LYs—or about 0.95 QALYs over the patient's remaining lifetime. At an additional cost of about $56,000, the cost-effectiveness ratios are ∼$42,000 per LY gained and $59,000 per QALY gained. These are far below the cost-effectiveness ratios of other chemotherapeutic agents in common use [22, 23]. The projected incremental LYs and incremental QALYs with VMP are somewhat higher (0.047 and 0.043) than with MPT and much higher (0.778 and 0.566) than with MPR-R. Because the mean incremental costs are less for VMP in these indirect comparisons (i.e., ∼$23,000 less than with MPT and $129,000 less than with MPR-R), the results suggest that the VMP regimen would be considered a “dominant” treatment. From a cost-effectiveness perspective, lenalidomide maintenance therapy provided minimal benefit in this patient population, primarily as a result of the absence of any survival advantage with the MPR-R regimen. If, however, as data from the MM-015 trial mature, a significant survival benefit for MPR-R over MP becomes apparent, a re-estimation of the incremental cost-effectiveness of VMP versus MPR-R would be warranted.
Subcutaneous (s.c.) administration of bortezomib was recently approved by the U.S. Food and Drug Administration and included in the prescribing information [24]. This administration route has the potential to reduce the cost associated with VMP because i.v. administration and hydration costs would not be required, and AE costs would be reduced with less toxicity seen with s.c. injection. In addition, long-term survival data from the VISTA study were recently presented, demonstrating that a persistent 13.3-month benefit in the median survival time versus MP (p = .0004) is maintained after 5 years of follow-up, despite substantial use of novel-agent-based salvage therapies [17]. In addition, no emerging safety signal was observed for second primary malignancies. Bortezomib is currently the only novel agent with survival data available for this length of follow-up.
This type of economic analysis has several limitations. First, the analysis is driven by the response, disease progression, and patient survival outcomes observed in randomized clinical trials—these are not “real-world” data. On the other hand, patients in these trials have a choice of a wide range of second-line or salvage therapies after progression, which better reflects the real-world situation. In addition, the modeling represents an extrapolation beyond the observed trial period.
Another limitation is the requirement for indirect comparisons. As a result of the design of the source trials, a direct comparison was only possible between VMP and MP. Indirect comparisons of VMP and MPT and of VMP and MPR-R were made using patient-level data for VMP and published study data for MPT and MPR-R. The IFM 99–06 study was selected as the most appropriate data source for MPT because of the superior activity and outcomes demonstrated in that study compared with other available MPT studies. This provided the most conservative comparison to evaluate cost-effectiveness rather than using data from studies that demonstrated lower activity. It should be noted that no comparison of MP with MPR (i.e., without lenalidomide maintenance) was conducted in this study because there are currently no data demonstrating a difference in outcomes, and thus no advantage to using MPR over MP.
Third, like all trial-based cost-effectiveness analyses, this analysis addresses the cost-effectiveness for the typical elderly patient in these trials in the first-line treatment setting. Lacking the patient-level data for each of these trials, the indirect comparison could not directly adjust for systematic differences across the trials. Indirect comparisons are inherently limited by potential differences in study populations in terms of prognostic factors and disease stages, for which differential survival projections cannot be adjusted. It should also be mentioned that our model did not explicitly consider specific second-line and salvage therapies.
