Abstract
The juvenile hormone analogs (JHA) are known to disrupt insect development but the molecular mechanisms of their action have been studied only in a few model insects belonging to orders Diptera and Lepidoptera. Here, we investigated the mechanisms of JHA action in red flour beetle, Tribolium castaneum, belonging to the order Coleoptera. Application of JHA during penultimate and final instar larval stages blocked larval-pupal metamorphosis and induced supernumerary larval molts. When compared to the control insects undergoing larval-pupal molt, down-regulation of expression of transcription factor, Broad, and up-regulation of other genes involved in 20-hydroxyecdysone (20E) action (FTZ-F1, E74) were observed in JHA-treated larvae undergoing supernumerary larval molts. The presence of JHA during the final instar larval stage blocked the midgut remodeling wherein programmed cell death (PCD) of larval cells and proliferation and differentiation of imaginal cells to pupal gut epithelium were impaired. The comparative analysis of 20E-induced gene expression in the midguts of JHA-treated and control insects revealed that JHA suppressed the expression of EcRA, EcRB, Broad, E74, E75A, and E75B, resulting in a block in PCD as well as proliferation and differentiation of imaginal cells.
Keywords: gene expression, hydroprene, midgut, pupal commitment, Tribolium castaneum
INTRODUCTION
Insect development is regulated by several hormones including the steroid, 20-hydroxyecdysone (20E), and sesquiterpenoid, juvenile hormone (JH). During larval/nymphal development in lepidopteran and hemimetabolous insects, JH prevents metamorphosis (Williams, 1961; Zhou and Riddiford, 2002). At the end of larval development, JH titers decrease, enabling 20E to trigger metamorphosis (Riddiford, 1996). In most insects the addition of JH at this time causes the formation of a supernumerary larva (Truman and Riddiford, 2002). However, the scenario is different in dipteran insects such as Drosophila melanogaster and mosquitoes, wherein the application of JH analog (JHA) did not block puparium formation or pupation (Wilson, 2004; Wu et al., 2006). Though the molecular mechanisms by which JHA affects metamorphosis is well understood in D. melanogaster, Manduca sexta, and Aedes aegypti (Riddiford, 1996; Zhou et al., 1998a; Nishiura et al., 2005), the information gained may not be applicable to all insect species since the JH actions or sensitivity to JH varies among insect species.
We studied the molecular mechanisms of JHA during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. T. castaneum is a coleopteran insect representing 25% of animal kingdom species. Like other holometabolous insects, it develops from egg to adult through the intermediate larval and pupal stages. Besides being a stored grain pest, T. castaneum is amenable to molecular genetic studies. Completion of whole genome sequencing (Tribolium Genome Sequencing Consortium, 2008) and functioning of systemic RNAi (Tomoyasu and Denell; 2004; Arakane et al., 2005; Parthasarathy et al., 2008b) make T. castaneum an ideal model insect. When compared to other model insects, detailed analysis of the development and molecular mechanisms of hormonal regulation on metamorphosis are not available for T. castaneum.
We investigated the role of JH on the metamorphosis of T. castaneum by using JHA to mimic JH action. Application of JHA blocked larval-pupal metamorphosis and prolonged larval life-span by inducing supernumerary larval molts. The gene expression varied significantly between insects undergoing supernumerary larval molt or larval-pupal molt. Based on the sensitivity to JHA, the critical period of pupal commitment most likely occurred between 72–96 h after ecdysis to the final instar larval stage. The presence of JHA during the final instar larvae blocked midgut remodeling and suppressed the expression of genes involved in 20E action in the midgut. Thus, this study provides a basis to understand the molecular mechanisms of hormonal regulation of metamorphosis in coleopteran insects.
MATERIALS AND METHODS
Rearing and Staging
The rearing and staging of GA-1 strain of T. castaneum was done as described in Parthasarathy et al. (2008a). The final instar larvae were identified as soon as they molted by untanned white cuticle, designated as 0 h AEFL (after ecdysis into the final instar larval stage). The following days of final instar larvae were designated as L24, L48, L72, and L96 h AEFL. The beginning of the quiescent stage was designated as Q0 and was determined based on cessation of feeding and movement. The following days were recognized by characteristic “C”-shaped larvae and were collected at 12-h intervals from Q0. White pupae were designated as 0 h AEPS (after ecdysis into the pupal stage) and staged at 24-h intervals. The supernumerary larval stage was designated as L’.
