This claims-based, retrospective analysis examines total health care costs and patient burden associated with bortezomib, thalidomide, and lenalidomide treatment versus other therapies for multiple myeloma. Total health care costs and patient out-of-pocket costs were found to be higher for thalidomide and lenalidomide than for bortezomib and other therapies.
Keywords: Multiple myeloma, Antineoplastic agents, Cost analysis, Medicare, Cost of illness
Abstract
Background.
Treatment of multiple myeloma has dramatically improved with the introduction of bortezomib (BOR), thalidomide (THAL), and lenalidomide (LEN). Studies assessing health care costs, particularly economic burden on patients, are limited. We conducted a claims-based, retrospective analysis of total health care costs as well as patient burden (patient out-of-pocket costs and number of ambulatory/hospital visits) associated with BOR/THAL/LEN treatment versus other therapies (OTHER).
Methods.
Treatment episodes starting between January 1, 2005 and September 30, 2010 were identified from the claims database of a large U.S. health plan. Health care costs and utilization were measured during 1 year after initiation and analyzed per treatment episode. Multivariate analyses were used to adjust for patient characteristics, comorbidities, and line of treatment.
Results.
A total of 4,836 treatment episodes were identified. Mean adjusted total costs were similar between BOR ($112,889) and OTHER ($111,820), but higher with THAL ($129,412) and LEN ($158,428). Mean adjusted patient out-of-pocket costs were also similar for BOR ($3,846) and OTHER ($3,900) but remained higher with THAL ($4,666) and LEN ($4,483). Mean adjusted rates of ambulatory visits were similar across therapies (BOR: 69.67; THAL: 66.31; LEN: 65.60; OTHER: 69.42).
Conclusions.
Adjusted analyses of real-world claims data show that total health care costs, as well as patient out-of-pocket costs, are higher with THAL/LEN treatment episodes than with BOR/OTHER therapies. Additionally, similar rates of ambulatory visits suggest that any perceived advantage in patient convenience of the orally administered drugs THAL/LEN is not supported by these data.
Implications for Practice:
In addition to efficacy and safety considerations, economic factors associated with novel treatments are important issues for payers and patients. Claims data provide real-world insight into health care costs associated with treatment for multiple myeloma. In this study, total health care costs and patient out-of-pocket costs were measured during one year post-initiation and analyzed per treatment episode. Adjusted for patient characteristics, comorbidities, and line of treatment, the costs of “other” therapies (i.e., other chemotherapy or radiotherapy) and bortezomib were similar, but the costs of thalidomide or lenalidomide treatment were significantly higher. Despite different routes of administration for bortezomib (intravenous), and thalidomide and lenalidomide (oral), the number of ambulatory visits (i.e., clinic/physician office and hospital outpatient visits) appeared similar across therapies with the only significant difference being between the rates for lenalidomide and “other” treatment. These data show differential real-world costs and patient burden associated with these treatments and may support decision-making with regard to multiple myeloma treatment.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy after non-Hodgkin's lymphoma, with an estimated 20,520 patients diagnosed with the disease and an estimated 10,610 deaths from MM in the U.S. in 2011 [1, 2]. The estimated prevalence of MM in the U.S. is 64,615 patients [1]. The incidence of MM increases with age, with the median age at diagnosis being 69 years [1]. The most common signs and symptoms of MM include hypercalcemia, renal dysfunction, anemia, and bone lesions, the so-called CRAB criteria [3]. Bone pain is often an initial symptom of the disease [4]. Symptoms often require specific management in addition to direct anti-MM treatment strategies.
Treatment of MM has dramatically improved over the past decade with the introduction and approval by the U.S. Food and Drug Administration of three novel targeted agents: the proteasome inhibitor bortezomib (BOR) and the immunomodulatory agents (IMiDs) thalidomide (THAL) and its analog lenalidomide (LEN) [5, 6]. Furthermore, associated enhanced supportive care measures have also been developed to improve MM management [7, 8]. BOR is approved for the treatment of previously untreated and relapsed MM, THAL in combination with dexamethasone for the treatment of previously untreated MM, and LEN in combination with dexamethasone for the treatment of MM following at least one prior therapy. Additionally, multiple regimens anchored with one or more of these novel agents are recommended treatment options for both first-line and relapsed MM in clinical practice guidelines, including in the National Comprehensive Cancer Network Clinical Practice Guidelines [9], European Society of Medical Oncology guidelines [10], and the International Myeloma Working Group guidelines [5, 6].
The introduction of these novel agents has contributed to a significant improvement in recent years in outcomes for patients with MM, as seen in population-based analyses [11–14]. In addition, numerous large phase III randomized studies have demonstrated improved outcomes with novel-agent-based therapies versus other chemotherapies and previous standards-of-care, including in previously untreated patients with MM who were ineligible [15–18] or eligible [19–22] for high-dose therapy with stem cell transplantation (SCT), and in patients with relapsed or refractory disease [23, 24].
However, only a limited number of studies have assessed the relative costs associated with these novel therapies and their cost-effectiveness compared with other therapeutic approaches [25–37]. In particular, very few studies have been conducted using real-world cost data and assessing the economic burden on patients. This is important because novel-agent-based therapies may have substantial differences compared with other therapies in terms of treatment paradigms (e.g., treatment until progression, finite treatment course), route of administration, frequency of administration, safety profile, and frequency of follow-up. All of these factors may affect both costs and convenience for patients in terms of number of visits.
We therefore conducted the present retrospective analysis using real-world claims data from a large U.S. health plan to evaluate and compare total health care costs, including patient out-of-pocket costs, and health care resource utilization between episodes of treatment with BOR, THAL, LEN, and other chemotherapies or radiotherapy (OTHER) in patients with previously untreated or relapsed MM.
Patients and Methods
The objectives of this retrospective cohort study were to compare total health care costs, which included health plan and patient paid amounts for both medical and pharmacy utilization; and rates of health care resource utilization, which included ambulatory, inpatient, and emergency visits, per course of treatment with BOR, LEN, THAL, or OTHER therapy (defined as clarithromycin, cyclophosphamide, dexamethasone, doxorubicin, interferon alfa-2, interferon alfa-2b, melphalan, vincristine, pegylated liposomal doxorubicin, prednisone, and/or radiation), as calculated for 1 year from the beginning of each treatment course. Patient out-of-pocket costs, including copayment, coinsurance, and deductibles, were calculated as a subset of total health care costs. Thus, the patient burden of a treatment episode comprised the two specific factors of out-of-pocket costs and number of ambulatory visits.
