Abstract
Pulmonary cytolytic thrombi (PCT) is an uncommon complication after hematopoietic cell transplantation. Although the pathogenesis is unknown, patients typically respond to systemic corticosteroid treatment. Considering corticosteroids may impair graft versus leukemia reactions, we reviewed the records of 324 pediatric patients who received a transplant for leukemia and compared the outcomes of those with PCT (n=14) to those without PCT (n=310). PCT patients had a significantly more acute and chronic GVHD. Though 3 year non-relapse mortality and overall survival were similar, there was significantly less relapse in patients with PCT compared to those without PCT, regardless of the presence or absence of aGVHD (0% vs. 34% vs. 18%, p<0.01). In multivariate analysis, grade II–IV aGVHD (p=0.02), cGVHD (p=0.01) and development of PCT (p<0.01) were independently associated with less relapse. These data suggest that patients with PCT are at greater risk for GVHD, but a lower risk of leukemia relapse.
Keywords: Pulmonary Cytolytic Thrombi, Stem Cell Transplantation, Leukemia, Relapse
INTRODUCTION
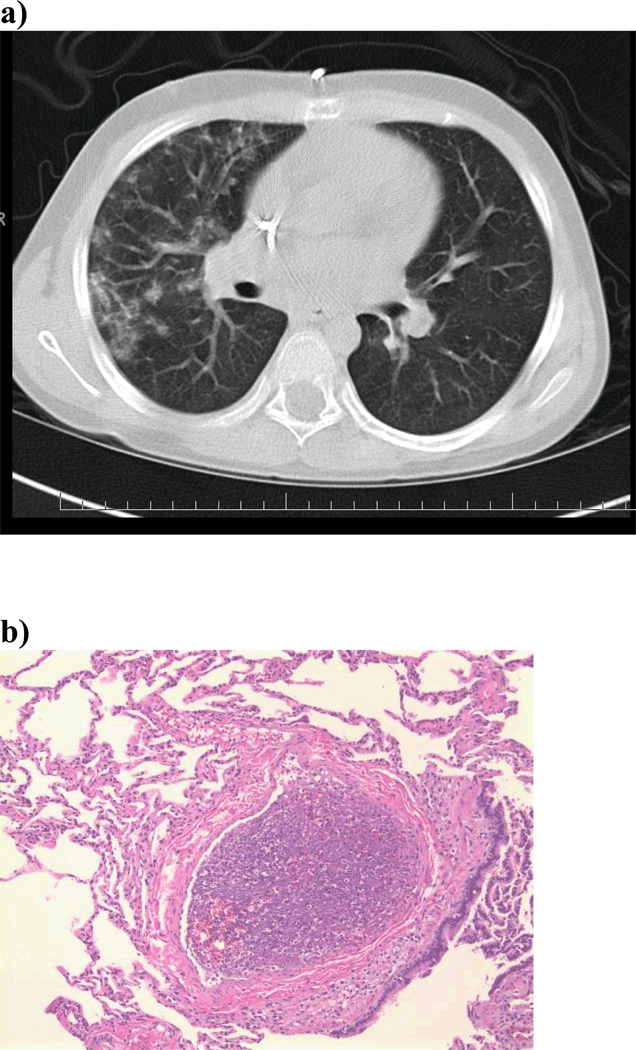
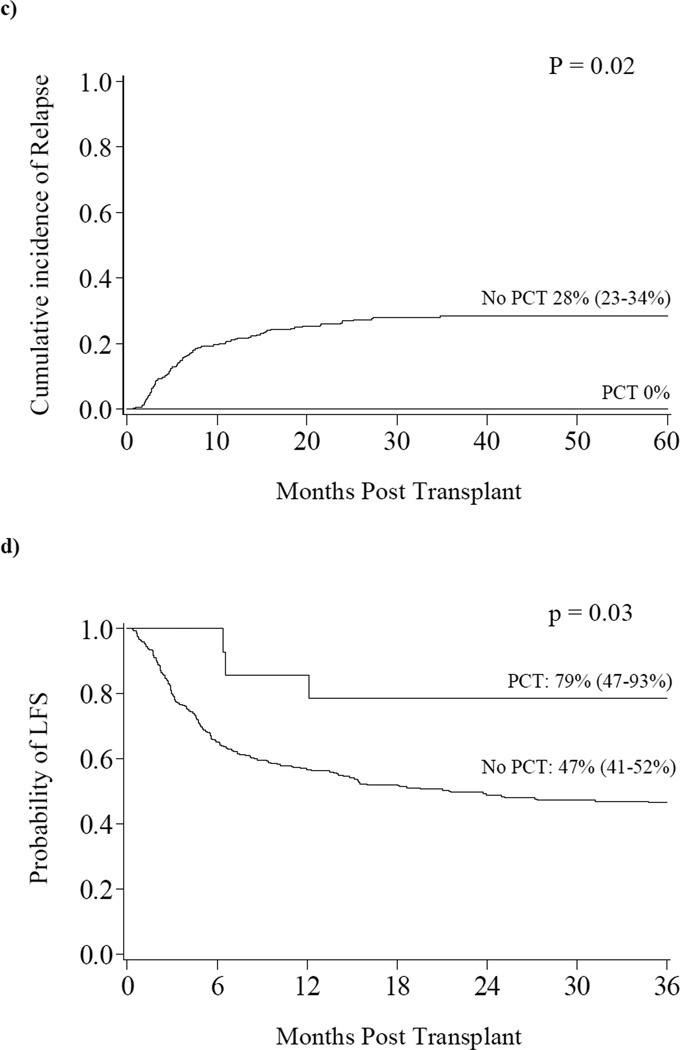
Both infectious and noninfectious lung complications occur frequently after allogeneic hematopoietic cell transplantation (allo-HCT). Pulmonary cytolytic thrombi (PCT) is a rare post-transplant lung complication that we have previously described. (1–3) PCT is more commonly reported in children, typically presenting with low grade fevers, cough and respiratory distress. Chest computed tomography findings range from small, peripheral nodules to large, diffuse infiltrates.