Chemotherapy effectiveness in clinical practice may differ from the efficacy demonstrated in clinical trials, particularly among populations underrepresented in clinical trials, such as elderly patients with cancer. This review suggests that without other reasons for withholding treatment, elderly patients with stage III colon cancer should receive chemotherapy as often as nonelderly patients.
Keywords: Colorectal neoplasms, Aged, Drug therapy, Comparative effectiveness research, Treatment outcome
CME Learning Objectives
Describe evidence of differential treatment response of chemotherapy in elderly versus nonelderly stage III colon cancer patients.
Synthesize differences in evidence of effectiveness and safety of chemotherapy between elderly and nonelderly stage III colon cancer patients to inform patient decision making and physician prescribing practices.
Abstract
Background.
Chemotherapy effectiveness in clinical practice may differ from the efficacy demonstrated in clinical trials, particularly among populations underrepresented in clinical trials, such as elderly patients with cancer. This review aims to examine the relative effectiveness of chemotherapy for stage III colon cancer in elderly versus nonelderly patients.
Methods.
A systematic literature review was conducted using the Agency for Healthcare Research and Quality approach. Literature searches were performed in Medline and Evidence-Based Medicine Reviews databases. Chemotherapy regimens approved for stage III colon cancer were reviewed. Four effectiveness and 15 safety outcomes were extracted.
Results.
From 708 identified articles, 25 articles provided data on the relative effectiveness and safety of chemotherapy among elderly versus nonelderly patients. Four of 14 studies showed lower overall survival treatment effects, whereas one of five and one of four studies indicated more favorable treatment effects for time to progression and overall response rate. Grade 3 or 4 adverse events were higher among elderly patients for cardiac disorder (2/5 studies), leukopenia (1/5), neutropenia (4/16), thrombocytopenia (2/13), febrile neutropenia (1/4), infection (2/10), dehydration (2/6), diarrhea (6/20), and fatigue (6/13). Grade 3 or 4 adverse events were lower for neutropenia (2/16 studies), nausea/vomiting (1/16), and neuropathy (1/9).
Conclusion.
The majority of the evidence suggests that chemotherapy has similar relative effectiveness and safety for patients >65 years of age versus younger patients with stage III colon cancer. When differences are reported, treatment effects are more often worse among the elderly. This review suggests that without other reasons for withholding treatment, elderly patients should receive chemotherapy as often as nonelderly patients.
Implications for Practice:
The underrepresentation of elderly patients from clinical trials has led to uncertainty regarding the efficacy of chemotherapy agents among the elderly. This uncertainty has contributed to underuse in the elderly population. The evidence from this systematic review suggests that colon cancer chemotherapy effectiveness and safety generally are similar in elderly and nonelderly patients; however, there is some evidence of a higher incidence of adverse events in elderly versus nonelderly patients. This systematic review concludes that chemotherapy prescribing decisions for colon cancer should not be based upon age, but rather on other factors such as performance status. Overall, the evidence does not suggest lower chemotherapy effectiveness among elderly patients. Thus, this review does not support observed lower chemotherapy utilization in the elderly population. In the absence of other reasons for withholding treatment, elderly patients should be given chemotherapy as often as nonelderly patients.
Introduction
Comparative effectiveness research (CER) is defined as the “generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition, or to improve the delivery of care” [1]. The purpose of CER is to provide consumers, clinicians, payers, and policy makers with information so they can make better health care decisions and use health care resources more effectively [1]. CER promotes personalized medicine and helps patients and their health care providers know if a treatment will work specifically for them. This is a challenge for elderly patients because much of this evidence comes from clinical trials, and elderly patients are often underrepresented in trials. A systematic approach to reviewing evidence may provide CER for a broad array of patients.
Systematic reviews assist in developing evidence-based treatment protocols and health care decision making. They are good tools that objectively gather and summarize all available evidence for decision makers. They can be used to develop practice guidelines, as well as risk assessments, economic analyses, and decision analyses [1–4]. They also point out the gaps in medical research [5, 6]. Thus, clinicians can read them to keep up with advancements in their fields, policy makers use them to decide what types of health care to provide, and granting agencies may require them to justify further research. Systematic reviews have been used to address the CER priorities set by the Institute of Medicine (IOM) [7]. Three of the IOM top 25 priorities for CER involve cancer. One priority is to compare the effectiveness of imaging techniques to help with diagnosing, staging, and monitoring the cancer [7]. For colorectal cancer (CRC), numerous systematic reviews answered this priority, looking at the use and quality of CRC screening, including symptom patterns and additional diagnostic tests, positron emission tomography and computed tomography (CT) screening, CT colonography and optical colonoscopy, and a diagnostic sentinel lymph node procedure [8–12].
A second IOM top 25 priority is to compare the effectiveness of genetic and biomarker testing and usual care in preventing and treating cancer [7]. In response to this, a systematic review found that microsatellite instability can be used to predict nonresponse of adjuvant chemotherapy for CRC [13]. A third IOM top 25 priority is to compare the effectiveness of interventions to reduce health disparities in cancer outcomes [7]. Numerous systematic reviews have been conducted on the effectiveness of various interventions, including salvage surgery, intraoperative radiotherapy, neoadjuvant chemotherapy, perioperative chemotherapy, adjuvant chemotherapy, and specific chemotherapeutic drugs, such as irinotecan and bevacizumab [14–21].
Management of elderly (>65 years old) cancer patients has been challenging because elderly patients are frequently excluded from clinical trials; therefore, clinicians are often unsure how elderly cancer patients will respond to treatments. For colorectal cancer, more than 40% of new cases occur in patients older than 75 years of age [22].
There is limited evidence regarding how chemotherapy impacts the prognosis of elderly patients compared to nonelderly patients. Chemotherapy could have a lower or higher effectiveness in elderly versus nonelderly patients. Chemotherapy could also result in a higher or lower incidence of specific adverse events in elderly versus nonelderly patients. The aim of this systematic review is to synthesize the available evidence and determine if there is a differential relative effectiveness in chemotherapy for stage III colon cancer between elderly and nonelderly patients.
Methods
This systematic review of clinical trials and effectiveness studies follows the approach recommended by the Agency for Healthcare Research and Quality [23]. These guidelines discuss topic refinement, analytic frameworks, study eligibility criteria, searching for relevant articles, when to select observational studies as evidence, data extraction, assessing the quality of individual studies, assessing applicability, presentation of findings, quantitative synthesis, grading strength of evidence, and reporting the review.
Search Strategy and Selection Criteria
Seven searches were performed in Medline and Evidence-Based Medicine Reviews (Cochrane Database of Systematic Reviews, American College of Physicians Journal Club, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Health Technology Assessment, National Health Service Economic Evaluation Database) databases on June 15–16, 2011. The searches used combinations of medical subject heading search terms: “colorectal neoplasms,” “adenomatous polyposis coli,” “colonic neoplasms,” “colorectal neoplasms, hereditary nonpolyposis,” “therapeutics,” “efficacy,” “effectiveness,” “treatment outcome,” “survival benefit,” “surgery,” “chemotherapy,” “drug therapy,” “elderly,” “all aged (65 and over),” “age factors,” “Medicare,” “SEER Program,” “cost,” “cost analysis,” “economics,” and “health care economics and organizations.” Articles were limited to studies written in English, pertaining to humans, and published from 2001 to 2011. The search strategies are provided as supplemental online data.
