This study assesses the incidence of breast cancer recurrence (locoregional and distant) and contralateral breast cancer by age at diagnosis among postmenopausal patients with hormone-sensitive breast cancer.
Keywords: Breast cancer, Elderly, Outcome, Recurrence, Hormone receptor positive
Abstract
Introduction.
For postmenopausal patients with hormone-sensitive breast cancer, outcome is worse with increasing age at diagnosis. The aim of this study was to assess the incidence of breast cancer recurrence (locoregional and distant), and contralateral breast cancer by age at diagnosis.
Methods.
Patients enrolled in the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial were included. Primary endpoints were locoregional recurrence, distant recurrence, and contralateral breast cancer. Age at diagnosis was categorized as younger than 65 years, 65–74 years, and 75 years or older.
Results.
Overall, 9,766 patients were included, of which 5,349 were younger than 65 years (reference group), 3,060 were 65–74 years, and 1,357 were 75 years or older. With increasing age, a decreased administration of radiotherapy after breast conserving surgery (94%, 92%, and 88%, respectively) and adjuvant chemotherapy (51%, 23%, and 5%, respectively) was observed. Risk of distant recurrence increased with age at diagnosis; multivariable hazard ratio for patients aged 65–74 years was 1.20 (95% confidence interval [CI]: 1.00–1.44), hazard ratio for patients aged 75 years or older was 1.39 (95% CI: 1.08–1.79). Risks of locoregional recurrence and contralateral breast cancer were not significantly different across age groups.
Conclusion.
Elderly patients with breast cancer were at increased risk for distant recurrence. Other studies have shown that the risk of distant recurrence is mainly affected by adjuvant systemic therapy. All TEAM patients received adjuvant endocrine treatment; however, chemotherapy was administered less often in elderly patients. These findings are suggestive for consideration of chemotherapy in relatively fit elderly breast cancer patients with hormone-sensitive disease.
Implications for Practice:
In this study, we analyzed 9,766 postmenopausal breast cancer patients with hormone-sensitive disease who were included in the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial. We demonstrated a higher incidence of distant breast cancer recurrence with increasing age at diagnosis. Thus, the common belief that the clinical course of breast cancer in older women may be more indolent is rejected in this study. All patients received endocrine therapy, while radiotherapy after breast-conserving surgery and administration of chemotherapy decreased with increasing age. As distant recurrence may reflect under use of systemic therapy, these findings hint at under treatment of systemic therapy, and chemotherapy in particular. Consequently, chemotherapy may be considered more often in relatively fit elderly breast cancer patients with hormone-sensitive disease.
Introduction
Breast cancer is the most common type of cancer in women in Western societies. Worldwide, nearly a third of all breast cancer patients are 65 years or older, and in more developed countries this proportion increases to over 40% [1]. Because of an increasing life expectancy and raised breast cancer incidence with increasing age, the disease will progressively affect the lives of elderly women [2].
Many researchers have published on the worse prognosis of premenopausal compared with postmenopausal patients with breast cancer [3–5]. However, evidence is lacking on age-specific breast cancer outcome within postmenopausal women. Recently, we reported that breast cancer outcome in postmenopausal patients deteriorated with increasing age [6]. To gain further insight in the relationship between age at diagnosis and breast cancer outcome, we studied the incidence of breast cancer recurrence (locoregional and distant), and contralateral breast cancer by age at diagnosis for patients included in the Tamoxifen Exemestane Adjuvant Multinational (TEAM) Trial.
Methods
Study Population
The TEAM trial has been described extensively in previous reports [6, 7]. In short, 9,766 postmenopausal women with estrogen (ER) and/or progesterone receptor (PR)-positive breast cancer who completed local therapy with curative intent were randomized to receive either exemestane 25 mg daily for 5 years or a sequential regimen consisting of tamoxifen 20 mg daily for 2.5–3 years, followed by exemestane 25 mg daily for 2.5–2 years. Adjuvant chemotherapy, if indicated, was given before the start of endocrine therapy, and radiotherapy was administered according to local practice. Participants commenced the assigned endocrine study treatment within 10 weeks of completion of surgery and chemotherapy, if indicated.