This study used claims data from a large national U.S. health plan affiliated with OptumInsight (formerly Ingenix) covering approximately 14 million commercially insured, Medicare Advantage (Medicare Part C), and managed Medicaid members annually with both medical and pharmacy benefits. Claims data from this data source have been used in several analyses of health care costs associated with cancer treatment [38–41]. Medical claims included multiple diagnosis codes (International Classification of Diseases, Ninth Edition [ICD-9]), procedure records (ICD-9 Procedure, Current Procedural Terminology [CPT], and Healthcare Common Procedure Coding System [HCPCS]), and revenue codes. Pharmacy claims were submitted electronically by the pharmacy. Information included the drug name, dosage form, drug strength, and days supplied.
Treatment Episodes
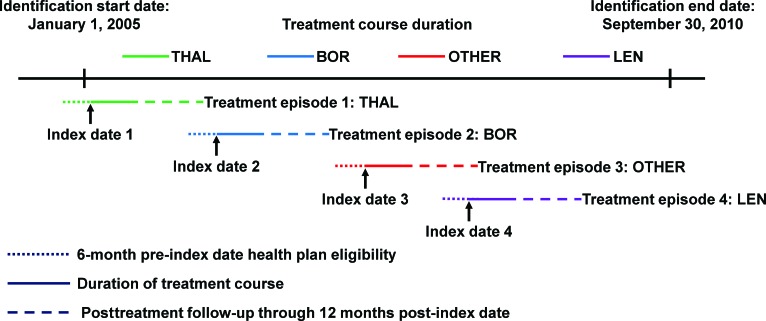
Patients with a diagnosis of MM (ICD-9 code 203.0x) who received at least one course of treatment (treatment episode) with BOR, THAL, LEN, or OTHER between January 1, 2005 and September 30, 2010 were considered for inclusion in this study. Treatment episodes were defined as unique courses of therapy (as identified from patient records) that incorporated BOR, THAL, LEN, or OTHER treatment. For each treatment episode, the date of first observation (which was when a new treatment [BOR, THAL, LEN, or OTHER] was begun) was defined as the index date for that episode. Treatment episodes that included more than one of the agents BOR, THAL, or LEN on the index date were excluded (e.g., BOR-THAL-dexamethasone; BOR-LEN-dexamethasone). Some patients could have received multiple treatment episodes within the time period of this study (Fig. 1), each of which was included in this analysis if the specified eligibility criteria were met. For each treatment episode included in this analysis, patients had to be ≥18 years of age on the index date, and required 6 months of health plan eligibility before the index date, as well as 12 months of health plan eligibility after the index date. Episodes without 12 months of eligibility following the index date, but ending in death, were retained.
Figure 1.
Example of identification of multiple treatment episodes in an individual patient. This patient received four treatment episodes, one each with bortezomib, thalidomide, lenalidomide, and other chemotherapy/radiotherapy, within the study period of January 1, 2005 to September 30, 2010.
Abbreviations: BOR, bortezomib; LEN, lenalidomide; OTHER, other chemotherapies or radiotherapy; THAL, thalidomide.
For each treatment episode, pre-index date patient demographics and clinical characteristics were recorded, including age, sex, U.S. geographic region, insurance type, cytogenetics, Deyo-Charlson comorbidity score, and history of deep vein thrombosis (DVT), pulmonary embolism (PE), peripheral neuropathy (PN), and dialysis. Whether patients had undergone a SCT pre- and post-index date for each treatment episode was also recorded. These conditions and tests/procedures were identified based on diagnosis codes (ICD-9), procedure records (ICD-9 procedure, CPT, and HCPCS), and revenue codes.
Health Care Costs
To assess the direct financial burden of MM treatment, total health care costs were evaluated for 1 year after the index date for each treatment episode, or until death if a patient died within 1 year of the index date. This 1-year follow-up period was not truncated if a patient discontinued treatment or switched therapy (Fig. 1). The study period allows for a 6-month lag to ensure all medical claims were captured and adjudicated in the claims prior to the analysis. Total health care costs, representing the combined patient and insurer 1-year paid amounts for each treatment episode, were adjusted for inflation.
Patient Burden
To assess the direct financial burden of MM treatment, health care resource utilization evaluated patient out-of-pocket costs (including copayment, coinsurance, and deductibles), as a subset of total health care costs. Indirect burden is difficult to measure in claims data but was examined in this study by all patient visits/hospitalizations occurring within 1 year of the index date as the volume of visits/hospitalizations is likely correlated with indirect costs. Patient visits/hospitalizations included all ambulatory visits, which were defined as any visit with a unique day and a unique provider identification number in an outpatient or office setting. Ambulatory visits included both clinic/physician office visits and hospital outpatient visits, and they did not necessarily result in contact with a physician.
Statistical Analyses
Mean (±SD) data were calculated per treatment episode for patient demographic and clinical characteristics, health care costs, and rates of health care resource utilization across all treatment episodes and according to treatment (BOR, THAL, LEN, OTHER). Descriptive analyses comparing data for BOR, THAL, and LEN with data for OTHER were conducted using a χ2 test (dichotomous variables) or t test (continuous variables).
In addition, multivariate regression analyses were conducted to control for possible confounding factors when comparing health care costs and health care resource utilization between treatments, including patient characteristics (age and gender), cytogenetic testing, comorbidities (DVT, PE, PN, Deyo-Charlson comorbidity score, dialysis, SCT), treatment patterns (prior treatments with OTHER, BOR, THAL, LEN), line of treatment (more prior lines of therapy are indicative of more advanced disease), and insurance type (commercial or public payer). Total health care costs were compared between BOR, THAL, and LEN versus OTHER using a generalized linear model with a gamma error distribution and log link. Similarly, rates of health care resource utilization were compared using a negative binomial model with a log link [42].
For ease of interpretation, adjusted health care costs and adjusted rates of health care resource utilization were predicted using the recycled predictions method [43]. This method calculates the mean prediction in the natural units (e.g., dollars) according to treatment, allowing a comparison of the outcomes, on average, while holding constant all other model covariates except treatment. All analyses were conducted using version 10.1 of the STATA/SE software package (Stata Corp, College Station, TX).
Results
A total of 6,762 patients with MM who received at least one treatment episode with BOR, THAL, LEN, or OTHER between January 1, 2005 and September 30, 2010 were identified. These patients had 10,623 unique treatment episodes during the study period. Approximately 46% of the treatment episodes (4,836 treatment episodes, in 2,642 patients) met all the eligibility criteria for this analysis. Of the excluded treatment episodes (n = 5,787), 2,503 and 3,055 were excluded due to patients not having 6 months of health plan eligibility before or 12 months of health plan eligibility after the index date, respectively. In addition, 218 treatment episodes were excluded due to missing demographic data or due to the index date being the same as the recorded date of death, and 11 treatment episodes were excluded due to patients being <18 years of age on the index date.