Reference sections of identified articles were scanned for additional relevant articles. Articles were kept if they met the following inclusion criteria: (a) Patients had stage III colon cancer, (b) treatment being studied was recommended chemotherapy for CRC as per National Comprehensive Cancer Network (NCCN) guidelines [24], (c) study looked at effectiveness of chemotherapy, (d) study included patients older than 65 years, and (e) study was a phase II, III, or IV trial or observational study with empirical analysis. Data were not extracted from reviews; instead, the studies reported in previous reviews were included in this study. Levels of evidence were not used in assessing the value of each publication selected for inclusion. Unpublished material was not included.
Data Extraction
Information regarding study characteristics (see supplemental online data) and study design were extracted. Point estimates, p values, and confidence intervals for effectiveness (overall survival, disease-free survival, time to progression, overall response rate, complete response, partial response, stable disease, progressive disease, tumor control rate) and safety outcomes (rate of adverse events) were extracted. It was decided a priori to accept age subgroups as presented in articles but to attempt to examine the effectiveness of chemotherapy among elderly (i.e., at least 65 years old) compared with nonelderly patients with stage III colon cancer. Authors were contacted for missing values if results were not broken down by age group.
Statistical Analysis
Data were not combined using a meta-analysis due to the heterogeneity of population and setting. Instead, each effectiveness outcome was examined within each study to determine whether the results showed statistically significant differences between elderly versus nonelderly patients with stage III colon cancer. A χ2 test for homogeneity was performed for safety outcome estimates if p values were not provided in the source article. Fisher's exact test was used if the χ2 test could not be used.
Determining Differential Relative Effectiveness
Differential relative effectiveness between elderly and nonelderly patients was determined by comparing a reported estimate for elderly patients to a reported estimate for nonelderly patients for the same effectiveness or safety parameter. For instance, given overall survival values, if the overall survival value reported for elderly patients was not statistically significantly different from that reported for nonelderly patients, then that study was indicative of similar effectiveness between elderly and nonelderly patients based on overall survival values.
If there was a statistically significant difference, then there were two possible results. If the overall survival value reported for elderly patients was lower than that reported for nonelderly patients, then that study was demonstrative of less effectiveness in elderly versus nonelderly patients. On the contrary, if the overall survival value reported for elderly patients was higher than that reported for nonelderly patients, then the study was indicative of more effectiveness in elderly versus nonelderly patients. The same process was repeated for all reported values of all effectiveness and safety parameters.
Color Coding in Figures
When displaying summary results, figures were color-coded to demonstrate how many studies reported effectiveness parameters indicating higher, similar, or lower relative effectiveness in elderly versus nonelderly patients. Red indicates that chemotherapy was less effective in elderly versus nonelderly patients based on comparing the reported estimates for a specific effectiveness parameter between elderly and nonelderly patients. Blue means that chemotherapy was similarly effective in elderly versus nonelderly patients because there was no statistically significant difference between the reported estimates for a specific effectiveness parameter for elderly versus nonelderly patients. Green means that chemotherapy was more effective in elderly versus nonelderly patients based on comparing the reported estimates for a specific effectiveness parameter between elderly and nonelderly patients.
Only four studies reported overall survival hazard ratios indicating less relative chemotherapy effectiveness in elderly versus nonelderly patients. The majority of studies reporting overall survival (10/14 studies) indicate similar chemotherapy effectiveness in elderly versus nonelderly patients.
A similar coding scheme was used to show how many studies reporting safety outcomes indicated higher, similar, or lower rates of adverse events in elderly versus nonelderly patients. Red indicates that there was a higher incidence of a specific adverse event in elderly versus nonelderly patients for that study. Blue means that there was no statistically significant difference in incidence of the specific adverse event in elderly versus nonelderly patients for that study. Green means that there was a lower incidence of the specific adverse event in elderly versus nonelderly patients for that study.
Results
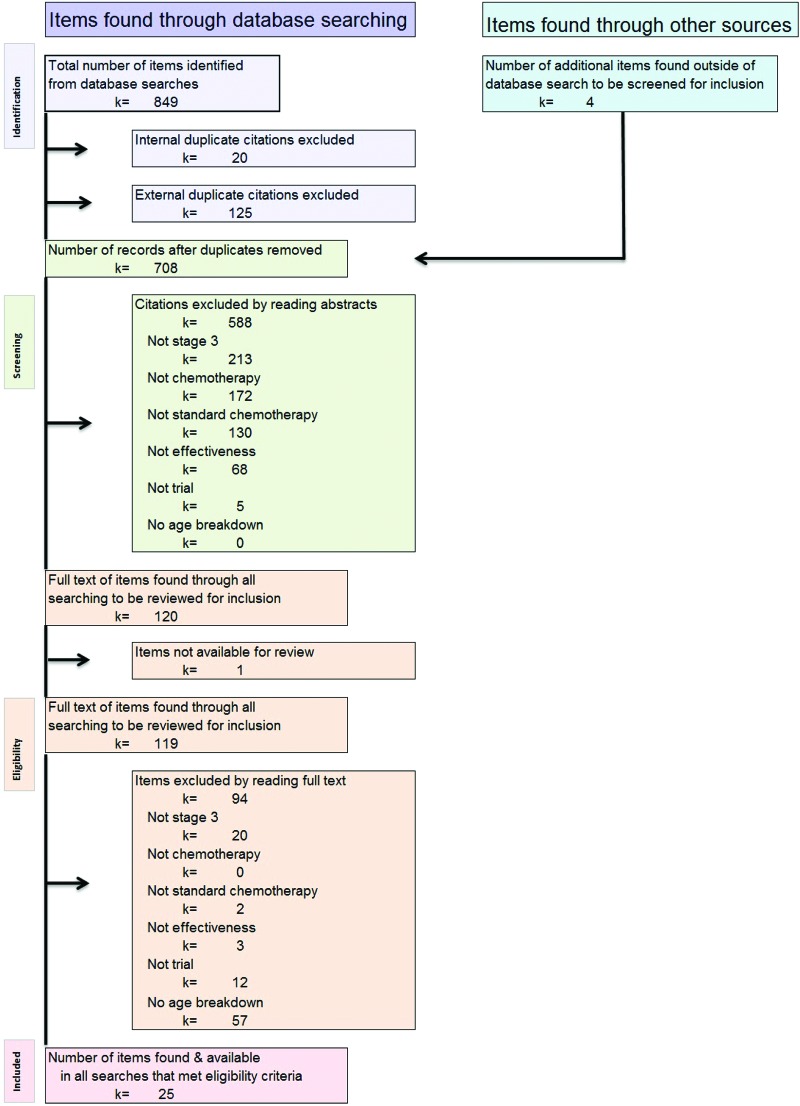
The systematic literature search identified 708 articles (after removing duplicates). Figure 1 shows the selection process. Data were extracted from the 25 articles that met all inclusion and exclusion criteria. Within the 25 articles, there were 39 studies administering various chemotherapy regimens. These studies were either observational studies or trials. Each study reported estimates of various effectiveness and/or safety parameters. Of the 39 studies, 27 were trials and 12 were observational studies.