Patients were ineligible if they had a malignancy within 5 years preceding breast cancer diagnosis, an Eastern Cooperative Oncology Group performance status of more than 2, or a significant cardiac disease or other illness interfering with study participation and adequate follow-up. Participants were enrolled in Belgium, The Netherlands, United Kingdom, Ireland, USA, Japan, Greece, Germany, and France. Similar protocols were used in these nine countries, with minor differences to accommodate the local treatment guidelines [8]. The trial was registered with ClinicalTrials.gov (NCT00279448, NCT00032136, and NCT00036270), Netherlands Trial Registry 267, Ethics Commission Trials (27/2001), and University hospital Medical Information Network (C000000057).
Because the final results of the TEAM trial showed no significant differences in efficacy endpoints between both treatment arms [7], we were able to investigate disease recurrence regardless of randomized treatment. The database was locked on October 7, 2010. The design of the current post hoc analysis was developed in July 2011.
Patients were categorized into three groups based on age at diagnosis (younger than 65 years, 65–74 years, and 75 years or older) as discussed at the Meeting of the International Society of Geriatric Oncology in 2009 and in line with other publications [6, 9, 10]. Study endpoints were as follows: (a) locoregional recurrence (recurrence in the ipsilateral breast or chest wall, recurrence in ipsilateral axillary or supraclavicular lymph node(s), or other locoregional localization), (b) distant recurrence (recurrence in bone, skin, liver, lung, brain, or other distant localization), and (c) contralateral breast cancer (new primary invasive tumor in the contralateral breast), whichever came first. In situ carcinoma was not considered to be a recurrence. For 61 patients with synchronously recurrent disease at more than one site, the localization most likely determining the prognosis was used as endpoint.
Statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL). Cox proportional hazard models were used to evaluate the association between age at diagnosis and the endpoints. Covariates were included in the multivariable model if they were judged to be clinically relevant, regardless of statistical significance. First, estimates were adjusted for country and tumor characteristics (country of residence, histological grade [Bloom Richardson grade I, II, III], T stage [T1, T2, T3/T4], nodal stage [negative, positive], estrogen receptor status [negative, positive], and progesterone receptor status [negative, positive]). Next, the fully adjusted model comprised both tumor and treatment characteristics (country of residence, histological grade, T stage, nodal stage, estrogen receptor status, progesterone receptor status, type of surgery [mastectomy, wide local excision], radiotherapy [yes, no], chemotherapy [yes, no], allocated endocrine therapy [tamoxifen followed by exemestane, exemestane] and persistence of endocrine therapy [discontinuation of allocated endocrine therapy because of either adverse events, intercurrent illness, patient refusal or other reasons; continuation of allocated endocrine therapy, or having an event while on study medication]). Patients with missing data were not included in the multivariable model. All statistical tests were two sided; p values <.05 were considered to be statistically significant.
Role of the Funding Source
The study investigators were responsible for the study design, collection, and interpretation of data. The study sponsor had no influence on the study design, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication. The authors had full access to all the data in the study. The corresponding author had the final responsibility for the decision to submit for publication.
Results
Overall, 9,766 patients (range: 35–96, median age: 64 years) were included; 5,349 were younger than 65 years (median: 58 years), 3,060 were 65–74 years (median: 69 years), and 1,357 were 75 years or older (median: 79 years). Baseline characteristics by age groups were shown in an earlier report [6]; elderly patients presented with larger tumors without differences in nodal status. With increasing age, the proportion of mastectomy increased significantly, whereas a marked decrease was observed in the administration of radiotherapy following a wide local excision (94%, 92%, and 88%, respectively; p < .001) and administration of chemotherapy (51%, 23%, and 5%, respectively; p < .001) [6].