Table 1 summarizes patient demographics and clinical characteristics for all treatment episodes and by therapy. The mean age at the treatment episode index date was 62.6 years, and 52% of treatment episodes were received by men. In total, 50% of treatment episodes were administered in the southern region of the U.S., and 76% of treatment episodes were for patients enrolled in commercial insurance plans. Reflecting the commercial nature of the health plan database used for this analysis, 30% of BOR treatment episodes were received by Medicare Advantage patients, compared with 25% of OTHER treatment episodes (p = .008); rates were lower for THAL (16%, p < .001) and LEN (21%, p = .024) treatment episodes compared with OTHER.
Table 1.
Patient demographics and clinical characteristics by treatment episode and treatment episode characteristics
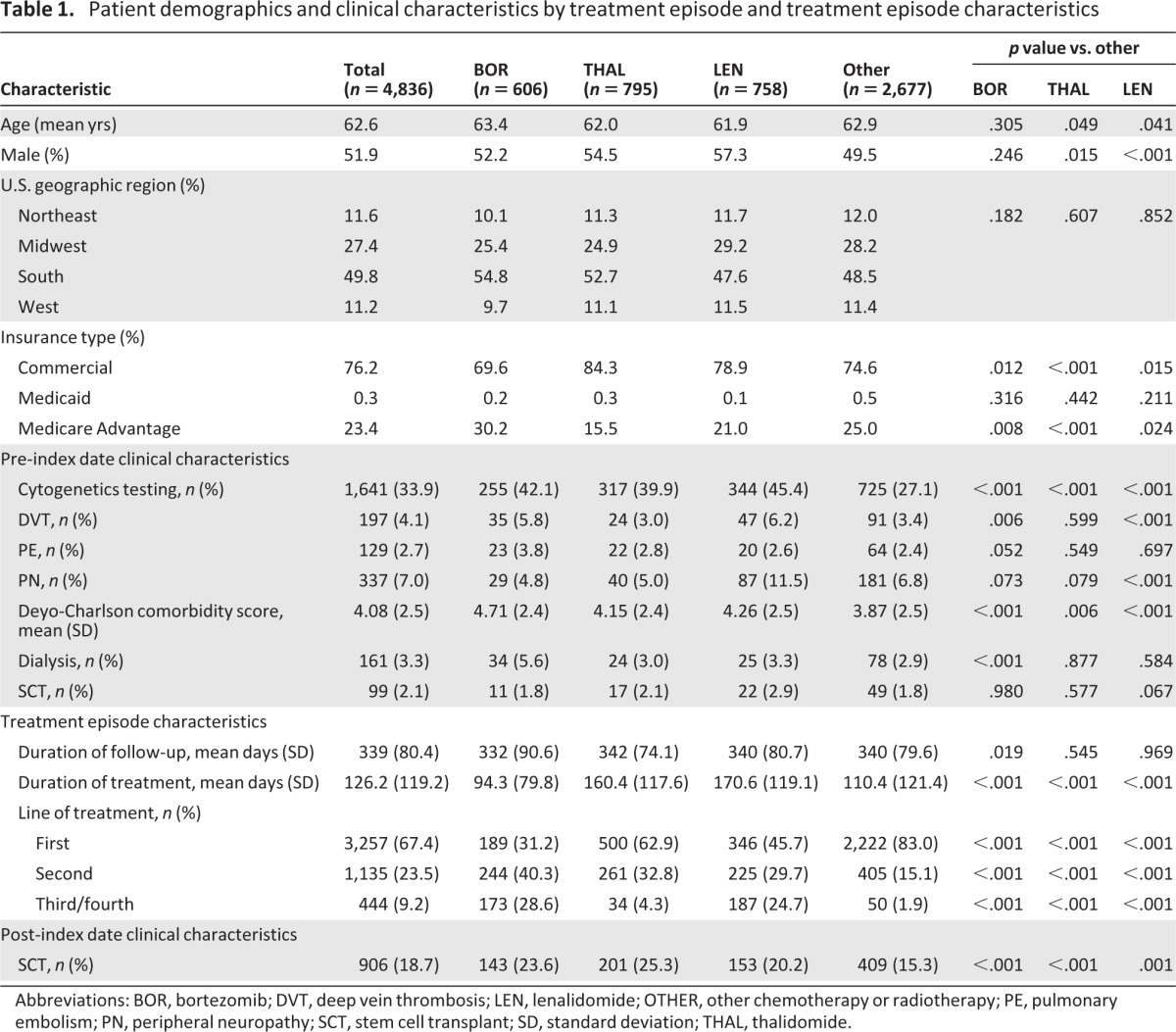
Abbreviations: BOR, bortezomib; DVT, deep vein thrombosis; LEN, lenalidomide; OTHER, other chemotherapy or radiotherapy; PE, pulmonary embolism; PN, peripheral neuropathy; SCT, stem cell transplant; SD, standard deviation; THAL, thalidomide.
There were some differences in pre-index date clinical characteristics between treatment episodes with BOR, THAL, LEN, and OTHER (Table 1). Patients receiving treatment episodes with BOR, THAL, or LEN were more likely to have undergone cytogenetic testing and to have a higher Deyo-Charlson comorbidity score than patients receiving treatment episodes with OTHER therapies. The rate of pre-index date DVT was significantly more common for treatment episodes with BOR or LEN versus OTHER and also appeared higher versus THAL, whereas the rate of pre-index date PN appeared higher with LEN compared with BOR and THAL and was significantly higher with LEN versus OTHER. The rate of post-index date SCT was significantly higher for treatment episodes with BOR, THAL, and LEN versus OTHER.
The median durations of treatment episodes were 94 (BOR), 160 (THAL), 171 (LEN), and 110 (OTHER) days, reflecting the different standard treatment paradigms for BOR (finite treatment course) and THAL/LEN (treat to progression). Overall, 67%, 24%, and 9% of all treatment episodes represented the first, second, and third/fourth lines of treatment, respectively; for BOR, THAL, LEN and OTHER treatment episodes, 31%, 63%, 46%, and 83%, respectively, were patients' first line of treatment (p < 0.001 for BOR, THAL, or LEN compared with OTHER).