(1–4) (Figure 1a) Because PCT mimics other post transplant pulmonary complications, including those with an infectious etiology, tissue biopsy is required for diagnosis. The pathologic appearance of PCT is unique and bears no resemblance to other infectious and non-infectious post transplant lung complications. Microscopically, PCT is characterized by occlusive vascular lesions in distal pulmonary vessels with entrapped leukocytes and amorphous material. There is associated endothelial disruption and infarction of adjacent lung tissue.(1) (Figure 1b) In samples where viable cells can be identified, immunohistochemistry shows mainly donor-derived macrophages.(3) Blood, bronchoalveolar fluid and lung biopsy are negative for infectious organisms. (1)
Figure 1. a) Radiographic Appearance of PCT b) Histologic Appearance of PCT c) Relapse d) Leukemia Free Survival.
a) CT scan shows numerous peripheral and pleural based nodular densities in the lung. b) PCT is characterized histologically by necrotic thromboemboli in the distal pulmonary vessels with entrapped leukocytes and amorphous material suggestive of cellular breakdown products. In samples where the cellular debris is still viable, immunohistochemistry shows mainly macrophages (i.e., cells staining for MPO and CD68). c) The cumulative incidence of relapse at three years was significantly lower in those with PCT compared to those without PCT (0% vs. 28%, p=0.02). d) The probability of LFS at three years was significantly higher in those with PCT compared to those without PCT (79% vs. 46%, p=0.03).
Though the pathogenesis of PCT is not currently known, endothelial injury secondary to GVHD, infections, chemotherapy and radiation therapy have all been implicated. (2, 3) Our previous studies have shown that patients with PCT frequently have concurrent acute GVHD (aGVHD) and that PCT responds to systemic steroid treatment.(1, 2) Considering that steroid therapy may impair graft versus leukemia (GVL) reactions and therefore theoretically increase the incidence of post-transplant relapse, we investigated the outcomes of patients who developed PCT after myeloablative HCT for hematologic malignancies.
