The decision to undergo major palliative surgery in end-stage gynecologic cancer is made when severe disease symptoms significantly hinder quality of life. This review discusses and summarizes the literature on surgical management of malignant bowel obstruction and palliative pelvic exenteration in gynecologic oncology.
Keywords: Palliative surgery, Gynecologic oncology, Bowel obstruction, Pelvic exenteration
CME Learning Objectives
Describe the range of palliative procedures utilized in gynecologic cancers.
Evaluate the indications for palliative bowel surgery in ovarian cancer.
Assess the clinical scenario for which palliative pelvic exenteration would be considered.
Compare the morbidities associated with palliative gynecologic surgeries to the potential benefits gained.
Abstract
The decision to undergo major palliative surgery in end-stage gynecologic cancer is made when severe disease symptoms significantly hinder quality of life. Malignant bowel obstruction, unremitting pelvic pain, fistula formation, tumor necrosis, pelvic sepsis, and chronic hemorrhage are among the reasons patients undergo palliative surgeries. This review discusses and summarizes the literature on surgical management of malignant bowel obstruction and palliative pelvic exenteration in gynecologic oncology.
Implications for Practice:
The decision to perform palliative surgery in gynecologic oncology is difficult. This review discusses the currently available data for the surgical management of malignant bowel obstruction and palliative pelvic exenteration. While there are no effective parameters to determine which patients will benefit from palliative surgery, surgery should be considered when severe disease symptoms hinder the quality of life.
Introduction
The decision to undergo major palliative surgery for advanced gynecologic malignancy is among the most difficult encounter by patients and doctors. Most women in this setting have undergone multiple therapies, are now refractory to treatment, and are facing very limited life expectancies. However, when severe symptoms and major disease complications obviate what little quality of life remains, major surgery for palliation becomes a serious option.
Palliative procedures in gynecologic oncology range from minimally invasive interventions to major complex surgeries. Minor procedures such as nasogastric tubes, decompressive percutaneous gastrostomy tubes, percutaneous nephrostomy tubes, large bowel stents, and indwelling pigtail catheters are critical in the management of advanced gynecologic cancer, but they are beyond the scope of this paper. This review focuses on the literature pertaining to two clinical scenarios requiring major surgical intervention to achieve palliation: malignant bowel obstruction requiring intestinal resection and/or diversion and severe pelvic sequelae leading to palliative pelvic exenteration.
Malignant Bowel Obstruction
Malignant bowel obstruction complicates approximately 3% of all advanced malignancies, among which ovarian cancer (5%–42%) and colorectal cancer (10%–28%) are most implicated [1, 2]. Other gynecologic malignancies, such as uterine sarcomas and aggressive endometrial cancers (papillary serous, mucinous, and carcinosarcoma), metastasize to the bowel and cause obstruction as well. Obstruction usually results from either occlusion of the bowel lumen or dysfunctional peristalsis. In ovarian cancer, bowel obstruction is most commonly associated with the presence of intestinal carcinomatosis at the time of initial surgery, suboptimal tumor debulking (>2 cm residual tumor), and stage III or IV disease [3]. Although intestinal obstructions are usually related to disease, nonmalignant causes, such as adhesions from prior surgery, intraperitoneal chemotherapy, or radiation therapy, are noted in 5%–24% of cases [2, 4–6].
The most common presentation of malignant bowel obstruction is luminal distension leading to nausea and vomiting in 68%–100% of cases [1, 7]. Approximately 92% percent of patients experience moderate to severe constant pain from bowel wall distention, whereas 72%–76% of patients feel “colicky” pain from peristaltic waves leading to muscle spasms and increased proximal bowel distension. Bowel obstructions can occur in the small or large intestine or both (15% of cases) and may be either partial or total occlusions (Fig. 1). Loose and watery stools are typical of partial obstruction, whereas total obstruction usually results in lack of bowel movements once the distal bowel clears of residual contents. Obstipation and constipation must be ruled out prior to making the diagnosis of total obstruction. Severe nausea and bilious emesis are typical of proximal intestinal obstruction, whereas more distal and colonic obstructions are more associated with abdominal distention and pain [8].
Figure 1.
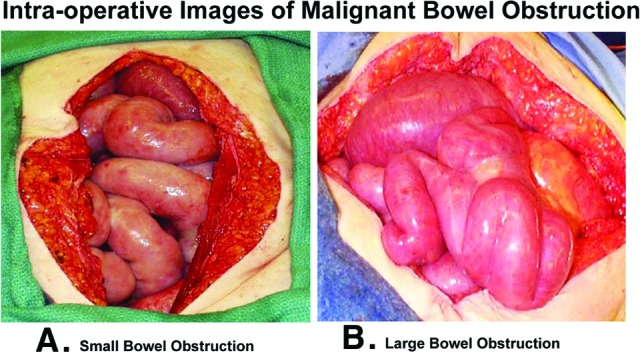
Intraoperative images of malignant bowel obstruction. Dilated, engorged, and hyperemic loops of small (A) and large (B) intestine typically found proximal to the site of obstruction.