Health Care Costs
Mean unadjusted 1-year total health care costs per treatment episode (i.e., costs not adjusted for differences in patient characteristics, comorbidities, or line of treatment, as assessed for 1 year from the start of treatment) were significantly higher for treatment episodes with any of the novel agents versus OTHER therapies (p < .001; Table 2). Similarly, mean unadjusted index medication costs and mean unadjusted ambulatory costs were significantly higher for treatment episodes with the novel agents versus OTHER therapies. Also, mean unadjusted inpatient hospitalization costs and retail pharmacy costs were significantly higher for treatment episodes with THAL and LEN versus OTHER therapies, but not for BOR versus OTHER. After adjusting for differences in patient characteristics, comorbidities, insurance type, and line of treatment, the predicted mean adjusted total health care costs per treatment episode (i.e., during 1 year after treatment initiation) were similar with BOR and OTHER, but significantly higher with THAL and LEN versus OTHER (Table 3). Findings were similar in an analysis restricted to treatment episodes in previously untreated patients with MM (Table 3). However, in an analysis restricted to treatment episodes for previously treated patients, only the predicted mean 1-year total health care costs associated with LEN were significantly higher versus OTHER (Table 3).
Table 2.
Unadjusted health care costs per treatment episode (i.e., during 1 year after treatment initiation), in U.S. dollars
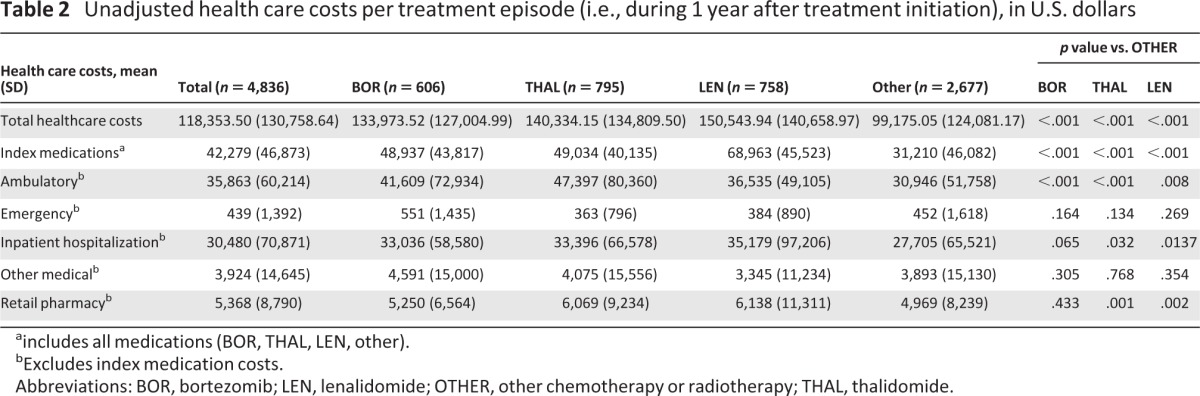
aincludes all medications (BOR, THAL, LEN, other).
bExcludes index medication costs.
Abbreviations: BOR, bortezomib; LEN, lenalidomide; OTHER, other chemotherapy or radiotherapy; THAL, thalidomide.
Table 3.
Generalized linear model, adjusting for differences in patient characteristics, comorbidities, and line of therapy, of total health care costs per treatment episode for 1 year after treatment initiation
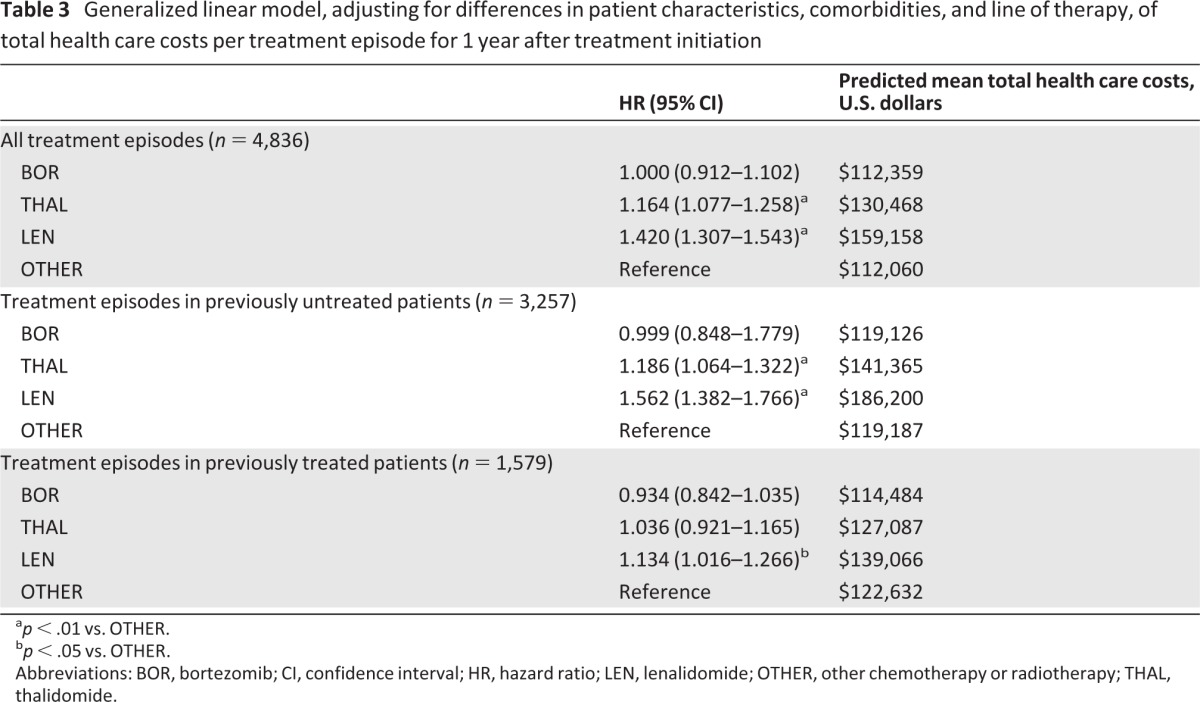
ap < .01 vs. OTHER.
bp < .05 vs. OTHER.
Abbreviations: BOR, bortezomib; CI, confidence interval; HR, hazard ratio; LEN, lenalidomide; OTHER, other chemotherapy or radiotherapy; THAL, thalidomide.
Patient Burden
Mean unadjusted patient out-of-pocket costs were significantly higher for treatment episodes with BOR ($4,422), THAL ($4,812), or LEN ($4,560) versus OTHER ($3,716; p < .001). The mean adjusted patient out-of-pocket costs per treatment episode remained higher with THAL and LEN versus OTHER (p < .01) but were not significantly different with BOR versus OTHER (Table 4). In an analysis restricted to treatment episodes in Medicare Advantage patients, the same differences were seen, but the mean unadjusted and adjusted costs with THAL ($7,559 and $7,315) and LEN ($6,457 and $6,682) appeared substantially higher overall relative to BOR ($4,573 and $4,057) and OTHER ($3,808 and $3,940) than in the analysis of all treatment episodes (Table 4). Mean unadjusted rates of health care resource utilization, in terms of numbers of ambulatory, hospital outpatient, and office visits, were significantly higher for treatment episodes with BOR, THAL, or LEN versus OTHER (Table 5). Mean numbers of emergency visits and inpatient hospitalizations were low across all therapies (Table 5).