Figure 1.
Flow diagram of systematic review article selection process, showing how articles were selected for inclusion in this review.
Chemotherapy Regimens
The NCCN-recommended chemotherapy regimens for stage III colon cancer were categorized into regimens including four of the following: 5-fluorouracil and leucovorin alone (5-FU/LV), 5-FU/LV and oxaliplatin (FOLFOX), capecitabine alone (Cap), or Cap and Ox (CapeOx) [25]. The fifth category of chemotherapy regimens consists of regimens using irinotecan (Iri) alone or with 5-FU/LV or Cap. The sixth category of chemotherapy regimens consists of regimens using bevacizumab (Bev) alone or with 5-FU/Ox. According to NCCN guidelines, irinotecan and bevacizumab are both recommended for metastasized colon cancer. However, in the studies that included both stage III and IV patients, it was unclear if irinotecan and bevacizumab were used for only stage IV or both stage III and IV cancer. The seventh category of chemotherapy regimens encompasses two studies that used multiple chemotherapy regimens. One study included 5-FU/Ox or 5-FU/Iri or 5-FU alone, or any one of the three options with bevacizumab or cetuximab. The second study included 5-FU/LV or CapeOx. Exact chemotherapy regimens for each of the 39 studies extracted are described in the supplemental online data along with other study characteristics, including author, year, study type, sample size, and age breakdown.
The number of studies producing estimates for various effectiveness parameters for each chemotherapy category is given in Table 1. Nine such studies provided effectiveness results for FOLFOX, four studies for CapeOx, three studies for Cap alone, and 14 studies for 5-FU/LV. Other regimens included irinotecan and bevacizumab. Five studies used Iri-based regimens, and two studies used regimens adding bevacizumab. Two studies used multiple regimens, as specified above. In total, 23 studies published effectiveness estimates and 28 studies published safety estimates (Table 1).
Table 1.
Number of studies reporting effectiveness and safety outcomes by chemotherapy type
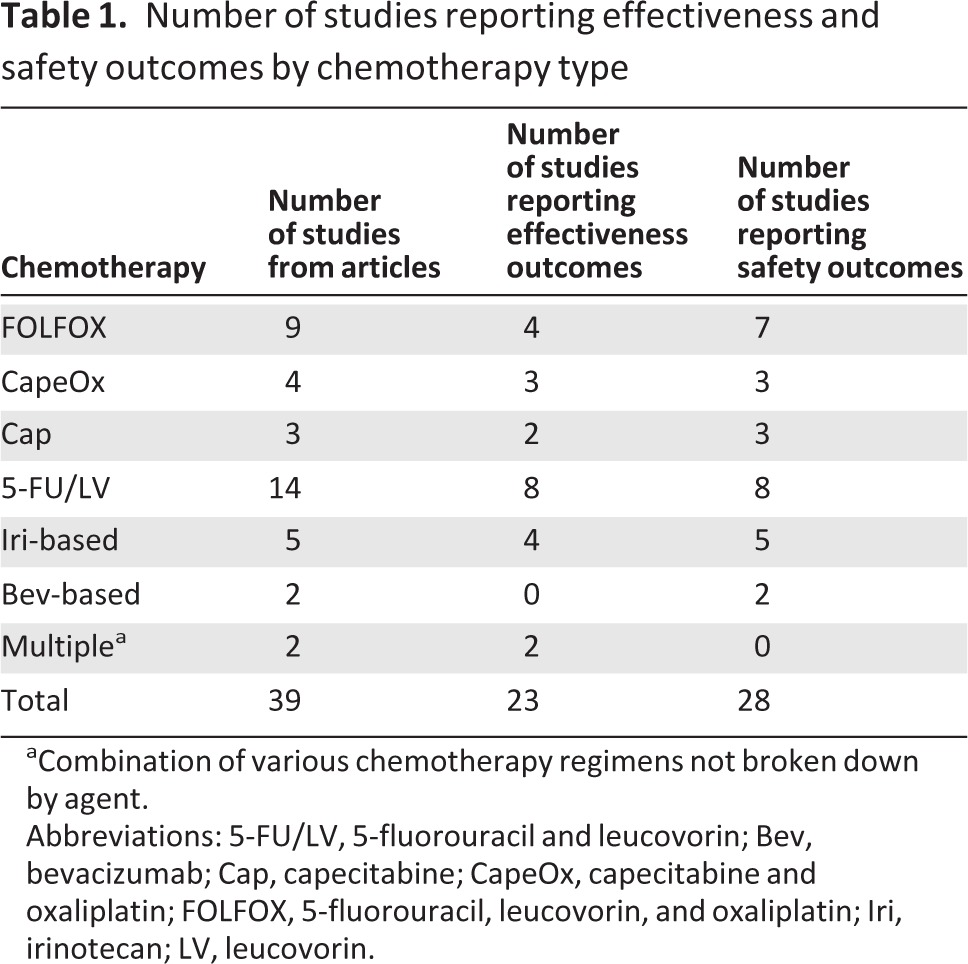
aCombination of various chemotherapy regimens not broken down by agent.
Abbreviations: 5-FU/LV, 5-fluorouracil and leucovorin; Bev, bevacizumab; Cap, capecitabine; CapeOx, capecitabine and oxaliplatin; FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; Iri, irinotecan; LV, leucovorin.
Overall Survival
Overall survival (OS) point estimates for different age groups were given in 20 studies. Figure 2 presents these values. The data from Figure 2 is presented in chart form in Figure 3. The six studies that did not directly compare OS estimates between elderly and nonelderly patients were omitted from Figure 3. Figure 3 shows the relative effectiveness of various chemotherapy regimens in elderly versus nonelderly patients based on reported overall survival values.
Figure 2.