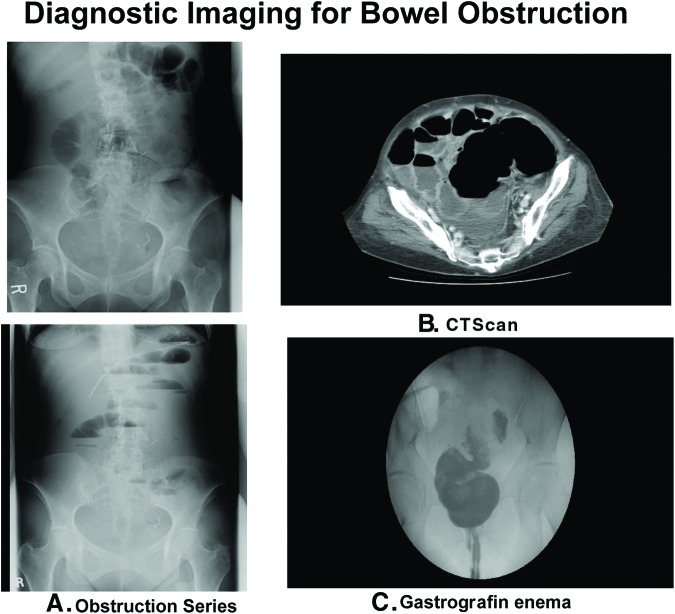
The simplest initial diagnostic test for obstruction is a supine and upright radiograph of the abdomen—the gastrointestinal obstruction series. Dilated loops of bowel, air fluid levels, and a paucity of air in the rectum are characteristic x-ray findings. A chest radiograph looking for free air under the diaphragm is also important to rule out bowel perforation. In the presence of ascites or diffuse abdominal tumor, the obstruction series may be insufficient and a computed tomography (CT) scan with oral and intravenous contrast is indicated. CT scan can often identify the point of obstruction by locating the transition point between proximal bowel dilation and distal luminal compression. If large bowel obstruction is suspected, the addition of a Gastrografin (diatrizoic acid) enema may be helpful to determine the site of obstruction (Fig. 2).
Figure 2.
Diagnostic imaging for bowel obstruction. (A): Gastrointestinal obstruction series includes a supine (top) and upright (bottom) image showing dilated bowel, air-fluid levels, and lack of air in rectum. (B): Computed tomography scan depicted massively dilated bowel juxtaposed with collapsed loops. (C): Gastrografin (diatrizoic acid) enema demonstrated the transition point in a large bowel obstruction.
Advanced-stage ovarian cancer, which progresses despite aggressive surgical debulking and multiple chemotherapies, carries a poor prognosis. In this scenario, when patients present with malignant bowel obstruction, the therapeutic framework shifts from curative to palliative. Conservative measures such as restricted oral feeding, intravenous hydration, and nasogastric tube placement may be attempted; however, once they fail, the physician and patient must broach the difficult question of palliative surgery (Fig. 3).
Figure 3.
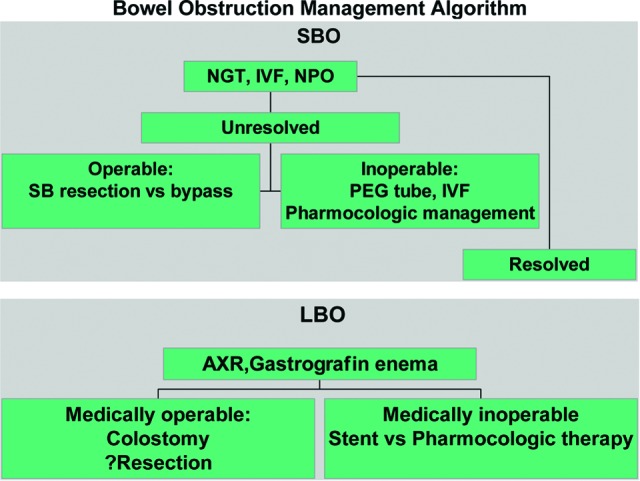
Management algorithm for small and large bowel obstructions in women with gynecologic malignancies. Unresolved indicates no relief of nausea, vomiting, or abdominal pain within 7 days of nasogastric tube placement. Resolved indicates relief of nausea, vomiting, or abdominal pain within 7 days of nasogastric tube placement.
Abbreviations: AXR, abdominal x-ray; IVF, intravenous fluids; LBO, large bowel obstruction; NGT, nasogastric food; NPO, nil per os (nothing by mouth); PEG, percutaneous endoscopic gastrostomy; SB, small bowel; SBO, small bowel obstruction.
Quality-of-life improvement should be the foremost goal of palliative surgery. Specifically, this means triaging patients into those who will be able to recover physically, regain bowel function, and tolerate oral intake postoperatively from those who will not. For the latter group, less invasive palliative treatments such as percutaneous gastrostomy tubes, stents for isolated large bowel obstructions, and pharmacologic management with narcotics, anticholinergics, and antiemetics should be considered.
The primary objective of palliative surgery is relief of obstruction. This goal may be accomplished by small bowel resection or bypass. A Gastrografin (diatrizoic acid) enema or CT scan with rectal contrast to rule out a large bowel obstruction should be performed prior to surgery, as approximately 15% percent of patients have a concomitant large and small bowel obstruction. If tumor resection is to be attempted, the surgeon must ensure that proximal and distal ends of bowel are healthy and devoid of tumor for a secure anastomosis. If resection is not feasible, bypass may be performed by leaving the tumor in situ and either internally linking healthy bowel proximal and distal to the obstruction or, alternatively, by bringing the proximal loop of bowel to the surface and creating an ostomy (Fig. 4). Also, in the event of extensive mesenteric involvement with tumor, if unable to bypass or perform an ileostomy, a gastrostomy tube should be placed, which unfortunately leads to a very poor prognosis and short life expectancy. In the case of a large bowel obstruction, a diverting colostomy is usually performed proximal to the site of obstruction. In select cases, resection may be performed. In the case of emergent surgery for large bowel obstruction presenting with acute peritoneal signs, perforation, or cecal dilation greater than 10 cm, a diverting colostomy is the indicated procedure.
Figure 4.