Table 4.
Unadjusted and adjusted patient out-of-pocket costs per treatment episode for 1 year after treatment initiation, in U.S. dollars
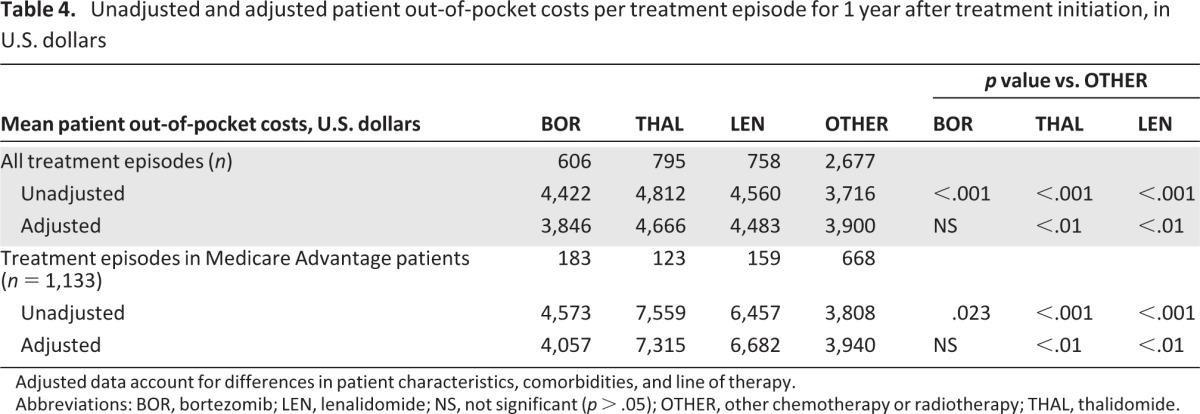
Adjusted data account for differences in patient characteristics, comorbidities, and line of therapy.
Abbreviations: BOR, bortezomib; LEN, lenalidomide; NS, not significant (p > .05); OTHER, other chemotherapy or radiotherapy; THAL, thalidomide.
Table 5.
Unadjusted rates of health care resource utilization per treatment episode (i.e., for 1 year after treatment initiation)
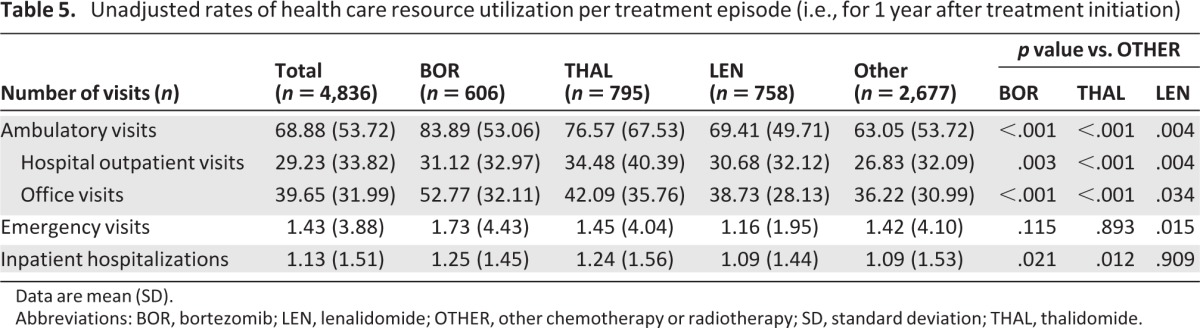
Data are mean (SD).
Abbreviations: BOR, bortezomib; LEN, lenalidomide; OTHER, other chemotherapy or radiotherapy; SD, standard deviation; THAL, thalidomide.
On multivariate regression analysis, accounting for differences in patient characteristics, comorbidities, or line of treatment, the predicted mean adjusted number of ambulatory visits per treatment episode, during 1 year after treatment initiation, appeared similar between therapies, with the rate significantly lower with LEN versus OTHER (Table 6). In an analysis restricted to treatment episodes in previously untreated patients with MM, the predicted mean adjusted rates were again similar between therapies, with no significant differences between BOR, THAL, and LEN versus OTHER (Table 6).
Table 6.
Multivariate regression analysis, adjusting for differences in patient characteristics, comorbidities, and line of therapy, of health care resource utilization per treatment episode for 1 year after treatment initiation
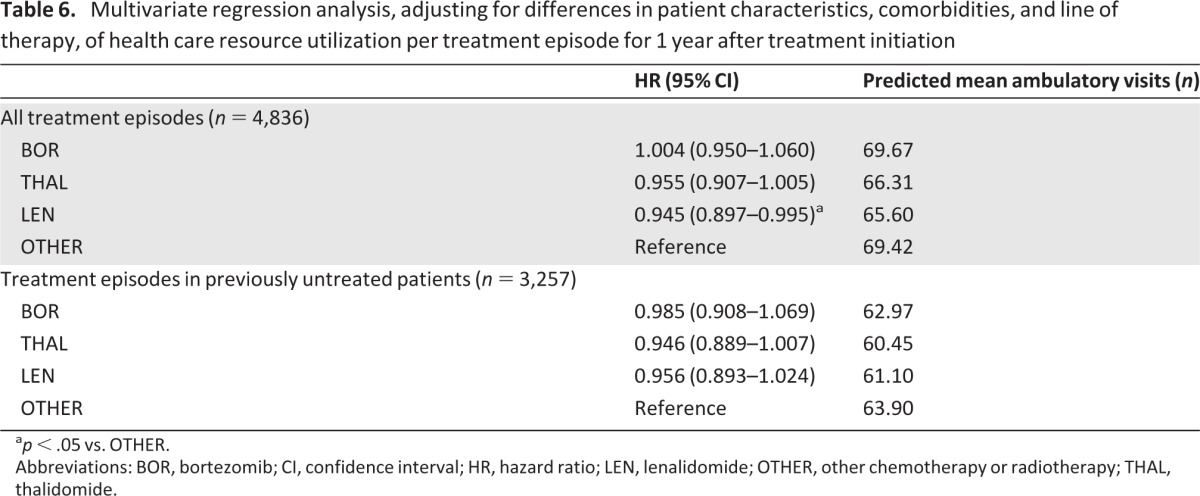
ap < .05 vs. OTHER.