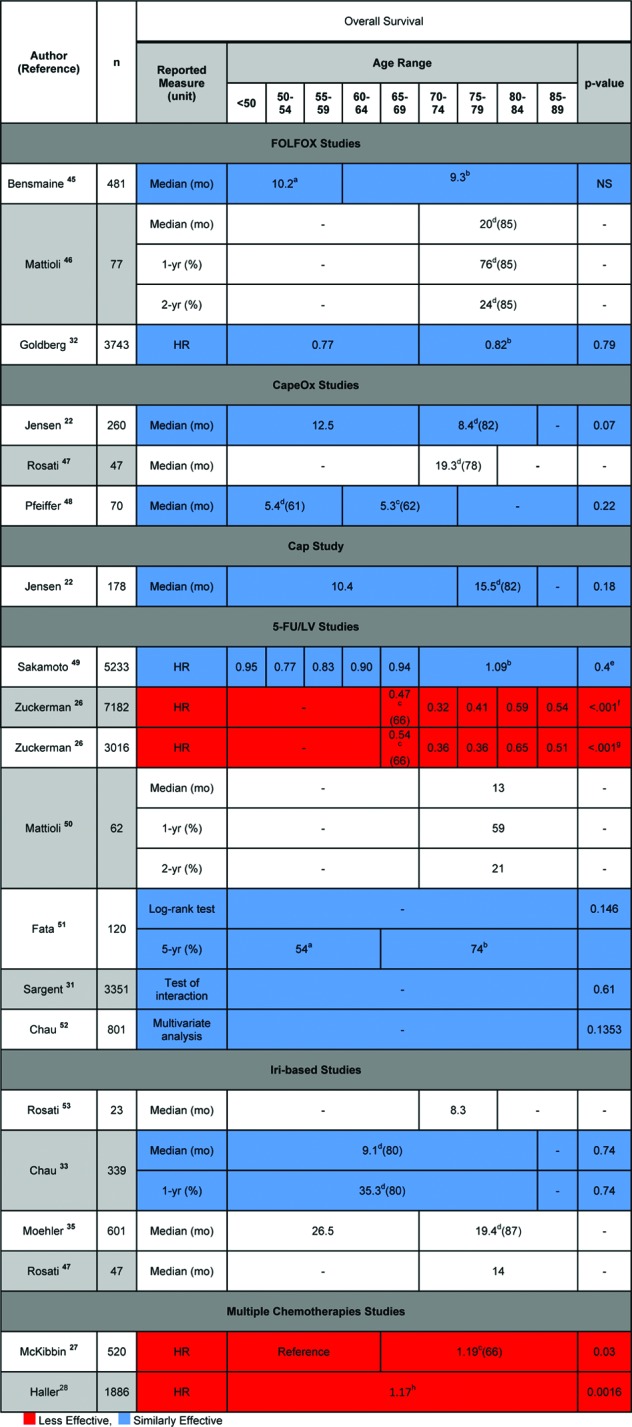
Reported estimates of overall survival for various chemotherapies by age group.
aLowest age unknown.
bHighest age unknown.
cMinimum age of subgroup given in parentheses following superscript.
dMaximum age of subgroup given in parentheses following superscript.
ep value for overall trend.
fp value for each age group.
gp value for each age group was reported as <.001, except for .006 for ages 65–69.
hHazard ratio of age using 10-year increments.
Abbreviations: 5-FU/LV, 5-fluorouracil and leucovorin; Bev, bevacizumab; Cap, capecitabine; FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio; Iri, irinotecan; LV, leucovorin; NS, not significant.
Figure 3.
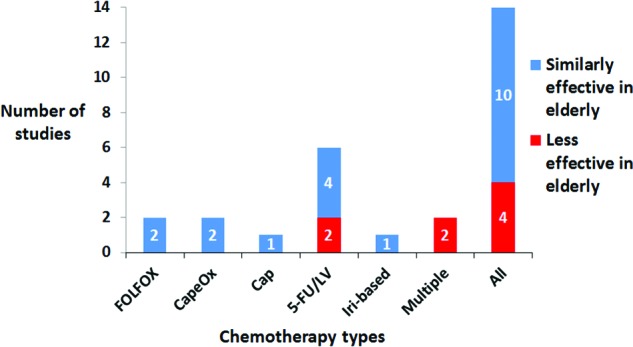
Relative overall survival impact of chemotherapies in elderly versus nonelderly patients with stage III colon cancer. The number of studies indicating differential relative effectiveness according to overall survival values for various chemotherapy regimens when comparing elderly and nonelderly patients with stage III colon cancer are shown.
Abbreviations: 5-FU/LV, 5-fluorouracil and leucovorin; Cap, capecitabine; CapeOx, capecitabine and oxaliplatin; FOLFOX, 5-fluorouracil, leucovorin, and oxaliplatin; Iri, irinotecan.
In addition to overall survival values, 1-year, 2-year, and 5-year survival percentage, and overall survival hazard ratio values are given. Only four studies (in red) reported overall survival hazard ratios indicating less relative chemotherapy effectiveness in elderly versus nonelderly patients. The majority of studies reporting overall survival (10/14 studies) indicate similar chemotherapy effectiveness (in blue) in elderly versus nonelderly patients.
There were four studies indicating less relative chemotherapy effectiveness in elderly versus nonelderly patients: two studies in which patients used 5-FU/LV chemotherapy and two studies in which patients used multiple chemotherapies. The two studies in which patients were given 5-FU/LV chemotherapy came from the same article by Zuckerman et al. [26]. The first study used the adjusted overall sample and the second study used the adjusted propensity score matched sample. In both studies, OS hazard ratios between various age groups (65–69, 70–74, 75–79, 80–84, and 85–89 years) were analyzed and p values were reported as <.001 for each age group (except for a reported p value of .006 for ages 65–69). In the first study, the OS hazard ratios were 0.47 (65–69), 0.32 (70–74), 0.41 (75–79), 0.59 (80–84), and 0.54 (85–89). In the second study, the OS hazard ratios were 0.54 (65–69), 0.36 (70–74), 0.36 (75–79), 0.65 (80–84), and 0.51 (85–89).
The third study by McKibbin et al. used multiple chemotherapies (either 5-FU/Ox or 5-FU/Iri or 5-FU alone, or any one of the three options with bevacizumab or cetuximab) [27]. McKibbin et al. compared OS adjusted hazard ratios between patients aged >65 (1.19 with a 95% confidence interval of 1.02–1.39) and ≤65 (reference value) and determined a p value of .03. The fourth study by Haller et al. used multiple chemotherapies (either CapeOx or 5-FU/LV given as a Mayo Clinic or Roswell Park regimen) [28]. Haller et al. found that age was associated with less chemotherapy benefit according to an overall survival hazard ratio (1.17, 95% confidence interval = 1.06–1.28, p = .0016).
Other Effectiveness Outcomes
The other effectiveness outcomes extracted in this systematic review included progression-free survival (PFS) or disease-free survival (DFS), time to progression (TTP), and overall response rate (ORR). Figure 4 gives a summary of effectiveness results and uses the color coding described earlier. PFS/DFS values were similar between elderly and nonelderly patients in all four studies. Most TTP (4/5 studies) and ORR (3/4 studies) values were similar between elderly and nonelderly patients.
Figure 4.
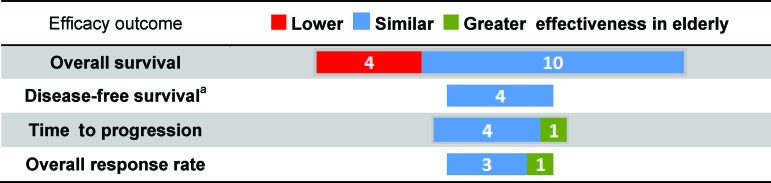
Relative effectiveness of chemotherapies in elderly versus nonelderly patients with stage III colon cancer. The number of studies indicating differential relative effectiveness between elderly and nonelderly patients with stage III colon cancer is shown according to reported overall survival, disease-free survival, time to progression, and overall response rates.
aOne study provided progression-free survival estimates.
One study by Jensen et al. indicated that capecitabine chemotherapy was more effective in elderly (≥75) than nonelderly (<75) patients according to both TTP and ORR values [22]. TTP values were reported as 8.4 and 4.1 months for elderly and nonelderly patients, respectively, with a p value of .001, hazard ratio of 0.35, and confidence interval of 0.29–0.80. ORR values were reported as 72% and 31% for elderly and nonelderly patients, respectively, with a p value of .0006.