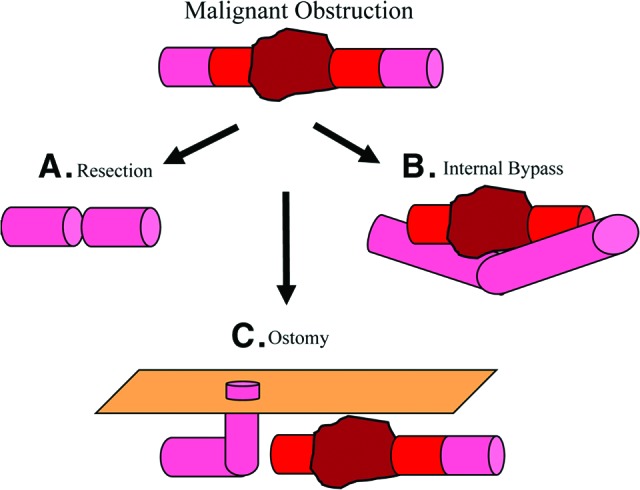
Operative options for malignant bowel obstruction. (A): Resection of tumor mass with anastomosis of healthy proximal and distal bowel. (B): Bypass of tumor mass without resection. (C): Bypass of tumor mass by creation of ostomy using bowel proximal to the malignant obstruction.
Initial surgical management for palliation of malignant bowel obstruction in ovarian cancer is well described. Since Castaldo reported the first series in 1981, there have been numerous other reports (Table 1) [3–6, 9–21]. From this data, palliative surgery is associated with a reported morbidity of 5%–49% and mortality of 5%–15%. Major operative complications including enterocutaneous fistula, anastomotic leaks, short bowel syndrome, and sepsis noted in 30% of cases (range, 7%–64%) [5–6, 12, 18].
Table 1.
Literature on intestinal obstruction in ovarian cancer
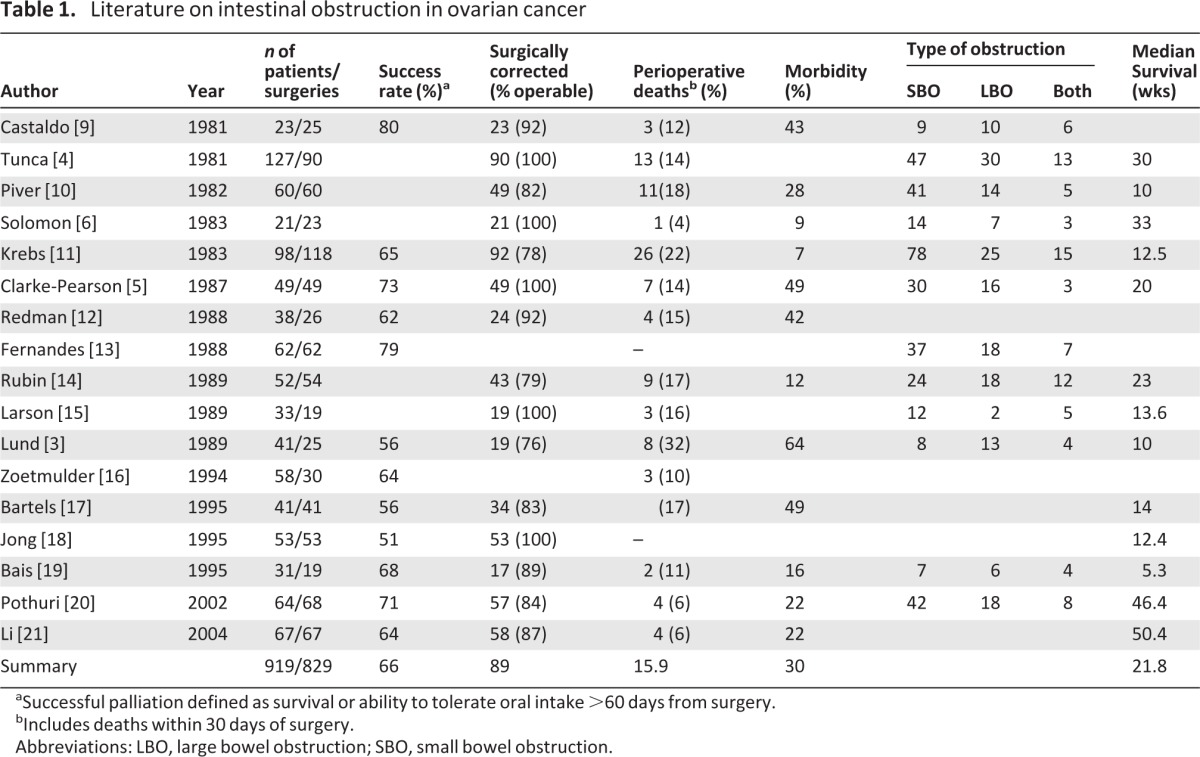
aSuccessful palliation defined as survival or ability to tolerate oral intake >60 days from surgery.
bIncludes deaths within 30 days of surgery.
Abbreviations: LBO, large bowel obstruction; SBO, small bowel obstruction.
In patients with bowel obstruction associated with recurrent disease, early case series report a median survival of 3–6 months after palliative surgery [14, 20]. However, in more recent series of patients who underwent palliative surgery for bowel obstruction in recurrent ovarian cancer, 64%–71% of patients were successfully palliated, with a median survival of 11.4–12.6 months versus 3.7–3.9 months for nonsurgical patients [20, 21]. This data, when compared to prior data, suggests that, if carefully selected, a majority of patients who choose to undergo palliative surgery can achieve quality-of-life improvements as defined by the ability to tolerate a low-residue or regular diet at least 60 days postoperatively.