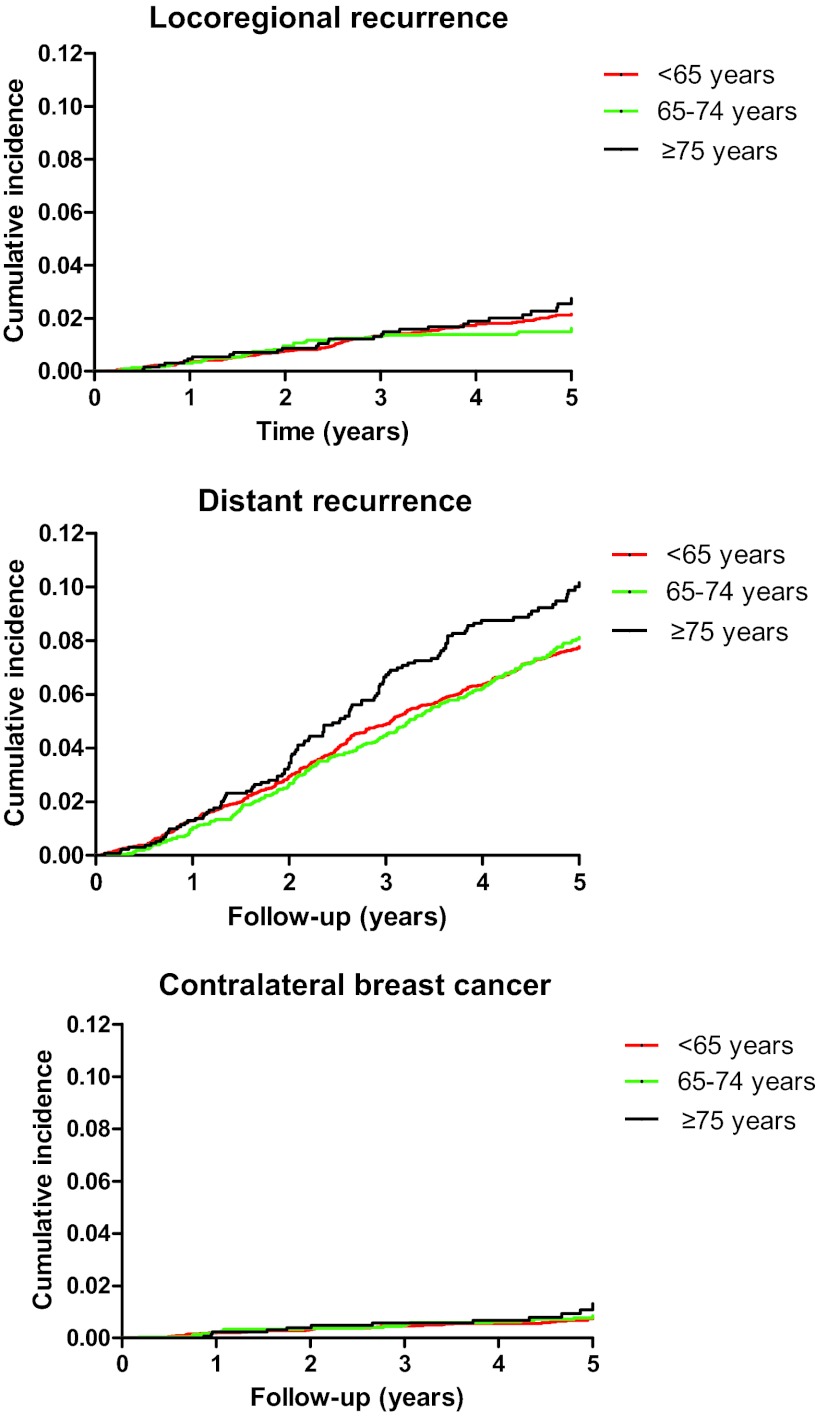
At database lock, median follow up (interquartile range) from randomization was 5.1 years (4.2–6.0 years), during which 1,062 first events were registered: 193 locoregional recurrences, 786 distant recurrences, and 83 contralateral breast cancers. As shown in Table 1, the distribution of locoregional recurrence and distant recurrence was similar across age groups. Figure 1 shows the cumulative incidence of endpoints by age at diagnosis. Cumulative incidence of locoregional recurrence was 2.1%, 1.6%, and 2.4%, respectively; cumulative incidence of distant recurrence increased from 7.6% in patients younger than 65 years, 8.1% in patients aged 65–74 years of age, to 9.6% in patients aged 75 years or older. Cumulative incidences of contralateral breast cancer were 0.8%, 0.8%, and 1.0%, respectively.