Symptoms and Safety Outcomes
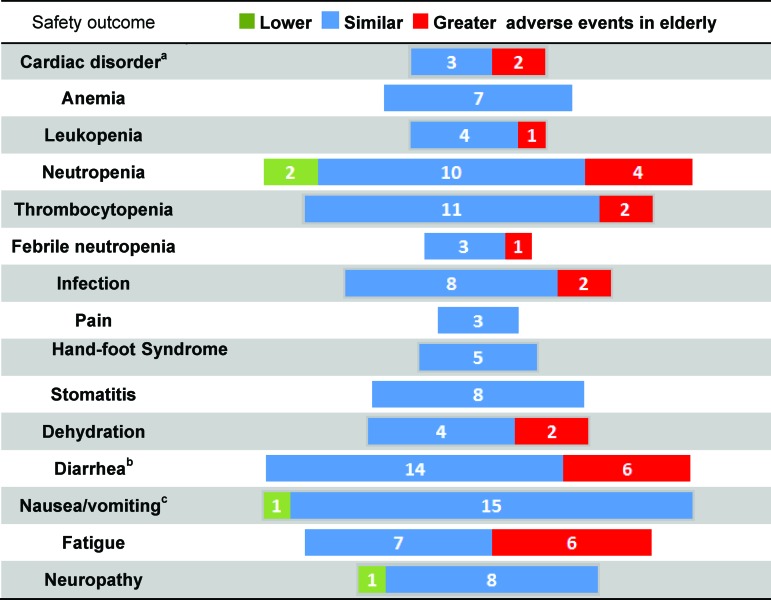
Symptoms and safety outcomes were extracted based on those reported in the studies. They include grade 3 or 4 cardiac disorder, anemia, leukopenia, neutropenia, thrombocytopenia, febrile neutropenia, infection, pain, hand-foot syndrome, stomatitis, dehydration, diarrhea, nausea/vomiting, fatigue, and neuropathy. Figure 5 gives a summary of the results, showing how many studies demonstrated chemotherapy having lower (in green), similar (in blue), or higher (in red) incidences of adverse events in the elderly versus nonelderly patients.
Figure 5.
Relative reporting of grade 3 or 4 adverse events from chemotherapies in elderly versus nonelderly patients with stage III colon cancer. The number of studies indicating a difference in the relative incidence of grade 3 or 4 adverse events between elderly and nonelderly patients with stage III colon cancer is shown.
aCardiac disorders consist of cardiac disorders, myocardial infarction, arterial thrombosis, and venous thrombosis. One study represented by red indicated higher incidence of arterial thrombosis, but similar incidence of venous thrombosis in the elderly. Another study represented by red indicated the opposite.
bOne study represented by red indicated higher incidence of late-onset diarrhea but similar incidence of early-onset diarrhea in the elderly.
cFour studies reported nausea only.
Maculopapular rash, anorexia, constipation, dyspnea, anxiety, depression, insomnia, and psychological distress are also common symptoms of colon cancer chemotherapy. However, no studies reported these symptoms, so they were not reported in this review.
Adverse Events More Frequently Seen in Elderly Patients
The majority of all adverse events occurred at a similar rate in elderly versus nonelderly patients. However, there were studies reporting higher incidences of adverse events in 9 out of 15 grade 3 or 4 adverse events: cardiac disorder (2/5 studies) [29, 30], leukopenia (1/5) [31], neutropenia (4/16) [32–34], thrombocytopenia (2/13) [32, 34], febrile neutropenia (1/4) [34], infection (2/10) [29, 32], dehydration (2/6) [29, 34], diarrhea (6/20) [22, 27, 29, 34–35], and fatigue (6/13) [27, 32, 34–35].
Fatigue was seen more frequently in elderly versus nonelderly patients according to six studies. Goldberg et al. reported values of fatigue incidence from three different trials indicating higher incidence of fatigue in elderly (≥70 years) versus nonelderly (<70 years) patients receiving FOLFOX [32]. Reported incidences of fatigue were 7% and 4% (p = .08 or .003 when age modeled as a continuous variable), 19% and 9% (p = .04), and 19% and 9% (p = .03) in elderly and nonelderly patients, respectively.
Moehler et al. reported fatigue as 5.0% and 1.3% (p = 0.03) in elderly (≥70 years) and nonelderly (<70 years) patients receiving Iri/5-FU/LV chemotherapy, respectively [35]. McKibbin et al. reported fatigue (grade unspecified) as 36% and 17% (p < .01) in elderly (>65 years) and nonelderly (≤65 years) patients receiving Iri/5-FU chemotherapy, respectively [27]. Allegra et al. reported fatigue (grade 3) as 15.2% and 6.9% (p < .001) in elderly (≥70 years) and nonelderly (<70 years) patients receiving 5-FU/Ox with or without bevacizumab as chemotherapy [34].
Diarrhea had a higher incidence in elderly versus nonelderly patients according to six studies. McKibbin et al. reported higher incidences of diarrhea (grade unspecified) in elderly (>65 years) versus nonelderly (≤65 years) patients twice [27]. In one study, patients were given 5-FU/Ox chemotherapy. The incidence of diarrhea was 32% and 19% (p < .01) in elderly and nonelderly patients respectively. In a second study, patients were given 5-FU/Iri chemotherapy. The incidence of diarrhea was 56% and 31% (p = .001) in elderly and nonelderly patients, respectively.
Jensen et al. reported diarrhea as 18% and 6% (p = .01) in elderly (≥70 years) and nonelderly (<70 years) patients receiving CapeOx chemotherapy [22]. Schmoll et al reported diarrhea as 23% and 17% (p < .05) in elderly (≥65 years) and nonelderly (<65 years) patients receiving CapeOx chemotherapy [29]. Moehler et al. reported late-onset diarrhea as 16% and 7.1% in elderly (≥70 years) and nonelderly (<70 years) patients receiving Iri/5-FU/LV chemotherapy [35]. Allegra et al reported diarrhea (grade 3) as 16.4% and 9.5% (p < .001) in elderly (≥70 years) and nonelderly (<70 years) patients receiving 5-FU/Ox with or without bevacizumab chemotherapy [34].
Neutropenia was seen more often in elderly versus nonelderly patients according to four studies. Goldberg et al. reported a higher rate of neutropenia in elderly (≥70 years) versus nonelderly (<70 years) patients receiving FOLFOX chemotherapy in two separate trials [32]. Rates of neutropenia were reported as 49% and 43% (p = .04 or p < .001 when age was modeled as a continuous variable) and 60% and 43% (p = .02) in elderly and nonelderly patients, respectively. Chau et al. reported neutropenia as 35% and 22% (p = .0228) in elderly (≥70 years) and nonelderly (<70 years) patients receiving irinotecan chemotherapy [33]. Allegra et al. reported grade 3 neutropenia as 42.2% and 28.8% (p < .001) and grade 4 neutropenia as 13% and 6% (p < .001) in elderly (≥70 years) and nonelderly (<70 years) patients receiving 5-FU/Ox with or without bevacizumab as chemotherapy [34].