Several authors have attempted to design a systematic method to determine which patients would benefit from surgery. Krebs and Gopelrud formulated a prognostic index based on age, nutritional status, tumor spread, ascites, and previous chemotherapy or radiation therapy in which a score of ≤6 was associated with 84% survival 60 days postsurgery, whereas a score of ≥7 had a 20% survival [11]. Larson et al. confirmed these findings, with 38% of patients with a score ≤6 surviving 1 year after obstruction, and no patients with higher scores surviving [15]. Fernanades et al. also studied prognostic factors influencing survival 1 year after obstruction and added the age at diagnosis, time from primary diagnosis obstruction, and abnormal albumin, blood urea nitrogen, and alkaline phosphatase levels [13]. Rubin et al., however, were unable to validate these prognostic factors nor define exclusion criteria allowing selection of patients unlikely to benefit from surgery [14].
Data are scarce for guiding management decisions regarding repeat malignant small bowel obstruction in ovarian cancer. Few reports exist describing reoperation for palliation of recurrent obstruction in ovarian carcinoma. In two small series of highly preselected patients chosen to undergo repeat surgery, only 30%–33% were successfully palliated, and 67% developed another obstruction within 3–6 months [22, 23]. Furthermore, survival was only 4–6 months in those successfully palliated and morbidity was significant, with 30%–40% developing enterocutaneous fistulas. These series suggest that repeat surgery for recurrent bowel obstruction leads to significant morbidity, rapid development of subsequent bowel obstructions, and limited survival. In this subgroup of patients, nonsurgical management approaches should be considered.
Palliative Pelvic Exenteration
One of the largest, most morbid procedures performed in gynecologic oncology is the pelvic exenteration. First described by Brunschwig in 1948, total pelvic exenteration involves the evacuation of all pelvic organs, including the bladder, rectum, vagina, urethra, vulva, clitoris, and anus [24]. It requires the creation of a urinary conduit (either continent or noncontinent) for urine and a colostomy for stool, as well as the reconstruction of the pelvic floor and vagina using flaps from areas such as the rectus abdominus or gracilis muscles (Figure 5). Less extensive variations such as anterior (rectum sparing) and posterior (bladder sparing) pelvic exenterations are well described in instances where these structures are clearly free of tumor. Recently, laparoscopic and robotic approaches have been reported [25, 26].
Figure 5.
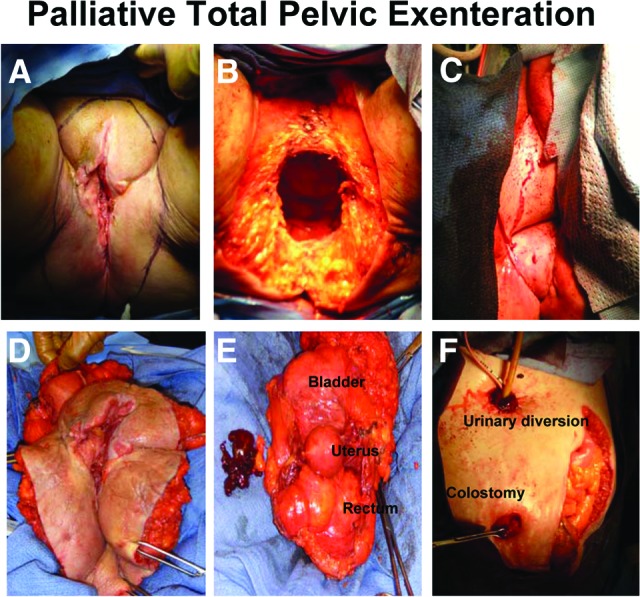
Palliative total pelvic exenteration. (A): Outline of perineal incision encompassing vulvar lesion. (B): Perineum after removal of pelvic structures. (C): Perineum after reconstruction with ventral rectus abdominus myocutaneous flap. (D): External view of specimen including vulva, vagina, perineal body, and anus. (E): Internal view of specimen including bladder, uterus, and rectum removed en masse. (F): Abdomen after creation of ileal conduit and diverting end colostomy.
Regardless of approach, operative times are long (5–14 hours) and potential complications innumerable [27–38]. Reported blood loss ranges from 2,300–4,000 cm3 (median: 3,000 cm3). Wound sepsis and dehiscence (12%), urinary fistula/obstruction (6%), intestinal leak (8%), small bowel obstruction (5%), postoperative hemorrhage (2.5%), and pulmonary embolus (1.5%) are among the most commonly reported complications [31–32, 37–41]. Other reported morbidities include deep vein thrombosis, myocardial infarction, stroke, flap necrosis, flap and stomal necrosis, septicemia, drug reactions, and pneumonia. In the earliest series, perioperative mortality was as high as 24%, but with effective use of critical care, antibiotics, and thromboembolic prophylaxis, these numbers have improved to less than 5% (range: 2%–14%) in most case series [24, 27–32, 34–36, 40–53].
In the classic teaching, the pelvic exenteration was a procedure reserved for the rare circumstance in which other treatment options were exhausted, and yet the glimmer of cure remained present in patients with a central pelvic recurrence. Contraindications to exenteration included indicators of incurability such as distant metastases, pelvic bone invasion, major blood vessel or pelvic wall musculature involvement, and intraperitoneal disease [54]. However, palliative exenterations are also performed and reported by gynecologic oncologists when patient suffering is enormous and quality-of-life is obliterated by disease symptoms such as tumor necrosis, fistulae, local sepsis, chronic bleeding, and severe localized pain [54, 55].