Abbreviations: BOR, bortezomib; CI, confidence interval; HR, hazard ratio; LEN, lenalidomide; OTHER, other chemotherapy or radiotherapy; THAL, thalidomide.
Discussion
The improvements in clinical outcomes for patients with MM associated with the introduction and increasingly widespread use of the novel agents BOR, THAL, and LEN are well established; indeed, based on the evidence from large randomized studies, novel-agent-based regimens are among the recommended standards of care for MM treatment in the first-line [5–6, 44–45] and relapsed [46] settings, especially for SCT-eligible individuals. However, the economic factors associated with the use of these novel agents are also important considerations in today's health care environment, and the data from this retrospective cohort study using claims data from a large national U.S. commercial health plan provide real-world information regarding treatment with BOR, THAL, LEN, or OTHER therapies and the associated costs from a payer's and patient's perspective.
With this type of claims analysis, the vast majority of costs incurred by the health plan are captured. Thus, the data from this study reflect not only the specific costs and health care resource utilization (patient visits) associated with the course of treatment itself, but also the costs and visits associated with subsequent management of MM-associated symptoms, the need for further treatment, and the adverse effects of treatment within a 1-year window after the start of each of the treatment episodes with BOR, THAL, LEN, or OTHER therapies. For example, with regards to adverse effects, BOR and THAL are known to be associated with PN; THAL and LEN in combination with dexamethasone are associated with an increased risk of DVT/PE, for which specific management recommendations have been developed [47, 48]. Management of these adverse effects may have been required during treatment episodes with the respective agents.
Indeed, our pre-index date demographic and clinical characteristics data appear to reflect some of the known issues associated with specific therapies; for example, the rate of pre-index date DVT appeared lower for THAL treatment episodes, and the rate of pre-index date PN appeared lower for BOR and THAL than for LEN, possibly due to selection of treatments not associated with these side effects in an attempt to avoid exacerbation of pre-existing co-morbidities. However, a similar apparent selection bias for LEN due to pre-index date DVT was not seen, which was unexpected based on disease-management guidelines. In addition, the rate of pre-index date dialysis appeared higher for BOR treatment episodes, likely related to preferred treatment with BOR-based therapies for patients with renal impairment [49].
As might be expected, the data from this analysis showed that the mean unadjusted total health care costs, as assessed for 1 year after treatment initiation, associated with treatment episodes with novel-agent-based therapies were significantly higher than with OTHER therapies. However, after adjustment for patient characteristics, comorbidities, and line of therapy, the predicted mean total health care costs for 1 year from the start of each treatment episode remained significantly higher with THAL and LEN versus OTHER therapies, but they were similar for BOR and OTHER therapies. These findings were similar in an analysis restricted to previously untreated and previously treated patients. This suggests that the perceived higher costs of BOR as a novel therapy compared with OTHER conventional therapies are not reflected in the overall health care costs associated with each type of treatment episode.
Regarding patient out-of-pocket costs, as with total health care costs, the mean unadjusted data showed a significantly greater patient cost burden with the novel-agent-based treatment episodes compared with OTHER treatment episodes. However, the adjusted data again revealed similar patient cost burdens between BOR and OTHER treatment episodes, while THAL and LEN remained significantly higher versus OTHER. These differences for IMiD-based therapies were particularly evident in an analysis restricted to Medicare Advantage patients; this was likely due to the coverage gap, commonly known as the “donut hole,” in Medicare Part D.
Reflecting the findings for total health care and patient out-of-pocket costs, the unadjusted data on health care resource utilization in terms of ambulatory visits (including hospital outpatient and office visits) during 1 year post-treatment initiation showed a significantly higher mean rate of visits associated with BOR, THAL, and LEN versus OTHER treatment episodes. The mean number of ambulatory visits appeared somewhat higher with BOR, which is administered intravenously or subcutaneously, than with THAL and LEN, which are administered orally, as might be expected given the different routes of administration. However, after adjustment for the potential confounding factors of patient characteristics, comorbidities, and line of therapy, the predicted mean number of ambulatory visits within 1 year per treatment episode appeared similar across all therapies, with the rate for LEN being significantly lower than for OTHER. Findings were similar in an analysis restricted to the first-line setting, with no significant differences between novel-agent-based and OTHER therapies, perhaps due to treating physicians preferring to closely monitor all newly diagnosed patients regardless of therapeutic approach. Thus, although it may be thought that orally administered THAL or LEN treatment regimens might be more convenient than a BOR regimen that includes an intravenously administered agent, in terms of patient visits, the findings of this analysis show that any such perceived economic or health care utilization advantage is not borne out overall due to the need for frequent patient assessments associated with MM management and the management of treatment-related side effects.
Our analysis of real-world health care costs data from a claims database sometimes differs from other previous studies of novel-agent-based therapies for MM. For example, budget impact analyses and other modeling studies have projected the costs for various treatments for relapsed/refractory MM based on data from large-scale clinical trials [26–28] and also evaluated the cost-effectiveness of the new treatments in terms of quality-of-life years [29, 30, 33, 35–37]. Using budget impact modeling, Fullerton et al. found that the total costs associated with treatment for relapsed/refractory MM were lowest with single-agent BOR compared with THAL-dexamethasone, LEN-dexamethasone, and BOR-liposomal doxorubicin [28]. This appears to reflect the findings of our analyses of adjusted total health care costs. Conversely, using a different model employing different assumptions, Durie et al. suggested that BOR and LEN drug costs over a 12-month period were comparable in the treatment of relapsed/refractory MM, but that medical services and other costs associated with adverse events were higher with BOR [26]. These discrepancies illustrate the value of real-world data.
Nevertheless, there are a number of limitations to the present retrospective analysis. Given the recent paradigm shift in the treatment of MM, it is likely that treatment episodes with the novel agents became increasingly common compared with OTHER therapies over the time period analyzed; additionally, these treatment episodes may also have increasingly been in previously untreated patients compared with in the relapsed setting, and this may have had an impact on the findings. Moreover, as evidenced in the evolution of treatment practice guidelines [5–6, 44–46], treatment patterns (particularly in the first-line setting) have evolved to include consolidation and maintenance therapy, which will have an additional impact on health care costs and resource utilization, and regimens incorporating combinations of these novel agents, such as BOR-THAL-dexamethasone, which were excluded from the current analysis due to the initially relatively small sample size.