Adverse Events Less Frequently Seen in Elderly Patients
Only neutropenia, nausea/vomiting, and neuropathy were seen less frequently in elderly versus nonelderly patients in 2/16 [35], 1/16 [32], and 1/9 [27] of all studies. A lower incidence of neutropenia was reported in patients receiving Iri/5-FU/LV chemotherapy by Moehler et al. [35]. Of the elderly patients (≥70 years), 0.6% experienced neutropenia, whereas 4.1% of the nonelderly patients (<70 years) experienced neutropenia. The p value was not provided, so it was calculated to be .02 by the χ2 test for homogeneity. McKibbin et al. reported the incidence of neutropenia (grade unspecified) as 18% and 28% in elderly and nonelderly patients, respectively, with a reported p value of 0.03 [27].
In Goldberg et al., the incidence of nausea/vomiting in patients receiving FOLFOX chemotherapy was reported as 7% in elderly patients (≥70 years) and 9% in nonelderly patients (<70 years) [32]. The reported p value in a model with age as a continuous variable was <.001, whereas the reported p value from a logistic regression model for age as a dichotomous variable (age <70 vs. ≥70) in a model adjusted for study, sex, and performance status was .38.
In McKibbin et al., the incidence of neuropathy (grade unspecified) in patients receiving 5-FU/Ox chemotherapy was reported as 15% in elderly patients (>65 years) and 26% in nonelderly patients (≤65 years), with a reported p value of .02 [27].
Discussion
Previous literature has indicated that colon cancer survival is better in nonelderly versus elderly patients [36]. However, it is unknown whether this is due to differential relative effect of chemotherapy among elderly versus nonelderly colon cancer patients or other factors. Elderly patients are less likely to receive chemotherapy and more likely to have other risks for reduced survival. Therefore, patients and their medical oncologists benefit from evidence that separates out the chemotherapy benefits and harms from confounding factors that affect relative survival and adverse events among elderly patients compared to nonelderly patients. This systematic review compares the relative effectiveness and incidence of adverse events of chemotherapy in elderly versus nonelderly stage III patients.
The vast majority of evidence in this review suggests that chemotherapy has similar relative effectiveness and safety outcomes in patients in their sixties, seventies, and eighties compared to younger patients. However, when looking at the evidence suggesting a difference, higher adverse event rates in elderly versus nonelderly patients are most observed. Fatigue, diarrhea, and neutropenia have the most reported differences in incidence when comparing elderly and nonelderly patients.
The higher incidence of fatigue, diarrhea, and neutropenia among elderly patients was seen for a wide variety of chemotherapy regimens, so no associations between a specific chemotherapy regimen and higher incidence of adverse event can be anticipated. This was also true for the higher incidences seen in elderly patients for other adverse events. In addition, when looking at the evidence suggesting a difference in outcomes in elderly versus nonelderly patients, a few studies reported lower incidence of adverse events, as well as lower and higher effectiveness of chemotherapy in elderly patients. However, these studies were even fewer in comparison to the number of studies reporting a higher incidence of adverse events in elderly versus nonelderly patients. A plausible explanation for the studies reporting lower incidence of adverse events in elderly patients is that these patients received lower doses or less aggressive chemotherapy treatment than nonelderly patients.
Any differences in safety outcomes when comparing elderly and nonelderly patients may be partially due to inherent discrepancies between the two populations. Because no major difference in treatment effects between elderly and nonelderly patients was found, this systematic review suggests that in the absence of other reasons for withholding treatment, elderly patients should receive chemotherapy as often as nonelderly patients.
Any differences in safety outcomes when comparing elderly and nonelderly patients may be partially due to inherent discrepancies between the two populations. Because no major difference in treatment effects between elderly and nonelderly patients was found, this systematic review suggests that in the absence of other reasons for withholding treatment, elderly patients should receive chemotherapy as often as nonelderly patients. Our systematic review does not support the lower treatment rates seen in patients in their sixties, seventies, and eighties.
Previous reviews have found similar effectiveness results. A review by Au et al. found that chemotherapy offered a similar benefit to elderly and nonelderly patients, but more data were needed regarding the toxicity of therapy [37]. Similarly, a review by Kohne et al. concluded that fit elderly patients could be given aggressive chemotherapy just as it would be given to nonelderly patients [38]. A review by Power et al. came to similar findings and added that older patients are more willing to take chemotherapy if they are fully informed of its potential toxicities and benefits [39].
Chemotherapy use among elderly patients has risen over time. Whereas approximately 22% of patients 80 years and older received treatment in 1990–1991, nearly 40% of patients in this age group received treatment a decade later [40]. However, treatment rates are still considered low [22, 40–41]. Elderly patients are less likely to receive treatment due to a variety of reasons including decreased functional and cognitive ability, financial barriers, lack of social support, comorbidities, patient preference, and clinician knowledge and attitudes [22, 41, 42]. Clinicians are often concerned with treating elderly patients because of the lack of generalizability of trial results [22].
There are likely other factors that affect the relative effectiveness of chemotherapy, some of which could be correlated with age. For instance, the likelihood of a poorer performance status is greater in an elderly than nonelderly patient. Future research should try to disentangle factors like performance status, comorbidities, and organ function from age alone when determining patient-centered outcomes associated with chemotherapy. It should be noted that there is a difference between performance status, functional status, and problems discovered in geriatric assessment in older patients with cancer [43]. Furthermore, even if elderly patients receive treatment, they are more likely to receive substandard treatment or discontinue treatment, often due to the higher incidence of specific treatment-related toxicities [22, 40]. It is important for clinicians to be aware of these specific toxicities so they can warn their elderly patients ahead of time.
Adjuvant chemotherapy is recommended for stage III colon cancer per NCCN guidelines; however, it remains controversial and underused in the elderly population. Recent studies have shown that older patients with stage III colon cancer, often with pre-existing comorbidities, were less likely to receive adjuvant chemotherapy, when the survival benefit was comparable across age groups [44, 45].
This systematic review is limited by the heterogeneity of the study populations and the quality of the articles studied. Ideally, a systematic review would uncover sufficient evidence to perform a meta-analysis or weighted average of estimates. Due to the paucity of studies that address this topic and the inconsistency in metrics used to report benefits of chemotherapy, such a synthesis was unfeasible.
Additionally, the lower age limit for an elderly patient varied between 60 and 75 years amongst different trials. However, most elderly patients were younger than 80 years and considered fit. The studied effects of older patients in these articles may not be applicable to all older patients because those who received treatment may reflect a selective and small subset of the total elderly population. A recent trial was designed to include frail and elderly patients with metastatic colorectal cancer by using reduced-dose chemotherapy options [46]. However, it is one of the only trials that includes this population of patients, so CER is still very important in helping determine the evidence for this population of patients. The CER movement is aimed at not only providing information on the relative effectiveness of one chemotherapeutic agent versus another, but also on providing evidence on subpopulations of patients, including the elderly. This systematic review presents various effectiveness and safety outcomes in a way that is applicable for elderly patients.