The literature on palliative exenteration stems back to 1976, when Deckers et al. challenged traditional dogma and argued that pelvic exenteration as intentional palliation can benefit selected patients. Since that time, there has been little consensus as to both the definition of and indications for palliative exenteration (Table 2) [41, 49, 56–61]. Magrina et al. defined palliative exenteration as tumor extension to the lateral pelvic wall or positive pelvic and para-aortic lymph nodes and reported a 5-year survival of 27% in this cohort [49]. Stanhope et al. used similar criteria with the addition of bony involvement or distant metastases, reporting a median survival of 19 months with 47% (28/59 patients) surviving 2 years [41]. Lambrou et al. used symptoms such as fistula, cystitis, and proctitis refractory to other treatments as indications for palliative exenteration and reported a 5-year survival of 17% [57]. Most recently, Marnitz et al. reported on 18 patients who underwent palliative exenteration for advanced cervical cancer, with a 2-year survival of only 10.5% compared to 60% for their curative intent counterparts [58]. In the multivariate survival analysis, these authors found a direct correlation between the presence of positive resection margins and the designation of palliative intent. From this finding, they suggest that preoperative assessment of resectability is an effective discriminator between curative and palliative intent exenterative procedures.
Table 2.
Literature on palliative pelvic exenteration
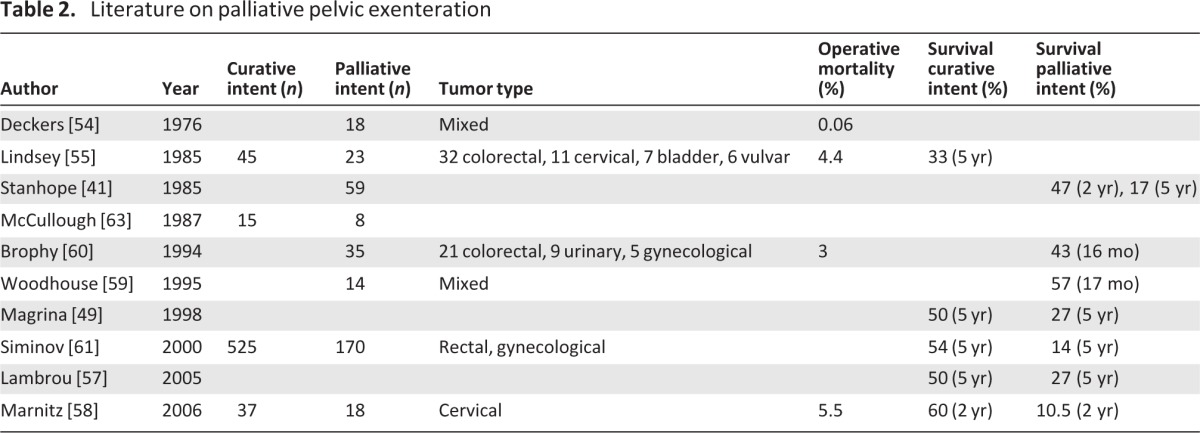
In general, the literature on palliative pelvic exenteration is hard to compare because of inconsistencies in cancer types, surgical indications, and patient selection criteria. Two-year survival rates range from 10%–47% for palliative exenterations compared to 5-year survivals of 20%–60% for pelvic exenterations in general [41, 49, 56–61]. However, survival may not be the best measure of success. The reason for these palliative procedures is to alleviate suffering and not necessarily to prolong life. Although some authors have argued that exenterations should never be considered palliative given that recovery can take 3–6 months and patients may not live long enough to benefit [53], others have found that 67%–90% of patients do find improvement in quality of life and symptom relief following palliative exenteration [56].
In general, the literature on palliative pelvic exenteration is hard to compare because of inconsistencies in cancer types, surgical indications, and patient selection criteria. Two-year survival rates range from 10%–47% for palliative exenterations compared to 5-year survivals of 20%–60% for pelvic exenterations in general. However, survival may not be the best measure of success. The reason for these palliative procedures is to alleviate suffering and not necessarily to prolong life.
Balancing Morbidity and Quality of Life
The palliative interventions described in this article are not without morbidity. Well over 50% of patients undergoing surgery for malignant bowel obstruction will end up with permanent diversions. Patients undergoing palliative exenteration will often have two noncontinent ostomies and significant loss of nascent anatomy. In addition, the infectious, hematologic, and healing complications in these highly pretreated patients can completely undermine the desired gain in quality of life that initially motivated the intervention. Furthermore, review of the literature reveals no consensus of selection criteria for patients likely to benefit from surgery.
Despite these cautions, evidence described in this review demonstrates that successful palliation is possible in advanced gynecologic cancer. Surgical palliation can also open the door to other life-prolonging interventions. In a recent case series, surgical correction of malignant bowel obstruction enabled chemotherapy to be administered in 79% of overall cases and 92% of successfully palliated cases [20]. In addition, the added chemotherapy conferred a statistically significant survival benefit (9.6 versus 2.5 months; p < .01) [20]. Additionally, in a prospective outcomes analysis of palliative procedures performed for malignant intestinal obstruction because of recurrent ovarian cancer, 26 patients underwent either operative intervention (n = 14) or endoscopic procedures (n = 12), it was noted that at 60 days, 10 (71%) of 14 patients who underwent operative procedures and 6 (50%) of 12 patients who had endoscopic procedures had symptom control [62]. Median survival for those undergoing surgical intervention was 191 days versus 78 days in patients undergoing endoscopic procedures. This data is important for helping patients to weigh the significant risks of a palliative surgery with the potential benefits.
The decision for palliative intervention in end-stage gynecologic cancers is among the most difficult decisions that a patient, her family, and a gynecological oncologist will face. There is no right or wrong answer. There is no level I data to guide decision making. Two patients, along with their physicians, in identical situations may make opposite and equally valid choices. In select situations, major surgery may enhance the quality of the life remaining for patients with advanced gynecologic cancer.