In addition, it should be noted that claims data are collected for the purposes of payment and not research. Claims data are subject to possible coding errors, coding for the purpose of rule-out rather than actual disease, and undercoding. Furthermore, the data used for this study came from a commercially insured and managed Medicare/Medicaid population and may not be applicable to patients in non-managed-care settings, especially in fee-for-service Medicare populations. Because MM is primarily a disease of the Medicare-age population, this group may be underrepresented in this claims analysis. Thus, the distribution of treatment episodes between therapies may not be representative of the overall MM patient population. However, it should be noted that approximately 48% of all patients in this dataset were 65 years of age or older, with an equal split between Medicare Advantage and commercially insured (primarily retirees with benefits). Additionally, extrapolation of these findings to the real-world overall population may magnify the cost differentials identified in this analysis.
It is also important to keep in mind that these results are most generalizable to the U.S. health care system because the cost differences reported here reflect U.S. unit prices. For example, a comparison of unit prices for THAL in the U.S. versus the U.K., Italy, Germany, and France over the past year revealed a unit price that was about eight times higher in the U.S. ($135.14 [€107.56]) than in these other countries (range: €12.36–14). In contrast, the unit prices for BOR and LEN were similar in the U.S. and Europe, with ranges of €962.39–1,295.89 for BOR and €199.61–300.96 for LEN in the U.K., Italy, Germany, France, and Spain versus $1,436 [€1,142.95] and $356.81 [€283.34] for BOR and LEN in the U.S. Consequently, if the unit drug prices in the U.S. had been within the range of unit prices in Europe, the cost of care in the THAL cohort would have been lower given that drug costs represent a significant proportion of total health care spending. In addition, reimbursed amounts for other health care services and treatment patterns, such as site of care, likely differs between the U.S. and other countries.
However, despite these limitations, these retrospective claims data enable real-world examination of health care costs and resource utilization in contrast to the highly controlled environment of clinical trials. In addition, administrative data also have the advantage of large sample sizes with diverse medical histories, as demonstrated in the present study.
Conclusion
Total health care costs, patient out-of-pocket costs, and resource utilization, as assessed for 1 year from the start of treatment, differ between treatment episodes with the novel agents BOR, THAL, and LEN and OTHER therapies. Adjusted analyses of real-world claims data, accounting for differences in patient characteristics, comorbidities, and line of treatment, show that total health care costs are similar for BOR but significantly higher for THAL and LEN treatment compared with OTHER therapies. Importantly, these differences are also seen in the patient out-of-pocket costs associated with each treatment. Additionally, adjusted analyses show similar rates of patient ambulatory visits per treatment episode across therapies, suggesting that any perceived advantage in patient convenience of the orally administered drugs THAL and LEN, in terms of fewer hospital outpatient/office visits, is not supported by these data from the real-world setting.
Acknowledgments
The authors would like to acknowledge the writing assistance of Tara Cowling, of Medlior Health Outcomes, and Steve Hill, of FireKite, during the development of this manuscript, which was supported by Millennium Pharmaceuticals.
Footnotes
Author Contributions
Conception and design: April Teitelbaum, Abbie Ba-Mancini, Henry J. Henk
Provision of study materials or patients: Henry J. Henk
Collection and/or assembly of data: Henry J. Henk
Data analysis and interpretation: April Teitelbaum, Abbie Ba-Mancini, Hui Huang, Henry J. Henk
Manuscript writing: April Teitelbaum, Abbie Ba-Mancini, Hui Huang, Henry J. Henk
Final approval of manuscript: Abbie Ba-Mancini, Hui Huang, Henry J. Henk
Disclosures
Abbie Ba-Mancini: Millennium Pharmaceuticals (E); Hui Huang: Millennium Pharmaceuticals (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. [Accessed October 20, 2012]. Available at http://seer.cancer.gov/csr/1975_2008/
- 2.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Rajkumar SV. Treatment of multiple myeloma: A comprehensive review. Clin Lymphoma Myeloma. 2009;9:278–288. doi: 10.3816/CLM.2009.n.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Cavo M, Rajkumar SV, Palumbo A, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117:6063–6073. doi: 10.1182/blood-2011-02-297325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation. Leukemia. 2009;23:1716–1730. doi: 10.1038/leu.2009.122. [DOI] [PubMed] [Google Scholar]
- 7.Bertolotti P, Bilotti E, Colson K, et al. Management of side effects of novel therapies for multiple myeloma: Consensus statements developed by the International Myeloma Foundation's Nurse Leadership Board. Clin J Oncol Nurs. 2008;12:9–12. doi: 10.1188/08.CJON.S1.9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snowden JA, Ahmedzai SH, Ashcroft J, et al. Guidelines for supportive care in multiple myeloma 2011. Br J Haematol. 2011;154:76–103. doi: 10.1111/j.1365-2141.2011.08574.x. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology Multiple Myeloma (V1.2012) [Accessed October 20, 2012]. Available at http://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdfy. [DOI] [PubMed]
- 10.Harousseau JL, Dreyling M. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v155–v157. doi: 10.1093/annonc/mdq178. [DOI] [PubMed] [Google Scholar]
- 11.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 12.Brenner H, Gondos A, Pulte D. Expected long-term survival of patients diagnosed with multiple myeloma in 2006–2010. Haematologica. 2009;94:270–275. doi: 10.3324/haematol.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): An analysis of the Greek Myeloma Study Group (GMSG) Leukemia. 2009;23:1152–1157. doi: 10.1038/leu.2008.402. [DOI] [PubMed] [Google Scholar]
- 14.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fayers PM, Palumbo A, Hulin C, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: Meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118:1239–1247. doi: 10.1182/blood-2011-03-341669. [DOI] [PubMed] [Google Scholar]
- 16.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: A randomized controlled trial. J Clin Oncol. 2010;28:5101–5109. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 17.Palumbo A, Adam Z, Kropff M, et al. A phase 3 study evaluating the efficacy and safety of lenalidomide (len) combined with melphalan and prednisone followed by continuous lenalidomide maintenance (MPR-R) in patients (pts) 65 years (yrs) with newly diagnosed multiple myeloma (NDMM): Updated results for pts aged 65–75 yrs enrolled in MM-015. Blood. 2011;118:220–221. [Google Scholar]