METHODS
Study Design
324 consecutive children with acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML) or chronic myelogenous leukemia (CML) underwent allo-HCT at the University of Minnesota between 1993 and 2006. All protocols were approved by the Institutional Review Board (IRB) and patients/guardians provided informed consent.
Preparative Therapy and Supportive Care
All patients received myeloablative conditioning therapy and GVHD prophylaxis as previously described. (5, 6) Supportive care included HEPA filtered rooms, herpes simplex and Pneumocystis (carinii) jiroveci pneumonia prophylaxis, cytomegalovirus (CMV) surveillance, post-transplant GCSF and therapy for acute and chronic GVHD as previously described.(5, 7, 8)
Outcome Endpoints and Definitions
Endpoints included aGVHD (grades II–IV and grades III–IV), cGVHD, overall survival (OS), leukemia free survival (LFS), relapse and treatment related mortality (TRM). High risk leukemia was defined as ALL in relapse, CR1, CR2 (relapse <36 months from diagnosis) or ≥ CR3, AML in relapse or ≥ CR2 or CML in accelerated phase or blast crisis. GVHD was graded according to published criteria with histopathological confirmation when possible.(8, 9) Patients with engraftment were considered evaluable for GVHD. LFS was defined as the time from transplant until disease recurrence, death or last patient contact, whichever came first. Survival was defined as the time from transplant until death or last contact. Relapse was defined as morphologic evidence of leukemia after HCT. TRM was defined as death in the first 3 years after HCT for any reason other than relapse.
Statistical Analysis
Patient and transplant characteristics by donor type were analyzed using the Chi-square test for categorical data and the Wilcoxon Rank-Sum test for continuous data. The cumulative incidence of GVHD, TRM and relapse were calculated by treating deaths from other causes as competing risks.(10) LFS and OS were estimated by the Kaplan-Meier method.(11) Event times were measured from the date of transplantation to the event or the date of last contact. Time-to-event curves were completed by the Log-Rank test or Gray method. Multiple regression analysis was performed with Cox regression and the Gray and Fine competing hazards method.(12, 13)
RESULTS
Patient demographics are presented in Table 1. The median age at HCT was 12 years (range 4–17) in those with PCT and 9 years (range 0–18) in those without PCT. There were 178 patients with ALL, 128 with AML and 18 with CML. Stem cell sources included BM (n=206, 64%) and UCB (n=118, 36%). Unrelated donor grafts (either BM or UCB) were used in 64% of subjects. Fourteen subjects (4%, 95% CI 2–7%) developed biopsy proven PCT at a median time of 3 months (range 1.3–11.3) after allo-HCT. Ten patients (71%) with PCT and 211 (68%) patients without PCT had high risk leukemia (p=0.79). There were no significant differences between those with and without PCT with respect to age, gender, type of leukemia or CMV serostatus. Likewise, there were no significant differences with respect to donor source (related vs. unrelated), stem cell source (BM vs. UCB), degree of HLA disparity, conditioning regimen or GVHD prophylaxis.
Table 1.