Author Contributions
Conception and design: Bhavana Pothuri
Provision of study materials or patients: Joanie Mayer Hope, Bhavana Pothuri
Collection and/or assembly of data: Joanie Mayer Hope, Bhavana Pothuri
Data analysis and interpretation: Joanie Mayer Hope, Bhavana Pothuri
Manuscript writing: Joanie Mayer Hope, Bhavana Pothuri
Final approval of manuscript: Bhavana Pothuri
Disclosures
The authors indicated no financial relationships.
Section Editors: Dennis Chi: None; Peter Harper: Sanofi, Roche, Imclone, Pfizer, GlaxoSmithKline, Lilly, Genentech (C/A); Lilly, Novartis, Sanofi, Roche (H)
Reviewer “A”: Intuitive Surgical (C/A); Vermillion, Intuitive Surgical (H)
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Baines MJ. The pathophysiology and management of malignant intestinal obstruction. In: Doyle D, Hanks GWC, MacDonald N, editors. Oxford Textbook of Palliative Medicine. Oxford, U.K.: Oxford University Press; 1998. pp. 526–534. [Google Scholar]
- 2.Storey PS. Obstruction of the GI tract. Am J Hospice Palliat Care. 1991;8:5. doi: 10.1177/104990919100800310. [DOI] [PubMed] [Google Scholar]
- 3.Lund B, Lundvall F, Hansen HH. Intestinal obstruction in patients with advanced carcinoma of the ovaries treated with combination chemotherapy. Sug Gynecol Obstet. 1989;169:213–218. [PubMed] [Google Scholar]
- 4.Tunca JC, Buchler DA, Mack EA, et al. The management of ovarian-cancer caused bowel obstruction. Gynecol Oncol. 1981;12:186–192. doi: 10.1016/0090-8258(81)90148-7. [DOI] [PubMed] [Google Scholar]
- 5.Clarke-Pearson DL, Chin N, DeLong ER, et al. Surgical management of intestinal obstruction in ovarian cancer. Gynecol Oncol. 1987;26:11–18. doi: 10.1016/0090-8258(87)90066-7. [DOI] [PubMed] [Google Scholar]
- 6.Solomon HJ, Atkinson KH, Coppleson JV, et al. Bowel complications in the management of ovarian cancer. Aust N Z J Obstet Gynecol. 1983;23:65–68. doi: 10.1111/j.1479-828x.1983.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 7.Ventafridda V, Ripamonti C, Caraceni A, et al. The management of inoperable gastrointestinal obstruction in terminal cancer patients. Tumori. 1990;76:389–392. doi: 10.1177/030089169007600417. [DOI] [PubMed] [Google Scholar]
- 8.Muir JC. Malignant bowel obstruction. In: Berger AM, Portenoy RK, Weissman DE, editors. Principles and Practices of Supportive Oncology Updates. 5. Vol. 2. Philadelphia: Lippincott, Williams & Wilkins; 1999. pp. 1–6. [Google Scholar]
- 9.Castaldo TW, Petrilli ES, Ballon SC, et al. Intestinal operations in patients with ovarian carcinoma. Am J Obstet Gynecol. 1981;139:80–84. doi: 10.1016/0002-9378(81)90416-6. [DOI] [PubMed] [Google Scholar]
- 10.Piver MS, Marlow JJ, Lele SB, et al. Survival after ovarian cancer induced intestinal obstruction. Gynecol Oncol. 1982;13:44–49. doi: 10.1016/0090-8258(82)90007-5. [DOI] [PubMed] [Google Scholar]
- 11.Krebs HB, Gopelrud DR. Surgical management of bowel obstruction in advanced ovarian carcinoma. Obstet Gynecol. 1981;61:327–330. [PubMed] [Google Scholar]
- 12.Redman CW, Shafi MI, Ambrose S, et al. Survival following intestinal obstruction in ovarian cancer. Eur J Surg Oncol. 1988;14:383–386. [PubMed] [Google Scholar]
- 13.Fernandes JR, Seymour RJ, Suissa S. Bowel obstruction in patients with ovarian cancer: A search for prognostic factors. Am J Obstet Gynecol. 1988;158:244–249. doi: 10.1016/0002-9378(88)90131-7. [DOI] [PubMed] [Google Scholar]
- 14.Rubin SC, Hoskins WJ, Benjamin I, et al. Palliative surgery for intestinal obstruction in advanced ovarian cancer. Gynecol Oncol. 1989;34:16–19. doi: 10.1016/0090-8258(89)90097-8. [DOI] [PubMed] [Google Scholar]
- 15.Larsen JE, Podczawki ES, Manetta A, et al. Bowel obstruction in patients with ovarian carcinoma: Analysis of prognostic factors. Gynecol Oncol. 1989;35:61–65. doi: 10.1016/0090-8258(89)90012-7. [DOI] [PubMed] [Google Scholar]
- 16.Zeotmulder A, Helmerhosrts TJ, van Coevorden F, et al. Management of bowel obstruction in patients with advanced ovarian cancer. Eur J Cancer. 1994;30A:1625–1628. doi: 10.1016/0959-8049(94)e0131-m. [DOI] [PubMed] [Google Scholar]
- 17.Bartels CJ, Reed E, Sindelar WF. Intestinal obstruction due to advanced ovarian cancer: Results of surgical intervention. Surg Forum. 1995:564–566. [Google Scholar]
- 18.Jong P, Sturgeon J, Jamieson CG. Benefit of palliative surgery for bowel obstruction in advanced ovarian cancer. Cancer J Surg. 1995;38:454–457. [PubMed] [Google Scholar]
- 19.Bais JJ, Schilthuis MS, Slors JM, et al. Intestinal obstruction in patients with ovarian cancer. Int J Gynecol Cancer. 1995;5:346–350. doi: 10.1046/j.1525-1438.1995.05050346.x. [DOI] [PubMed] [Google Scholar]