Table 1.
Distribution of locoregional recurrence and distant recurrence by age at diagnosis
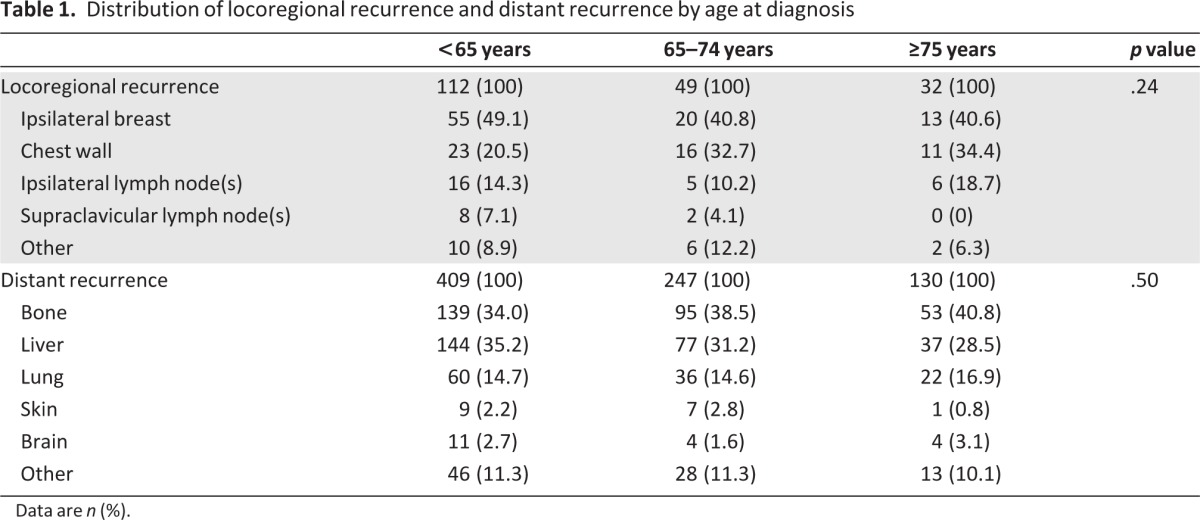
Data are n (%).
Figure 1.
Cumulative incidence of locoregional recurrence, distant recurrence, and contralateral breast cancer by age at diagnosis.
Table 2 shows the results of Cox regression analyses. In both univariate and multivariable analyses, the risk of locoregional recurrence was similar across age categories. Contrary, the risk of distant recurrence increased with increasing age at diagnosis. Patients aged younger than 65 years functioned as a reference, univariate hazard ratio (HR) for patients aged 65–74 was 1.06 (95% confidence interval [CI]: 0.91–1.24) and HR for patients aged 75 years or older was 1.37 (95% CI: 1.13–1.68); p = .006. Both the partly and fully adjusted model showed comparable results; the fully adjusted HR for patients aged 65–74 was 1.20 (95% CI: 1.00–1.44). The HR for patients aged 75 years or older was 1.39 (95% CI: 1.08–1.79); p = .024. The risk of contralateral breast cancer was not significantly different across age categories.
Table 2.