Patient Characteristics
| Patient Factors | PCT (n=14) |
No PCT (n=309) |
p value |
|---|---|---|---|
|
Time to Development of PCT (months) Median (range) |
2.98 (1.25–11.25) |
na |
na |
|
Age at Transplant (years) Median (range) |
12 (4–17) |
9 (0–18) |
.43 |
|
Gender Male Female |
10 (71%) 4 (29%) |
198 (64%) 111 (36%) |
.57 |
|
Diagnosis ALL AML CML |
9 (64%) 3 (21%) 2 (14%) |
168 (54%) 125 (40%) 16 (5%) |
.18 |
|
Disease Risk Group High Intermediate |
10 (71%) 4 (29%) |
211 (68%) 99 (32%) |
.79 |
|
Graft Source BM UCB |
10 (71%) 4 (29%) |
195 (63%) 114 (37%) |
.61 |
|
Donor Unrelated Sibling Identical Twin Parent/Grandparent |
9 (64%) 4 (29%) 0 1 (7%) |
197 (64%) 96 (31%) 2 (1%) 14 (5%) |
0.97 |
|
HLA Match 6/6 5/6 4/6 Missing |
8 (57%) 6 (43%) 0 0 |
163 (53%) 98 (32%) 45 (15%) 3 (1%) |
.44 |
|
Recipient CMV Serostatus Negative Positive Missing |
9 (64%) 5 (36%) 0 |
153 (49%) 150 (50%) 6 (2%) |
.52 |
|
Conditioning Intensity MA NMA |
14 (100%) 0 |
310 (99%) 1 (<1%) |
1.00 |
|
GVHD prophylaxis CSA based Elutriation MTX based Other |
11 (79%) 2 (14%) 1 (7%) 0 |
250 (81%) 34 (11%) 18 (6%) 8 (2%) |
.99 |
na = not applicable, ALL = acute lymphocytic leukemia, AML = acute myelocytic leukemia, CML = chronic myelocytic leukemia, BM = bone marrow, UCB = umbilical cord blood, HLA = human leukeocyte antigen, CMV = cytomegalovirus, MA = myeloablative, NMA = nonmyeloablative, GVHD = graft-versus-host disease, CSA = cyclosporine, MTX = methotrexate, aGVHD = acute graft-versus-host disease, cGVHD = chronic graft-versus-host disease.
The cumulative incidence of grades II–IV aGVHD for the entire cohort was 40%. Grade II–IV aGVHD was significantly higher in PCT patients (86%, n=12) when compared to those without PCT (38%, n=110) (p<0.01). The cumulative incidence of grades III–IV aGVHD for the entire cohort was 16% and was significantly greater in patients with PCT (43% vs. 15%, p=0.01). Similarly, cGVHD was more common in PCT patients (36% vs. 14%, p=0.02).
At 3 years, 86 patients had leukemia recurrence [27% (95% CI 22 – 32%)]. In univariate analysis, the probability of relapse at 3 years was 0% in PCT patients and 28% (95% CI 23–34%) in patients without PCT (p=0.02). (Figure 1c) In multivariate regression analysis, the only factors with a significant impact on relapse were the development of PCT (RR 0.0, p<0.01), grades II–IV aGVHD (RR 0.53, 95% CI 0.32–0.88, p=0.01) and cGVHD (RR 0.15, 95% CI 0.04–0.59, p=0.01). (Table 2) The protective effect of PCT on relapse persisted in multivariate analysis when only the patients with acute (grade II–IV) or chronic GVHD (n=137) were analyzed (RR 0.0, p<0.01). The probability of LFS for the entire cohort was 48% (95% CI 42–53%) at 3 years. PCT patients had a significantly higher probability of LFS when compared to those without PCT (79% [95% CI 47–93%] vs. 47% [95% CI 41–52%], p=0.03). (Figure 1d)
Table 2.
Multivariate Analysis of Factors Contributing to the Risk of Relapse
| Patient Factors | RR of relapse (95% C.I.) | p-value |
|---|---|---|
|
PCT No* Yes |
1.00 0 |
<.01 |
|
Chronic GVHD No* Yes |
1.00 0.15 (0.04–0.59) |
.01 |
|
Acute GVHD (grades IIIV) No* Yes |
1.00 0.53 (0.32–0.88) |
.01 |
Reference Group
The above model is the result of multiple regression analysis on relapse after testing the following independent variables: PCT, donor source, HLA match, underlying disease, cGVHD and aGVHD.
RR = relative risk, PCT = pulmonary cytolytic thrombi, BM = bone marrow, UCB = umbilical cord blood, HLA = human leukocyte antigen, ALL = acute lymphoblastic leukemia, AML = acute myelogenous leukemia, CML = chronic myelogenous leukemia, GVHD = graft versus host disease.