- 20.Pothuri B, Vaidya A, Aghajanian C, et al. Palliative surgery for bowel obstruction in recurrent ovarian cancer: An updated series. Gynecol Oncol. 2003;89:306–331. doi: 10.1016/s0090-8258(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 21.Li ZT, Wu XH, Fu SL. Benefit of palliative surgery for bowel obstruction in recurrent ovarian carcinoma. Zhonghua Fu Chan Ke Za Zhi. 2004;39:260–263. [PubMed] [Google Scholar]
- 22.Pothuri B, Meyer L, Gerardi M, et al. Reoperation for palliation of recurrent malignant bowel obstruction. Gynecol Oncol. 2004;95:193–195. doi: 10.1016/j.ygyno.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Caprotti R, Bonardi C, Crippa S, et al. Palliative surgery for recurrent bowel obstruction due to advanced ovarian cancer. Minerva Ginecologica. 2006;58:239–244. [PubMed] [Google Scholar]
- 24.Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma. Cancer. 1948;1:177–183. doi: 10.1002/1097-0142(194807)1:2<177::aid-cncr2820010203>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Lin MY, Fan EW, Chiu AW, et al. Laparoscopy-assisted transvaginal total exenteration for locally advanced cervial cancer with bladder invasion after radiotherapy. J Endourol. 18:867–870. doi: 10.1089/end.2004.18.867. 204. [DOI] [PubMed] [Google Scholar]
- 26.Pomel C, Ruzier R, Pocard M, et al. Laparoscopic total pelvic exenteration for cervical cancer relapse. Gynecol Oncol. 2003;91:616–618. doi: 10.1016/j.ygyno.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Berek SJ, Howe C, Lagasse LD, Hacker NF. Pelvic exenteration for recurrent gynaecologic malignancy: Survival and morbidity analysis of the 45-year experience at UCLA. Gynecol Oncol. 2005;99:153–159. doi: 10.1016/j.ygyno.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 28.Hatch KD, Shingelton HM, Soong SJ, et al. Anterior pelvic exenteration. Gynecol Oncol. 1988;31:205–213. doi: 10.1016/0090-8258(88)90294-6. [DOI] [PubMed] [Google Scholar]
- 29.Höckel M. Laterally extended endopelvic resection—Novel surgical treatment of locally recurrent cervical carcinoma involving the pelvic side wall. Gynecol Oncol. 2003;91:369–377. doi: 10.1016/s0090-8258(03)00502-x. [DOI] [PubMed] [Google Scholar]
- 30.Ketcham AS, Deckers PJ, Sugarbaker EV, et al. Pelvic exenteration for carcinoma of the uterine cervix—A 15-year experience. Cancer. 1970;26:513–521. doi: 10.1002/1097-0142(197009)26:3<513::aid-cncr2820260304>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Matthews CM, Morris M, Burke TW, et al. Pelvic exenteration in the elderly patient. Obstet Gynecol. 1992;79:773–777. [PubMed] [Google Scholar]
- 32.Morley GW, Hopkins MP, Lindenauer SM, et al. Pelvic exenteration, University of Michigan: 100 patients at 5 years. Obstet Gynecol. 1989;74:934–943. [PubMed] [Google Scholar]
- 33.Numa F, Ogata H, Suminami Y, et al. Pelvic exenteration for the treatment of gynecologic malignancies. Arch Gynecol Obstet. 1997;259:133–138. doi: 10.1007/BF02505321. [DOI] [PubMed] [Google Scholar]
- 34.Roberts WS, Cavanagh D, Bryson P, et al. Major morbidity after pelvic exenteration. A seven-year experience. Obstet Gynecol. 1987;69:617–621. [PubMed] [Google Scholar]
- 35.Sharma S, Odunsi K, Driscoll D, Lele S. Pelvic exenteration for gynecological malignancies: Twenty-year experience at Rosewell Park Cancer Institute. Int J Gynecol Cancer. 2005;15:475–482. doi: 10.1111/j.1525-1438.2005.15311.x. [DOI] [PubMed] [Google Scholar]
- 36.Soper JT, Berchuck A, Creasman WT, et al. Pelvic exenteration: factors associated with major surgical morbidity. Gynecol Oncol. 1989;35:93–98. doi: 10.1016/0090-8258(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 37.Curtin J, Morrow Surgery for cervical neoplasia. Gynecologic Cancer Surgery. 1995;1:555. [Google Scholar]
- 38.Avarette HE, Licktinger M, Seven BU, Girtanner RE. Pelvic exenteration: A 15-year experience in a general metropolitan hospital. Am J Obstet Gynecol. 1984;150:179–184. doi: 10.1016/s0002-9378(84)80013-7. [DOI] [PubMed] [Google Scholar]
- 39.Kraybill WG, Lopez MJ, Bricker EM. Total pelvic exenteration as a therapeutic option in advanced malignant disease of the pelvis. Surg Gynecol Obstet. 1988;44:259–263. [PubMed] [Google Scholar]
- 40.Robertson G, Lopes A, Beynon G, et al. Pelvic exenteration: A review of the Gateshead experience, 1974–1992. Br J Obstet Gynecol. 1994;101:529–531. doi: 10.1111/j.1471-0528.1994.tb13156.x. [DOI] [PubMed] [Google Scholar]
- 41.Stanhope CR, Webb JM, Podratz KC. Pelvic exenteration for recurrent cervical cancer. Clin Obstet Gynecol. 1990;33:897–909. doi: 10.1097/00003081-199012000-00026. [DOI] [PubMed] [Google Scholar]
- 42.Barber H. Relative prognostic significance of preoperative and operative findings in pelvic exenteration. Surg Clin North Am. 1969;49:431–447. doi: 10.1016/s0039-6109(16)38800-4. [DOI] [PubMed] [Google Scholar]