Breast cancer recurrence by age at diagnosis
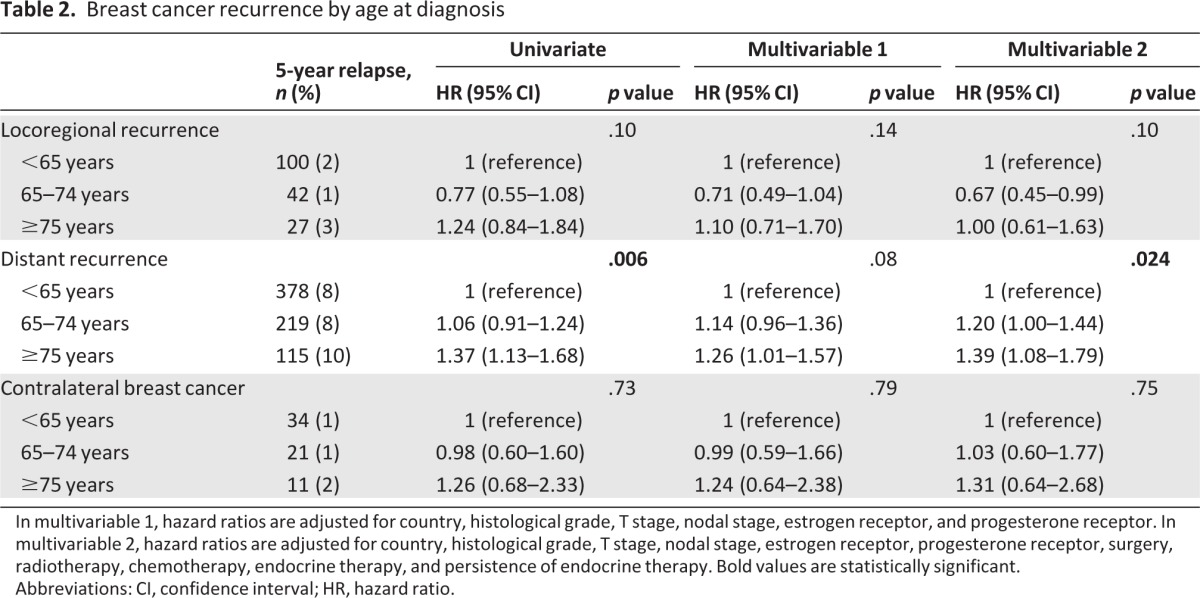
In multivariable 1, hazard ratios are adjusted for country, histological grade, T stage, nodal stage, estrogen receptor, and progesterone receptor. In multivariable 2, hazard ratios are adjusted for country, histological grade, T stage, nodal stage, estrogen receptor, progesterone receptor, surgery, radiotherapy, chemotherapy, endocrine therapy, and persistence of endocrine therapy. Bold values are statistically significant.
Abbreviations: CI, confidence interval; HR, hazard ratio.
To test the sensitivity of the endpoints, three alternative analyses were performed. The results of these alternative analyses were similar to the main results. First, survival analyses were repeated without restriction to the first site of recurrence; that is, all events irrespective of the sequence of occurrence were included in the analysis. Multivariable HR for locoregional recurrence was 0.79 for patients aged 65–74 years (95% CI: 0.57–1.09) and 0.94 for patients aged 75 years or older (95% CI: 0.62–1.43); p = .335. Multivariable HR for distant recurrence was 1.17 for patients aged 65–74 years (95% CI: 0.98–1.40) and 1.39 for patients aged 75 years or older (95% CI: 1.09–1.76); p = .024. Multivariable HR for contralateral breast cancer was 0.90 for patients aged 65–74 years (95% CI: 0.53–1.52) and 0.92 for patients aged 75 years or older (95% CI: 0.46–1.83); p = .917.
Second, synchronous endpoints (n = 61) were recoded as locoregional recurrence and contralateral breast cancer, respectively. Multivariable HR for locoregional recurrence was 0.76 for patients aged 65–74 years (95% CI: 0.54–1.06) and 0.92 for patients aged 75 years or older (95% CI: 0.59–1.42); p = .260. Multivariable HR for distant recurrence was 1.20 for patients aged 65–74 years (95% CI: 0.99–1.46) and 1.45 for patients aged 75 years or older (95% CI: 1.12–1.88); p = .015. Multivariable HR for contralateral breast cancer was 1.05 for patients aged 65–74 years (95% CI: 0.62–1.80) and 1.46 for patients aged 75 years or older (95% CI: 0.73–2.94); p = .546.