Conclusion
The vast majority of the evidence from this systematic review indicates that the relative treatment effect of chemotherapy for stage III cancer in terms of both effectiveness and safety is similar among older patients compared to younger patients. When differences in the relative treatment effect between elderly and nonelderly patients are reported, the prognosis is worse for the elderly. Furthermore, the reported chemotherapy effects on grade 3 or 4 adverse event rates are more often higher among elderly versus nonelderly patients. Nonetheless, because no major difference in treatment effects between elderly and nonelderly patients was found, this systematic review suggests that in the absence of other reasons for withholding treatment, elderly patients should receive chemotherapy as often as nonelderly patients. Our systematic review does not support the lower treatment rates seen in older patients. Comparative effectiveness research should continue to provide additional evidence for populations historically underrepresented in clinical trials, such as the elderly population.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institute on Aging Short-Term Training Program on Aging (1T35AG036679–02) through the Health Professions Student Training in Aging Research Program at the University of Maryland School of Medicine.
Author Contributions
Conception and design: Anna Hung, C. Daniel Mullins
Provision of study materials or patients: Anna Hung, C. Daniel Mullins
Collection and/or assembly of data: Anna Hung
Data analysis and interpretation: Anna Hung, C. Daniel Mullins
Manuscript writing: Anna Hung, C. Daniel Mullins
Final approval of manuscript: Anna Hung, C. Daniel Mullins
Disclosures
C. Daniel Mullins: Amgen, Bayer, Bristol-Myers Squibb, Eisai, Genentech, GlaxoSmithKline, Mitsubishi, Novartis, Pfizer, Sanofi-Aventis (C/A); Bayer, Pfizer, Sanofi-Aventis (RF). The other author indicated no financial relationships.
Section Editors: Hyman B. Muss: Wyeth/Pfizer (C/A); Arti Hurria: GTX, Seattle Genetics (C/A), Celgene (previously Abraxis Bioscience), GSK (RF); Matti Aapro: Sanofi (C/A)
Reviewer “A”: None
Reviewer “B”: None
Reviewer “C”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Sox HC, Greenfield S. Comparative effectiveness research: A report from the Institute of Medicine. Ann Intern Med. 2009;151:203–205. doi: 10.7326/0003-4819-151-3-200908040-00125. [DOI] [PubMed] [Google Scholar]
- 2.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bero LA, Jadad AR. How consumers and policymakers can use systematic reviews for decision making. Ann Intern Med. 1997;127:37. doi: 10.7326/0003-4819-127-1-199707010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Cook DJ, Greengold NL, Ellrodt AG, et al. The relation between systematic reviews and practice guidelines. Ann Intern Med. 1997;127:210–216. doi: 10.7326/0003-4819-127-3-199708010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bero LA, Grilli R, Grimshaw JM, et al. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317:465–468. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petticrew M. Why certain systematic reviews reach uncertain conclusions. BMJ. 2003;326:756–758. doi: 10.1136/bmj.326.7392.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Washington, DC: The National Academies Press; 2009. Initial National Priorities for Comparative Effectiveness Research. [Google Scholar]
- 8.Holden DJ, Jonas DE, Porterfield DS, et al. Systematic review: Enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 9.Jellema P, van der Windt DA, Bruinvels DJ, et al. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: Systematic review and meta-analysis. BMJ. 2010;340:1269. doi: 10.1136/bmj.c1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel S, McCall M, Ohinmaa A, et al. Positron emission tomography/computed tomographic scans compared to computed tomographic scans for detecting colorectal liver metastases: a systematic review. Ann Surg. 2011;253:666–671. doi: 10.1097/SLA.0b013e31821110c9. [DOI] [PubMed] [Google Scholar]
- 11.Pickhardt PJ, Hassan C, Halligan S, et al. Colorectal cancer: CT colonography and colonoscopy for detection: Systematic review and meta-analysis. Radiology. 2011;259:393–405. doi: 10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Pas MH, Meijer S, Hoekstra OS, et al. Sentinel-lymph-node procedure in colon and rectal cancer: A systematic review and meta-analysis. Lancet Oncol. 2011;12:540–550. doi: 10.1016/S1470-2045(11)70075-4. [DOI] [PubMed] [Google Scholar]
- 13.Des Guetz G, Schischmanoff O, Nicolas P, et al. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer. 2009;45:1890–1896. doi: 10.1016/j.ejca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Ho TW, Mack LA, Temple WJ. Operative salvage for retroperitoneal nodal recurrence in colorectal cancer: A systematic review. Ann Surg Oncol. 2011;18:697–703. doi: 10.1245/s10434-010-1322-7. [DOI] [PubMed] [Google Scholar]
- 15.Cantero-Munoz P, Urien MA, Ruano-Ravina A. Efficacy and safety of intraoperative radiotherapy in colorectal cancer: A systematic review. Cancer Lett. 2011;306:121–133. doi: 10.1016/j.canlet.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Chua TC, Saxena A, Liauw W, et al. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann Surg Oncol. 2010;17:492–501. doi: 10.1245/s10434-009-0781-1. [DOI] [PubMed] [Google Scholar]
- 17.Wieser M, Sauerland S, Arnold D, et al. Peri-operative chemotherapy for the treatment of resectable liver metastases from colorectal cancer: A systematic review and meta-analysis of randomized trials. BMC Cancer. 2010;10:309. doi: 10.1186/1471-2407-10-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: A systematic review and meta-analysis. JAMA. 2011;305:2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 19.Oostendorp LJ, Stalmeier PF, Pasker-de Jong PC, et al. Systematic review of benefits and risks of second-line irinotecan monotherapy for advanced colorectal cancer. Anticancer Drugs. 2010;21:749–758. doi: 10.1097/CAD.0b013e32833c57cf. [DOI] [PubMed] [Google Scholar]
- 20.Galfrascoli E, Piva S, Cinquini M, et al. Risk/benefit profile of bevacizumab in metastatic colon cancer: A systematic review and meta-analysis. Dig Liver Dis. 2011;43:286–294. doi: 10.1016/j.dld.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Welch S, Spithoff K, Rumble RB, et al. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: A systematic review. Ann Oncol. 2010;21:1152–1162. doi: 10.1093/annonc/mdp533. [DOI] [PubMed] [Google Scholar]
- 22.Jensen SA, Lonborg JT, Sorensen JB. Benefits and risks of palliative capecitabine based therapy to elderly patients with advanced colorectal cancer: Danish single centre experiences. Acta Oncol. 2006;45:67–76. doi: 10.1080/02841860500375213. [DOI] [PubMed] [Google Scholar]