- 18.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 19.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 20.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: Results of the IFM 2005–01 phase III trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 21.Rosinol L, Cibeira MT, Mateos MV, et al. A phase III PETHEMA/GEM study of induction therapy prior autologous stem cell transplantation (ASCT) in multiple myeloma: superiority of VTD (bortezomib/thalidomide/dexamethasone) over TD and VBMCP/VBAD plus bortezomib. Blood. 2010;116:139a–140a. [Google Scholar]
- 22.Sonneveld P, Schmidt-Wolf I, van der Holt B, et al. HOVON-65/GMMG-HD4 randomized phase III trial comparing bortezomib, doxorubicin, dexamethasone (PAD) vs VAD followed by high-dose melphalan (HDM) and maintenance with bortezomib or thalidomide in patients with newly diagnosed multiple myeloma (MM) Blood. 2010;116:23a–24a. [Google Scholar]
- 23.Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23:2147–2152. doi: 10.1038/leu.2009.147. [DOI] [PubMed] [Google Scholar]
- 24.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: Final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 25.Blank PR, Levin R, Pestalozzi BC, et al. Cost differences among treatment options for patients with refractory myeloma previously treated with high-dose chemotherapy and autologous stem-cell transplantation: An analysis from the U.S. and Swiss perspectives. J Clin Oncol. 2011;29:e16569. [Google Scholar]
- 26.Durie BG, Borrello I, Binder G, et al. Treatment cost comparison in relapsed multiple myeloma. Haematologica. 2011;96:S140. [Google Scholar]
- 27.Fullerton DS, Huleatt H, Marantz JL, et al. Treatment of relapsed myeloma: A budget impact model comparing single agent bortezomib with combination lenalidomide and high-dose dexamethasone. J Manag Care Pharmacy. 2007;13:706–707. [Google Scholar]
- 28.Fullerton DSP, Trautman H, Huang H, et al. A budget impact model comparing resource utilization of four approved therapies for multiple myeloma (MM) in the US. Blood. 2007;110:3324. [Google Scholar]
- 29.Moller J, Nicklasson L, Murthy A. Cost-effectiveness of novel relapsed-refractory multiple myeloma therapies in Norway: Lenalidomide plus dexamethasone vs bortezomib. J Med Econ. 2011;14:690–697. doi: 10.3111/13696998.2011.611841. [DOI] [PubMed] [Google Scholar]
- 30.Schey S, Stern S, Dhanasiri S, et al. Cost-effectiveness of lenalidomide in multiple myeloma patients with 1 prior therapy in England and Wales. Blood. 2011;118:1789. doi: 10.1007/s10198-012-0395-6. [DOI] [PubMed] [Google Scholar]
- 31.Messori A, Maratea D, Nozzoli C, et al. The role of bortezomib, thalidomide and lenalidomide in the management of multiple myeloma: An overview of clinical and economic information. Pharmacoeconomics. 2011;29:269–285. doi: 10.2165/11585930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Armoiry X, Fagnani F, Benboubker L, et al. Management of relapsed or refractory multiple myeloma in French hospitals and estimation of associated direct costs: A multi-centre retrospective cohort study. J Clin Pharm Ther. 2011;36:19–26. doi: 10.1111/j.1365-2710.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 33.Hornberger J, Rickert J, Dhawan R, et al. The cost-effectiveness of bortezomib in relapsed/refractory multiple myeloma: Swedish perspective. Eur J Haematol. 2010;85:484–491. doi: 10.1111/j.1600-0609.2010.01526.x. [DOI] [PubMed] [Google Scholar]
- 34.Koleva D, Cortelazzo S, Toldo C, et al. Healthcare costs of multiple myeloma: An Italian study. Eur J Cancer Care (Engl) 2011;20:330–336. doi: 10.1111/j.1365-2354.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 35.Schey S, Higginson I. Cost-effectiveness of lenalidomide in multiple myeloma. Expert Rev Pharmacoecon Outcomes Res. 2010;10:229–238. doi: 10.1586/erp.10.19. [DOI] [PubMed] [Google Scholar]
- 36.Wang S-T, Huang H, Shi H, et al. The cost-effectiveness of bortezomib for the initial treatment of multiple myeloma in the United States. Blood. 2009;114:561–562. [Google Scholar]
- 37.Wang S-T, Huang H, Ba-Mancini A, et al. The cost-effectiveness of bortezomib plus melphalan and prednisone versus lenalidomide plus melphalan and prednisone with continuous lenalidomide maintenance treatment for the initial treatment of multiple myeloma In the United States. Blood. 2010;116:1060–1061. [Google Scholar]
- 38.Amonkar MM, Chastek B, Samant N, et al. Economic burden of resected squamous cell carcinoma of the head and neck in a US managed-care population. J Med Econ. 2011;14:421–432. doi: 10.3111/13696998.2011.584096. [DOI] [PubMed] [Google Scholar]
- 39.Baser O, Wei W, Henk HJ, et al. Patient survival and healthcare utilization costs after diagnosis of triple-negative breast cancer in a United States managed care cancer registry. Curr Med Res Opin. 2012;28:419–428. doi: 10.1185/03007995.2011.628649. [DOI] [PubMed] [Google Scholar]
- 40.Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: A retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25:481–496. doi: 10.2165/00019053-200725060-00004. [DOI] [PubMed] [Google Scholar]
- 41.Lash S, Teitelbaum A, Henk HJ, et al. Costs associated with second-line therapies for lung cancer. Am J Pharmacy Benefits. 2011;3:21–29. [Google Scholar]
- 42.Cameron AC, Trivedi PK. Econometric Society Monograph No. 30. New York: Cambridge University Press; 1998. Regression analysis of count data. [Google Scholar]
- 43.Basu A, Rathouz PJ. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6:93–109. doi: 10.1093/biostatistics/kxh020. [DOI] [PubMed] [Google Scholar]
- 44.Ludwig H, Beksac M, Blade J, et al. Multiple myeloma treatment strategies with novel agents in 2011: A European perspective. The Oncologist. 2011;16:388–403. doi: 10.1634/theoncologist.2010-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palumbo A, Attal M, Roussel M. Shifts in the therapeutic paradigm for patients newly diagnosed with multiple myeloma: Maintenance therapy and overall survival. Clin Cancer Res. 2011;17:1253–1263. doi: 10.1158/1078-0432.CCR-10-1925. [DOI] [PubMed] [Google Scholar]
- 46.van de Donk NW, Lokhorst HM, Dimopoulos M, et al. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev. 2011;37:266–283. doi: 10.1016/j.ctrv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595–608. doi: 10.1038/leu.2011.346. [DOI] [PubMed] [Google Scholar]
- 48.Zamagni E, Brioli A, Tacchetti P, et al. Multiple myeloma, venous thromboembolism, and treatment-related risk of thrombosis. Semin Thromb Hemost. 2011;37:209–219. doi: 10.1055/s-0031-1273085. [DOI] [PubMed] [Google Scholar]
- 49.Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: A consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976–4984. doi: 10.1200/JCO.2010.30.8791. [DOI] [PubMed] [Google Scholar]