Third, contralateral breast cancer was recoded as locoregional recurrence. Multivariable HR for locoregional recurrence was 0.77 for patients aged 65–74 years (95% CI: 0.56–1.06) and 1.08 for patients aged 75 years or older (95% CI: 0.72–1.62); p = .164.
Also, two additional analyses were performed to diminish selection bias. First, survival analyses for distant recurrence were stratified by T stage because increasing age was associated with larger tumors (supplemental online Table 1). Although not significant, estimates were comparable to the main analysis. Second, survival analyses for locoregional recurrence were stratified by most extensive surgery because elderly patients more frequently underwent a mastectomy (supplemental online Table 2). Again, the results remained similar.
Discussion
We found that elderly patients with hormone-sensitive breast cancer participating in the TEAM trial had a higher risk of distant recurrence, although the risks of locoregional recurrence and contralateral breast cancer did not significantly differ across age groups. Additional analyses were performed to test the robustness of the endpoints and to explore whether our findings may have been biased. Inclusion of three alternative definitions of endpoints did not alter the results. Moreover, stratified analyses by T stage and most extensive surgery revealed comparable estimates.
Many studies have been published on predictors of breast cancer recurrence in premenopausal compared to postmenopausal patients. Virtually all studies observed a higher risk of locoregional breast cancer recurrence in premenopausal compared to postmenopausal women [4, 5, 11–14]. Few studies addressed breast cancer recurrence within postmenopausal patients, and again most focused on locoregional recurrence [15–18]. It is tempting to speculate on the possible mechanisms that may explain our findings. Based on the literature, we hypothesize that locoregional recurrence may reflect suboptimal local [19] and/or systemic treatment [20], whereas distant recurrence and contralateral breast cancer more likely reflect suboptimal systemic treatment [20–23]. Because all TEAM patients received endocrine treatment, the decreased administration of chemotherapy with increasing age may have contributed to a higher distant breast cancer recurrence in elderly patients. Of note, the hazard ratio for contralateral breast cancer for patients aged 75 years or older was comparable with the hazard ratio for distant recurrence, but the distribution of contralateral breast cancer was not significantly different across age groups, possibly due to a low number of events.
Few researchers have studied chemotherapy efficacy in elderly patients with breast cancer. In a meta-analysis from the Early Breast Cancer Trialists' Collaborative Group, not enough women older than 70 years were included to be able to draw conclusions about chemotherapy efficacy in this age group [20]. However, a review of randomized clinical trials on chemotherapy in patients with node-positive breast cancer revealed that older patients derived similar reductions in breast cancer mortality and recurrence compared to younger patients [24]. Recently, Muss et al. evaluated the efficacy of two regimens of adjuvant chemotherapy in older women with early-stage breast cancer. Standard chemotherapy was shown to be superior to oral capecitabine, especially in patients with hormone receptor-negative tumors. Two studies aimed to evaluate the benefit of chemotherapy in elderly patients with breast cancer, in which chemotherapy was compared with a no-treatment arm [25, 26]. Both trials failed to recruit and were closed early. The investigators suggested that a recruitment failure was due to the inability to convince patients to accept randomization in which a no-treatment arm was incorporated [25]. Our findings suggest that addition of chemotherapy might be of benefit in relatively fit patients with breast cancer and hormone receptor-positive breast tumors. This needs to be evaluated in future studies.
Because the association between age and distant recurrence was not eliminated by adjustment for both tumor and treatment characteristics, additional mechanisms may play a role, such as different tumor biology, resulting in worse breast cancer outcome [27]; interplay between tumor and patient characteristics, including immunosenescence, which may result in a higher risk of disease progression [28, 29]; or a different response to anticancer therapy due to interactions with comorbidity and polypharmacy [30].
One may argue that increasing age may be associated with a lower adherence to endocrine therapy and consequently may result in a higher rate of recurrence. No data were available on adherence by pill count. However, multivariable analyses were adjusted for nonpersistence, which was defined as discontinuing the assigned endocrine treatment because of adverse events, intercurrent illness, patient refusal, or other reasons. Previously, we reported a higher rate of nonpersistence of endocrine therapy with increasing age in the Dutch and Belgian patients included in the TEAM study. However, both in patients aged 65–74 as well as patients aged 75 years or older, survival was not affected by nonpersistence [31]. The absence of a consistent association between nonpersistence and outcome suggests that the current findings cannot adequately be explained by age-specific adherence.