The cumulative incidence of TRM at 3 years for the entire cohort was 25%. TRM was not different in those with or without PCT (21% vs. 25%, p=0.51). OS at 3 years was 49% (95% CI 43–54%) and was not different between those with and without PCT (71% vs. 50%, p=0.12). Of the 6 deaths in the PCT group, 5 subjects had pulmonary complications at the time of death.
DISCUSSION
To date, PCT has only rarely been reported as a complication following allo-HCT making this the largest series of such patients. Prior studies have shown that PCT is associated with aGVHD. (1–3) However, the influence of PCT on either cGVHD or relapse has not been addressed. Here we examined a cohort of patients transplanted for malignant hematological diseases who developed PCT. When compared to controls, PCT patients experienced more aGHVD (grade II–IV/III–IV) and cGVHD. Despite the increased probability of GVHD, a negative impact on either TRM or OS was not observed. Prior reports have demonstrated that both acute and chronic GVHD are associated with relapse protection. (14, 15) Here, using multivariate analysis, we show that PCT is independently associated with relapse protection.
While patients with PCT are at higher risk for developing cGVHD and pulmonary associated toxicity, they have similar overall TRM and a lower risk of relapse. Further observations in patients with PCT are needed to confirm these findings, but it appears that the underlying etiology of these lesions may provide a protective effect with regard to leukemia relapse after allo-HCT.
ACKNOWLEDGEMENTS
This research was in part supported by the Children’s Cancer Research Fund.
Footnotes
The authors have no financial disclosures.
REFERENCES
- 1.Gulbahce H, Manivel J, Jessurun J. Pulmonary cytolytic thrombi: a previously unrecognized complication of bone marrow transplantation. Am J Surg Pathol. 2000;24:1147–1152. doi: 10.1097/00000478-200008000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Woodard J, Gulbahce E, Shreve M, et al. Pulmonary cytolytic thrombi: a newly recognized complication of stem cell transplantation. Bone Marrow Transplant. 2000;25:293–300. doi: 10.1038/sj.bmt.1702137. [DOI] [PubMed] [Google Scholar]
- 3.Peters A, Manivel J, Dolan M, Gulbahce H, Baker K, Verneris M. Pulmonary cytolytic thrombi after allogeneic hematopoietic cell transplantation: a further histologic description. Biol Blood Marrow Transplant. 2005;11:484–485. doi: 10.1016/j.bbmt.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Morales I, Anderson P, Tazelaar H, Wylam M. Pulmonary cytolytic thrombi: unusual complication of hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. 2003;25:89–92. doi: 10.1097/00043426-200301000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Smith A, Baker K, Defor T, Verneris M, Wagner J, Macmillan M. Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant. 2009;15:1086–1093. doi: 10.1016/j.bbmt.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verneris M, Brunstein C, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft versus leukemia effect in recipients of two units. Blood. 2009 doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora M, Nagaraj S, Wagner J, et al. Chronic graft-versus-host disease (cGVHD) following unrelated donor hematopoietic stem cell transplantation (HSCT): higher response rate in recipients of unrelated donor (URD) umbilical cord blood (UCB) Biol Blood Marrow Transplant. 2007;13:1145–1152. doi: 10.1016/j.bbmt.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 8.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz ME, Sullivan KM. Chronic graft-versus-host disease. Blood reviews. 2006;20:15–27. doi: 10.1016/j.blre.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL MP. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 12.Fine JP GR. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.Cox DR. Regression Models and Life Tables. J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- 14.Valcárcel D, Martino R, Piñana J, Sierra J. Allogeneic stem cell transplantation after reduced-intensity conditioning for acute myeloid leukaemia: impact of chronic graft-versus-host disease. Curr Opin Oncol. 2009;21(Suppl 1):S35–S37. doi: 10.1097/01.cco.0000357474.66035.9b. [DOI] [PubMed] [Google Scholar]
- 15.Weisdorf D. GVHD the nuts and bolts. Hematology Am Soc Hematol Educ Program. 2007:62–67. doi: 10.1182/asheducation-2007.1.62. [DOI] [PubMed] [Google Scholar]