- 23.Agency for Healthcare Research and Quality. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- 24.Carreca I, Balducci L, Extermann M. Cancer in the older person. Cancer Treat Rev. 2005;31:380–402. doi: 10.1016/j.ctrv.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 25.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Colon Cancer. [Accessed October 19, 2012]. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 26.Zuckerman IH, Rapp T, Onukwugha E, et al. Effect of age on survival benefit of adjuvant chemotherapy in elderly patients with stage III colon cancer. J Am Geriatr Soc. 2009;57:1403–1410. doi: 10.1111/j.1532-5415.2009.02355.x. [DOI] [PubMed] [Google Scholar]
- 27.McKibbin T, Frei CR, Greene RE, et al. Disparities in the use of chemotherapy and monoclonal antibody therapy for elderly advanced colorectal cancer patients in the community oncology setting. The Oncologist. 2008;13:876–885. doi: 10.1634/theoncologist.2008-0061. [DOI] [PubMed] [Google Scholar]
- 28.Haller D, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 29.Schmoll HJ, Cartwright T, Tabernero J, et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: A planned safety analysis in 1,864 patients. J Clin Oncol. 2007;25:102–109. doi: 10.1200/JCO.2006.08.1075. [DOI] [PubMed] [Google Scholar]
- 30.Raman AK. Bevacizumab (BV) related adverse events among various age groups of elderly patients with advanced colorectal cancer. J Clin Oncol. 2007;25:145–146. [Google Scholar]
- 31.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 33.Chau I, Norman AR, Cunningham D, et al. Elderly patients with fluoropyrimidine and thymidylate synthase inhibitor-resistant advanced colorectal cancer derive similar benefit without excessive toxicity when treated with irinotecan monotherapy. Br J Cancer. 2004;91:1453–1458. doi: 10.1038/sj.bjc.6602169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allegra CJ, Yothers G, O'Connell MJ, et al. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27:3385–3390. doi: 10.1200/JCO.2009.21.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moehler M, Ababneh Y, Verpoort K, et al. Efficacy and safety of irinotecan-based chemotherapy for advanced colorectal cancer outside clinical trials: An observational study. Onkologie. 2010;33:684–690. doi: 10.1159/000322208. [DOI] [PubMed] [Google Scholar]
- 36.Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 37.Au HJ, Mulder KE, Fields AL. Systematic review of management of colorectal cancer in elderly patients. Clin Colorectal Cancer. 2003;3:165–171. doi: 10.3816/CCC.2003.n.022. [DOI] [PubMed] [Google Scholar]
- 38.Kohne CH, Folprecht G, Goldberg RM, Mitry E, Rougier P. Chemotherapy in elderly patients with colorectal cancer. The Oncologist. 2008;13:390–402. doi: 10.1634/theoncologist.2007-0043. [DOI] [PubMed] [Google Scholar]
- 39.Power D, Lichtman S. Chemotherapy for the elderly patient with colorectal cancer. Cancer. 2010;16:241–252. doi: 10.1097/PPO.0b013e3181e07690. [DOI] [PubMed] [Google Scholar]
- 40.Muss HB, Biganzoli L, Sargent DJ, et al. Adjuvant therapy in the elderly: Making the right decision. J Clin Oncol. 2007;25:1870–1875. doi: 10.1200/JCO.2006.10.3457. [DOI] [PubMed] [Google Scholar]
- 41.Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 42.Lemmens VE, van Halteren AH, Janssen-Heijnen ML, et al. Adjuvant treatment for elderly patients with stage III colon cancer in the southern Netherlands is affected by socioeconomic status, gender, and comorbidity. Ann Oncol. 2005;16:767–772. doi: 10.1093/annonc/mdi159. [DOI] [PubMed] [Google Scholar]
- 43.Extermann M, Overcash J, Lyman GH, et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 44.Chagpar R, Xing Y, Chiang Y, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. 2012;30:972–979. doi: 10.1200/JCO.2011.39.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham A, Habermann E, Rothenberger D, et al. Adjuvant chemotherapy for stage III colon cancer in the oldest old: Results beyond clinical guidelines. Cancer. 2012 doi: 10.1002/cncr.27755. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open-label, randomised factorial trial. Lancet. 2011;377:1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bensmaine MA, Marty M, de Gramont A, et al. Factors predicting efficacy of oxaliplatin in combination with 5-fluorouracil (5-FU) +/- folinic acid in a compassionate-use cohort of 481 5-FU-resistant advanced colorectal cancer patients. Br J Cancer. 2001;85:509–517. doi: 10.1054/bjoc.2001.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattioli R, Massacesi C, Recchia F, et al. High activity and reduced neurotoxicity of bi-fractionated oxaliplatin plus 5-fluorouracil/leucovorin for elderly patients with advanced colorectal cancer. Ann Oncol. 2005;16:1147–1151. doi: 10.1093/annonc/mdi222. [DOI] [PubMed] [Google Scholar]
- 49.Rosati G, Cordio S, Bordonaro R, et al. Capecitabine in combination with oxaliplatin or irinotecan in elderly patients with advanced colorectal cancer: Results of a randomized phase II study. Ann Oncol. 2010;21:781–786. doi: 10.1093/annonc/mdp359. [DOI] [PubMed] [Google Scholar]
- 50.Pfeiffer P, Sorbye H, Ehrsson H, et al. Short-time infusion of oxaliplatin in combination with capecitabine (XELOX30) as second-line therapy in patients with advanced colorectal cancer after failure to irinotecan and 5-fluorouracil. Ann Oncol. 2006;17:252–258. doi: 10.1093/annonc/mdj060. [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto J, Ohashi Y, Hamada C, et al. Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5-year results from three randomized trials. J Clin Oncol. 2004;22:484–492. doi: 10.1200/JCO.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 52.Mattioli R, Lippe P, Recchia F, et al. Advanced colorectal cancer in elderly patients: tolerance and efficacy of leucovorin and fluorouracil bolus plus continuous infusion. Anticancer Res. 2001;21:489–492. [PubMed] [Google Scholar]
- 53.Fata F, Mirza A, Craig G, et al. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colon carcinoma: A 10-year experience of the Geisinger Medical Center. Cancer. 2002;94:1931–1938. doi: 10.1002/cncr.10430. [DOI] [PubMed] [Google Scholar]
- 54.Chau I, Norman AR, Cunningham D, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005;16:549–557. doi: 10.1093/annonc/mdi116. [DOI] [PubMed] [Google Scholar]
- 55.Rosati G, Cordio S. Single-agent irinotecan as second-line weekly chemotherapy in elderly patients with advanced colorectal cancer. Tumori. 2006;92:290–294. doi: 10.1177/030089160609200405. [DOI] [PubMed] [Google Scholar]
- 56.Andre T, Boni C, Mounedji-Boudiaf L, et al. Multicenter international study of oxaliplatin/5-fluorouracil/leucovorin in the adjuvant treatment of colon cancer (MOSAIC) investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 57.Lee D. Highlights from 40th Annual Meeting of the American Society of Clinical Oncology New Orleans, LA, June 2004. Clin Colorectal Cancer. 2004;4:154–158. [PubMed] [Google Scholar]
- 58.Diaz Rubio E. Safety of capecitabine (X) compared to fluorouracil/leucovorin (5-FU/LV) for the adjuvant treatment of elderly colon cancer patients (pts) J Clin Oncol. 2004;22:3737. [Google Scholar]
- 59.Maughan TS, James RD, Kerr DJ, et al. Comparison of intermittent and continuous palliative chemotherapy for advanced colorectal cancer: A multicentre randomised trial. Lancet. 2003;361:457–464. doi: 10.1016/s0140-6736(03)12461-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.