A major strength of this study was the ability to study a large group of patients with breast cancer who were followed as part of a clinical trial on endocrine therapy. Trial data comprise highly standardized treatment algorithms and virtually complete follow-up. The TEAM trial had very few exclusion criteria, among which there was no upper age limitation. This enabled us to study age-specific breast cancer recurrence. However, although eligibility criteria of the TEAM trial were quite broad, it is known that trial populations generally comprise relatively healthy patients compared to the general population [32]. Additionally, as enrollment in the TEAM trial was restricted to postmenopausal patients with hormone receptor-positive disease, these results may not necessarily be extrapolated to all patients with breast cancer.
Conclusion
In conclusion, elderly patients with breast cancer included in the TEAM trial had a higher risk of distant recurrence. This risk may be due to underuse of chemotherapy, which therefore might be considered for relatively fit elderly patients.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors would like to thank Pfizer and the Dutch Cancer Society (2007–3968).
Author Contributions
Conception and design: Willemien van de Water, Esther Bastiaannet, Anton J.M. de Craen, Rudi G.J. Westendorp, Cornelis J.H. van de Velde, Gerrit-Jan Liefers
Provision of study material or patients: Caroline Seynaeve, Christos Markopoulos, Steve E. Jones, Daniel Rea, Annette Hasenburg, Elysée T.M. Hille, Robert Paridaens, Cornelis J.H. van de Velde
Collection and/or assembly of data: Caroline Seynaeve, Christos Markopoulos, Steve E. Jones, Daniel Rea, Annette Hasenburg, Elysée T.M. Hille, Robert Paridaens, Cornelis J.H. van de Velde
Data analysis and interpretation: Willemien van de Water, Esther Bastiaannet, Caroline Seynaeve, Christos Markopoulos, Steve E. Jones, Daniel Rea, Annette Hasenburg, Hein Putter, Elysée T.M. Hille, Robert Paridaens, Anton J.M. de Craen, Rudi G.J. Westendorp, Cornelis J.H. van de Velde, Gerrit-Jan Liefers
Manuscript writing: Willemien van de Water, Esther Bastiaannet, Caroline Seynaeve, Anton J.M. de Craen, Rudi G.J. Westendorp, Cornelis J.H. van de Velde, Gerrit-Jan Liefers
Final approval of manuscript: Willemien van de Water, Esther Bastiaannet, Caroline Seynaeve, Christos Markopoulos, Steve E. Jones, Daniel Rea, Annette Hasenburg, Hein Putter, Elysée T.M. Hille, Robert Paridaens, Anton J.M. de Craen, Rudi G.J. Westendorp, Cornelis J.H. van de Velde, Gerrit-Jan Liefers
Disclosures
Christos Markopoulos: AstraZeneca, Novartis, Pfizer (H); AstraZeneca, Novartis, Pfizer (RF); Steven E. Jones: Pfizer (C/A); Daniel Rea: Pfizer, Novartis, AstraZeneca (C/A); Pfizer, Novartis (H); Annette Hasenburg: Pfizer (C/A); Pfizer, GlaxoSmithKline, Sanofi Pasteur, AstraZeneca, Medconcept (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Cancer of the Breast. SEER Stat Fact Sheets. [Accessed September 6, 2012]. Available at http://seer.cancer.gov/statfacts/html/breast.html#incidence-mortality.