A cost-effectiveness analysis of VMP and MPT by Picot et al. [25] in previously untreated, transplant-ineligible patients was recently published, analyzing the data from models developed by Janssen-Cilag and Celgene and presenting the findings of an independent survival model. The Janssen-Cilag survival model estimated OS and PFS outcomes based on treatment effects from a mixed-treatment comparison of trials and included second- and third-line therapy. All treatments were found to be cost-effective; the ICER for VMP versus MP was estimated to be £10,498, and for VMP versus MPT it was £11,907. The Celgene Markov model included health states for preprogression, postprogression, and death; it was assumed that the survival outcome after progression was the same irrespective of first-line treatment. Treatment effects for disease progression were calculated using a random effect mixed-treatment comparison. The ICER for MPT versus MP was £23,381 per QALY gained. The independent survival model estimated ICERs for MPT and VMP of £9,135 and £29,820 per QALY gained versus MP, respectively. MPT dominated because it was cheaper but with similar efficacy. The results evaluated in the report by Picot et al. [25] varied widely, indicating a lack of consistency in the findings. In addition, their findings are in contrast to our report, which found that VMP was more cost-effective than MPT. The differing findings mainly stem from the different cost assumptions made for thalidomide. In our model, which adopted a U.S. payers' perspective, we used the price for branded thalidomide because thalidomide is still currently branded in the U.S. and is not forecast to have generic availability in the U.S. market until 2023. In contrast, the report by Picot et al. [25] adopted a U.K. perspective, and thalidomide is available generically in the U.K. The cost for a pack of 28 50-mg capsules is £298.48 in the U.K. (U.S. $472.46) and $3,555.74 in the U.S. (source: zenRx Research, Pharmaceutical Global Pricing Database, accessed May 3, 2012). In terms of QALYs, the report by Picot et al. [25] is consistent with our finding of a similarity between VMP and MPT (3.62 QALYs versus 3.64 QALYs in Picot et al. [25] and 2.994 QALYs versus 2.951 QALYs in our report). The incremental difference in QALYs between VMP and MP in the report by Picot et al. [25] is actually greater than that reported in our manuscript (1.2 QALYs in Picot et al. [25] and 0.945 QALYs in our report). Differences between the QALYs estimated by Picot et al. [25] and our analysis are likely a result of differences in the modeling method (partitioned survival model in Picot et al. [25] and a Markov model in our analysis), time horizon (30 years versus 20 years), and HRs for the OS outcome (time varying with a minimum of 0.62 in Picot et al. [25] and state varying with an average of 0.599 in our analysis) as well as inclusion of mortality resulting from non-MM causes (e.g., aging) in our model.
Other economic evaluations have been conducted for bortezomib-, thalidomide-, and lenalidomide-based regimens in different settings as summarized below. As would be expected, variations exist among different patient subgroups and for different lines of therapy. Hornberger et al. [26] evaluated bortezomib as second-line or salvage therapy versus dexamethasone in a Swedish setting. The mean cost-effectiveness ratio was higher (€95,000) than in our study, suggesting that bortezomib is more cost-effective as first-line therapy. Other economic evaluations of these novel agents have been reported in populations of previously untreated or relapsed or refractory MM patients [26–30], and have demonstrated the cost-effectiveness benefit of novel agents over the older regimens and in different patient populations. However, it is not possible to directly compare the studies because of the differing currencies, the discordant timing of the analyses, and the diverse patient populations studied. Additionally, drug prices differ among countries. For example, thalidomide is sold in generic form in Europe but not in the U.S., which could result in markedly lower drug costs in Europe than in the U.S. Deniz et al. [29] conducted a similar analysis using only data from the IFM 99–06 trial and concluded that the ICER for MPT compared with MP was within a range considered cost-effective in Scotland, at £17,847 per QALY. In the U.K., an analysis based on individual patient data from the Study of Uncontrolled Multiple Myeloma Managed with Proteasome Inhibition suggested that bortezomib monotherapy, when used as a third-line treatment for patients with relapsed or refractory MM, appeared to be cost-effective relative to best supportive care [27]. A noncomparative U.K. study analyzing the combination of lenalidomide and dexamethasone using data from the MM-009 and MM-010 trials reported that this two-drug combination yielded an estimated incremental cost per QALY that was within a cost-effective range (ICERs <£30,000 per QALY) [28].
The inevitable heterogeneity between our analysis, which was conducted from a U.S. payer's perspective, and the analyses mentioned above, which were conducted from numerous other perspectives and used differing currencies and drug costs, make comparisons between analyses difficult. However, although the findings of our analysis may not be directly comparable with previous—or future—analyses, the finding that VMP is more cost-effective than MPR-R or MPT in the first-line treatment of elderly MM patients may still be applicable to other health care systems if the relative costs across the regimens are similar to the pattern in the U.S.
Conclusion
Efficacy data have clearly shown the superiority of VMP, MPT, and MPR-R over MP; however, to date, no head-to-head clinical trials have been conducted to directly compare the effectiveness and economic impact of VMP with those of MPT or MPR-R. Our findings support the recommendation of VMP for previously untreated, transplant-ineligible patients with MM [3, 4] and suggest that, on the basis of the currently available data, VMP would be a cost-effective option compared to MP, MPT, or MPR-R for these patients when managed within the U.S. health care system.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the writing assistance of Natalie Beavan of FireKite during the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc. and Janssen Global Services.