- 43.Bricker EM. Bladder substitution after pelvic evisceration. Surg Clin North Am. 1950;30:1511–1521. doi: 10.1016/s0039-6109(16)33147-4. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg JM, Piver S, Hempling RE, et al. Improvement in pelvic exenteration: Factors responsible for reducing morbidity and mortality. Ann Surg Oncol. 1998;5:399–406. doi: 10.1007/BF02303857. [DOI] [PubMed] [Google Scholar]
- 45.Hawighorst-Knapstein S, Fusshoeller C, Franz C, et al. The impact of treatment for genital cancer on quality of life and body image—Results of a prospective longitudinal 10-year study. Gynecol Oncol. 2004;94:398–403. doi: 10.1016/j.ygyno.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Houvenaeghel G, Moutardier V, Karsenty G, et al. Major complications of urinary diversion after pelvic exenteration for gynecologic malignancies: A 23-year mono-institutional experience in 124 patients. Gynecol Oncol. 2004;92:680–683. doi: 10.1016/j.ygyno.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Karsenty G, Moutardier V, Lelong B, et al. Long-term follow up of continent urinary diversion after pelvic exenteration for gynecologic malignancies. Gynecol Oncol. 2005;97:524–528. doi: 10.1016/j.ygyno.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Lawhead RA, Clark D, Smith DH, et al. Pelvic exenteration for recurrent or persistent gynaecologic malignancies: A 10-year review of the Memorial-Sloan-Kettering Cancer Center experience (1972–1981) Gynecol Oncol. 1989;33:279–282. doi: 10.1016/0090-8258(89)90512-x. [DOI] [PubMed] [Google Scholar]
- 49.Magrina JF, Stanhope CR, Waever AL. Pelvic exenterations: Supralevator, infralevator, and with vulvectomy. Gynecol Oncol. 1997;64:130–135. doi: 10.1006/gyno.1996.4532. [DOI] [PubMed] [Google Scholar]
- 50.Roos EJ, van Eijkeren MA, Boon TA, et al. Pelvic exenteration as treatment of recurrent gynecologic and urologic cancer. Int J Gynecol Cancer. 2005;15:624–629. doi: 10.1111/j.1525-1438.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 51.Saunders N. Pelvic exenteration: By whom and for whom? Lancet. 1995;345:5–6. doi: 10.1016/s0140-6736(95)91146-4. [DOI] [PubMed] [Google Scholar]
- 52.Shingelton HM, Soong SJ, Gelder MS, et al. Clinical and histopathologic factors predicting recurrence and survival after pelvic exenteration for cancer of the cervix. Obstet Gynecol. 1989;73:1027–1034. doi: 10.1097/00006250-198906000-00024. [DOI] [PubMed] [Google Scholar]
- 53.Symmonds RE, Pratt JH, Webb MJ. Exenterative operations: Experience with 198 patients. Am J Obstet Gynecol. 1975;121:907–918. doi: 10.1016/0002-9378(75)90908-4. [DOI] [PubMed] [Google Scholar]
- 54.Deckers PJ, Olsson C, Williams LA, et al. Pelvic exenteration as palliation of malignant disease. Am J Surg. 1976;131:509–515. doi: 10.1016/0002-9610(76)90166-5. [DOI] [PubMed] [Google Scholar]
- 55.Lindsey WF, Wood DK, Briele HA, et al. Pelvic exenteration. J Surg Oncol. 1985;30:231–234. doi: 10.1002/jso.2930300409. [DOI] [PubMed] [Google Scholar]
- 56.Crowe PJ, Temple WJ, Lopez MJ. Ketcham AS. Pelvic exenteration for advanced pelvic malignancy. Semin Surg Oncol. 1999;17:152–160. doi: 10.1002/(sici)1098-2388(199910/11)17:3<152::aid-ssu3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 57.Lambrou NC, Pearson JM, Averette HE. Pelvic exenteration of gynecologic malignancy: Indications and technical and reconstructive considerations. Surg Oncol Clin N Am. 2005;14:289–300. doi: 10.1016/j.soc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Marnitz S, Kohler C, Marianne M, et al. Indications for primary and secondary exenterations in patients with cervical cancer. Gynecol Oncol. 2006;103:1023–1030. doi: 10.1016/j.ygyno.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 59.Woodhouse CR, Plail RO, Schlesinger PE, et al. Exenteration as palliation for patients with advanced pelvic malignancy. Br J Urol. 1995;76:315–320. doi: 10.1111/j.1464-410x.1995.tb07707.x. [DOI] [PubMed] [Google Scholar]
- 60.Brophy PF, Hoffman JP, Eisenberg BL. The role of palliative exenteration. Am J Surg. 1994;167:386–390. doi: 10.1016/0002-9610(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 61.Siminov NN, Guliawev AV, Maksimon S, et al. Effectiveness of combined resection and exenteration of the pelvic organs as part of the comprehensive treatment of extensive malignancies of the rectum and female genitals. Vopr Onkol. 2000;46:224–228. [PubMed] [Google Scholar]
- 62.Chi DS, Phaëton R, Miner TJ, et al. A prospective outcomes analysis of palliative procedures performed for malignant intestinal obstruction due to recurrent ovarian cancer. The Oncologist. 2009;14:835–839. doi: 10.1634/theoncologist.2009-0057. [DOI] [PubMed] [Google Scholar]
- 63.McCullough WM, Nahhas WA. Palliative pelvic exenteration–futility revisited. Gynecol Oncol. 1987;27:97–103. doi: 10.1016/0090-8258(87)90235-6. [DOI] [PubMed] [Google Scholar]