- 3.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92:550–556. doi: 10.1093/jnci/92.7.550. [DOI] [PubMed] [Google Scholar]
- 4.Mirza NQ, Vlastos G, Meric F, et al. Predictors of locoregional recurrence among patients with early-stage breast cancer treated with breast-conserving therapy. Ann Surg Oncol. 2002;9:256–265. doi: 10.1007/BF02573063. [DOI] [PubMed] [Google Scholar]
- 5.Elkhuizen PH, van de Vijver MJ, Hermans J, et al. Local recurrence after breast-conserving therapy for invasive breast cancer: High incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys. 1998;40:859–867. doi: 10.1016/s0360-3016(97)00917-6. [DOI] [PubMed] [Google Scholar]
- 6.van de Water W, Markopoulos C, van de Velde CJH, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA. 2012;307:590–597. doi: 10.1001/jama.2012.84. [DOI] [PubMed] [Google Scholar]
- 7.van de Velde CJH, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): A randomised phase 3 trial. Lancet. 2011;377:321–331. doi: 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 8.van Nes JGH, Seynaeve C, Jones S, et al. Variations in locoregional therapy in postmenopausal patients with early breast cancer treated in different countries. Br J Surg. 2010;97:671–679. doi: 10.1002/bjs.6962. [DOI] [PubMed] [Google Scholar]
- 9.International Society of Geriatric Oncology. [Accessed September 6, 2012]. Available at http://www.siog.org.
- 10.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 11.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 12.Clark RM, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: An update. Ontario Clinical Oncology Group. J Natl Cancer Inst. 1996;88:1659–1664. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 13.Courdi A, Largillier R, Ferrero J-M, et al. Early versus late local recurrences after conservative treatment of breast carcinoma: Differences in primary tumor characteristics and patient outcome. Oncology. 2006;71:361–368. doi: 10.1159/000107771. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi U, Marubini E, Del Vecchio M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: Partly independent events. J Natl Cancer Inst. 1995;87:19–27. doi: 10.1093/jnci/87.1.19. [DOI] [PubMed] [Google Scholar]
- 15.Livi L, Paiar F, Saieva C, et al. Breast cancer in the elderly: Treatment of 1500 patients. Breast J. 2006;12:353–359. doi: 10.1111/j.1075-122X.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 16.Forrest AP, Stewart HJ, Everington D, et al. Randomised controlled trial of conservation therapy for breast cancer: 6-year analysis of the Scottish trial. Scottish Cancer Trials Breast Group. Lancet. 1996;348:708–713. doi: 10.1016/s0140-6736(96)02133-2. [DOI] [PubMed] [Google Scholar]
- 17.Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 18.Fyles AW, McCready DR, Manchul LA, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351:963–970. doi: 10.1056/NEJMoa040595. [DOI] [PubMed] [Google Scholar]
- 19.Early Breast Cancer Trialists' Collaborative Group. Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 21.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 22.Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomised trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 23.Schaapveld M, Visser O, Louwman WJ, et al. The impact of adjuvant therapy on contralateral breast cancer risk and the prognostic significance of contralateral breast cancer: A population based study in the Netherlands. Breast Cancer Res Treat. 2008;110:189–197. doi: 10.1007/s10549-007-9709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 25.Leonard R, Ballinger R, Cameron D, et al. Adjuvant chemotherapy in older women (ACTION) study: What did we learn from the pilot phase? Br J Cancer. 2011;105:1260–1266. doi: 10.1038/bjc.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ring A, Reed M, Leonard R, et al. The treatment of early breast cancer in women over the age of 70. Br J Cancer. 2011;105:189–193. doi: 10.1038/bjc.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) Lancet Oncol. 2012;13:e148–e160. doi: 10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 28.Fulop T, Kotb R, Fortin CF, et al. Potential role of immunosenescence in cancer development. Ann N Y Acad Sci. 2010;1197:158–165. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- 29.Pawelec G, Derhovanessian E, Larbi A. Immunosenescence and cancer. Crit Rev Oncol Hematol. 2010;75:165–172. doi: 10.1016/j.critrevonc.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Lichtman SM, Wildiers H, Launay-Vacher V, et al. International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer. 2007;43:14–34. doi: 10.1016/j.ejca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 31.van de Water W, Bastiaannet E, Hille ETM, et al. Age-specific nonpersistence of endocrine therapy in postmenopausal patients diagnosed with hormone receptor-positive breast cancer: A TEAM study analysis. The Oncologist. 2012;17:55–63. doi: 10.1634/theoncologist.2011-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.