The study was presented previously at the 2009 Annual Meeting of the American Society of Hematology (abstract 1379) and at the 2010 Annual Meeting of the American Society of Hematology (abstract 2563).
Footnotes
Editor's Note: See the accompanying commentary on pages 5–7 and the related article on pages 37–45 of this issue.
Author Contributions
Conception/Design: Louis P. Garrison, Jr., Si-Tien Wang, Hui Huang, Abbie Ba-Mancini, Hongliang Shi, Kristina Chen, Caroline Korves, Ravinder Dhawan, Deyanira Corzo, Mei S. Duh
Provision of study material or patients: Andrew Cakana, Helgi van de Velde
Collection and/or assembly of data: Louis P. Garrison, Jr., Hongliang Shi, Andrew Cakana, Helgi van de Velde
Data analysis and interpretation: Louis P. Garrison, Jr., Si-Tien Wang, Hui Huang, Abbie Ba-Mancini, Hongliang Shi, Kristina Chen, Caroline Korves, Ravinder Dhawan, Andrew Cakana, Helgi van de Velde, Deyanira Corzo
Manuscript writing: Louis P. Garrison, Jr., Ravinder Dhawan, Deyanira Corzo
Final approval of manuscript: Louis P. Garrison, Jr., Si-Tien Wang, Hui Huang, Abbie Ba-Mancini, Hongliang Shi, Kristina Chen, Caroline Korves, Ravinder Dhawan, Andrew Cakana, Helgi van de Velde, Deyanira Corzo, Mei S. Duh
Disclosures
Louis P. Garrison, Jr.: Millennium, Janssen (RF); Si-Tien Wang: Millennium (CA, RF), Analysis Group (RF); Hui Huang: Millennium (E); Abbie Ba-Mancini: Millennium (E); Hongliang Shi: Millennium (E); Kristina Chen: Analysis Group (RF), Millennium (RF); Caroline Korves: Millennium (CA, H, RF), Analysis Group (RF); Ravinder Dhawan: Johnson & Johnson (E, OI), Janssen (E); Andrew Cakana: Janssen (E); Helgi van de Velde: Janssen (E, IP), Johnson & Johnson (E, OI); Deyanira Corzo: Millennium (E, OI); Mei Sheng Duh: Analysis Group (RF), Millennium (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laubach JP, Mitsiades CS, Mahindra A, et al. Novel therapies in the treatment of multiple myeloma. J Natl Compr Canc Netw. 2009;7:947–960. doi: 10.6004/jnccn.2009.0062. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Fort Washington, PA: National Comprehensive Cancer Network; 2012. NCCN Clinical Practice Guidelines in Cancer. Multiple Myeloma, Version 1.2012; pp. 1–59. [Google Scholar]
- 4.Engelhardt M, Kleber M, Udi J, et al. Consensus statement from European experts on the diagnosis, management, and treatment of multiple myeloma: From standard therapy to novel approaches. Leuk Lymphoma. 2010;51:1424–1443. doi: 10.3109/10428194.2010.487959. [DOI] [PubMed] [Google Scholar]
- 5.Beksac M, Haznedar R, Firatli-Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: Results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86:16–22. doi: 10.1111/j.1600-0609.2010.01524.x. [DOI] [PubMed] [Google Scholar]
- 6.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): A randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 7.Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27:3664–3670. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 8.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: Updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259–2266. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 9.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: Randomised controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 10.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: Updated results of a randomized, controlled trial. Blood. 2008;112:3107–3114. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 11.Palumbo A, Dimopoulos MA, Delforge M, et al. A phase III study to determine the efficacy and safety of lenalidomide in combination with melphalan and prednisone (MPR) in elderly patients with newly diagnosed multiple myeloma [oral presentation] Blood. 2009;114:613. [Google Scholar]
- 12.Palumbo A, Dimopoulos M, Delforge M, et al. A phase 3 study to determine the efficacy and safety of lenalidomide combined with melphalan and prednisone in patients >65 years with newly diagnosed multiple myeloma (NDMM) [abstract 0566] Haematologica. 2010;95(suppl 2):234. [Google Scholar]
- 13.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 14.Waage A, Gimsing P, Fayers P, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116:1405–1412. doi: 10.1182/blood-2009-08-237974. [DOI] [PubMed] [Google Scholar]
- 15.Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: The HOVON 49 Study. J Clin Oncol. 2010;28:3160–3166. doi: 10.1200/JCO.2009.26.1610. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig H, Beksac M, Bladé J, et al. Multiple myeloma treatment strategies with novel agents in 2011: A European perspective. The Oncologist. 2011;16:388–403. doi: 10.1634/theoncologist.2010-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.San Miguel JF, Schlag R, Khuageva NK, et al. Continued overall survival benefit after 5 years' follow-up with bortezomib-melphalan-prednisone (VMP) versus melphalan-prednisone (MP) in patients with previously untreated multiple myeloma, and no increased risk of second primary malignancies: Final results of the phase 3 VISTA trial. Blood. 2011;118:476. [Google Scholar]
- 18.Waage A, Palumbo AP, Fayers P, et al. MP versus MPT for previously untreated elderly patients with multiple myeloma: A meta-analysis of 1,682 individual patient data from six randomized clinical trials. J Clin Oncol. 2010;28:8130. [Google Scholar]
- 19.Kapoor P, Rajkumar SV, Dispenzieri A, et al. Melphalan and prednisone versus melphalan, prednisone and thalidomide for elderly and/or transplant ineligible patients with multiple myeloma: A meta-analysis. Leukemia. 2011;25:689–696. doi: 10.1038/leu.2010.313. [DOI] [PubMed] [Google Scholar]
- 20.Palumbo A, Delforge M, Catalano J, et al. A phase 3 study evaluating the efficacy and safety of lenalidomide combined with melphalan and prednisone in patients ≥ 65 years with newly diagnosed multiple myeloma (NDMM): Continuous use of lenalidomide vs fixed-duration regimens. Blood. 2010;116:622. [Google Scholar]
- 21.Philips Z, Ginnelly L, Sculpher M, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess. 2004;8:iii–iv. ix–xi, 1–158. doi: 10.3310/hta8360. [DOI] [PubMed] [Google Scholar]
- 22.Brock DW. How much is more life worth? Hastings Cent Rep. 2006;36:17–19. doi: 10.1353/hcr.2006.0036. [DOI] [PubMed] [Google Scholar]
- 23.Elkin EB, Weinstein MC, Winer EP, et al. HER-2 testing and trastuzumab therapy for metastatic breast cancer: A cost-effectiveness analysis. J Clin Oncol. 2004;22:854–863. doi: 10.1200/JCO.2004.04.158. [DOI] [PubMed] [Google Scholar]
- 24. Millennium Pharmaceuticals, Inc. Velcade® (bortezomib) for injection [prescribing information], Revision 13, January 2012.
- 25.Picot J, Cooper K, Bryant J, et al. The clinical effectiveness and cost-effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first-line treatment of multiple myeloma: A systematic review and economic evaluation. Health Technol Assess. 2011;15:1–204. doi: 10.3310/hta15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornberger J, Rickert J, Dhawan R, et al. The cost-effectiveness of bortezomib in relapsed/refractory multiple myeloma: Swedish perspective. Eur J Haematol. 2010;85:484–491. doi: 10.1111/j.1600-0609.2010.01526.x. [DOI] [PubMed] [Google Scholar]
- 27.Bagust A, Haycox AR, Boland A, et al. Economic evaluation of bortezomib (Velcade) for relapsed and refractory multiple myeloma. Blood. 2004;104:268. [Google Scholar]
- 28.Deniz B, Morgan G, Schey S, et al. Economic evaluation of lenalidomide combined with dexamethasone for the treatment of multiple myeloma in the UK. Blood. 2008;112:2400. [Google Scholar]
- 29.Deniz B, Facon T, Singer I, et al. Economic evaluation of thalidomide combined with melphalan and prednisone in previously untreated multiple myeloma in Scotland. Blood. 2008;112:2395. [Google Scholar]
- 30.Mehta J, Duff SB, Gupta S. Cost effectiveness of bortezomib in the treatment of advanced multiple myeloma. Manag Care Interface. 2004;17:52–61. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.