Hormonal Treatment
Methoprene (isopropyl (E,E)-RS)-11-methoxy-3,7,11-trimethyl-2,4-dodeca-dienoate) and Hydroprene (Ethyl (2E,4E,7S)-3,7,11-trimethyl-2,4-dodecadienoate) were a gift from Wellmark International (Dallas, TX). Technical grade compounds were dissolved in acetone and used at 0.1 ml/g of diet for all dosages in feeding bioassays. For topical application, 0.5 μl of Hydroprene (2 μg/μl) in acetone was applied on the dorsal side of the thorax and abdomen of final instar larvae prior to 24-h AEFL. All control larvae were treated with equivalent amounts of acetone alone.
cDNA Synthesis and Quantitative Real-Time Reverse-Transcriptase PCR (qRT-PCR)
Total RNA was extracted from whole body and midguts of staged larvae and pupae using TRI reagent (Molecular Research Center Inc., Cincinnati, OH). cDNA was synthesized using 2 μg of DNAse1 (Ambion, Austin, TX) -treated RNA and iScript cDNA synthesis kit (Biorad Laboratories, Hercules, CA) in a 20-μl reaction volume as per the manufacturer’s instructions. Real-time quantitative reverse-transcriptase PCR was performed using MyiQ single color real-time PCR detection system (Biorad Laboratories). PCR reaction components were: 1 μl of cDNA, 1 μl each of forward and reverse sequence-specific primers, 7 μl of H2O, and 10 μl of supermix (Biorad Laboratories). The sequences of primers used here have been reported in Parthasarathy et al. (2008a,b) and Tan and Palli (2008a,b). PCR conditions were: 95°C for 3 min followed by 45 cycles of 95°C for 10 sec, 60°C for 20 sec, 72°C for 30 sec. Both the PCR efficiency and R2 (correlation coefficient) values were taken into account prior to estimating the relative quantities. Relative expression levels of each gene were quantified using ribosomal protein, rp49, expression levels as an internal control.
Histology
The midguts from staged larvae and pupae were dissected in 1 × PBS (phosphate buffered saline, Sigma) and fixed in 4% paraformaldehyde (Sigma). Sectioning was done as previously described (Parthasarathy and Palli, 2007). The sections were deparaffinized through successive baths of Xylene, rehydrated through serial grades of ethanol, water, and 1 × PBS. Nuclear staining was done with DAPI (4′, 6-Diamidino-2-phenyl indole, Sigma) at 1 μg/ml concentration for 10 min. The slides were washed with 1 × PBS twice and mounted in 50% glycerol.
Imaging and Documentation
For light microscopy, the modular zoom system (Leica Z16 APO, Germany) fitted with JVC 3CCD Digital Camera KY-F75U was used. The images were documented using Cartograph version 6.1.0 (GT Vision Demonstration). Image processing was done using Archimed version 5.2.2 (Micovision Instruments).
For fluorescent images, an Olympus FV1000 laser scanning confocal microscope was used. DAPI was excited using a 405-nm laser line. The primary objective used was an Olympus water immersion PLAPO40XWLSM-NA1.0. Image acquisition was conducted at a resolution of 512 × 512 pixels and a scan-rate of 10 ms/pixel. Control of the microscope, as well as image acquisition and exportation as TIFF files, was conducted using Olympus Fluoview software version 1.5. Figures of all micrographs were assembled using Photoshop 7.0.
RESULTS
Juvenile Hormone Analogs (JHA) Block Larval-Pupal Metamorphosis
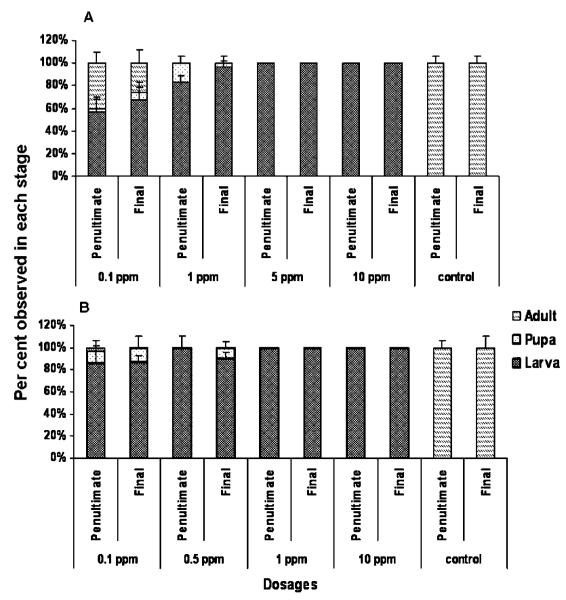
The newly molted penultimate and final instar larvae were starved for 4 h and fed with diet containing different doses of methoprene and hydroprene continuously and the developmental events were recorded (Fig. 1A and B). At higher dosages of 5 and 10 ppm, methoprene blocked larval to pupal metamorphosis completely (100%). Most of the larvae molted into supernumerary larval instar (Fig. 1A). At 1 ppm, methoprene blocked larval to pupal metamorphosis in 85% of larvae treated during the penultimate larval stage and more than 95% of larvae treated during the final instar larval stage. The remaining 5–15% of larvae treated during both stages pupated and died subsequently and no adults emerged from these pupae. Fifty to sixty percent of insects treated with a 0.1-ppm dose of methoprene during the penultimate and final instar larval stage molted into the supernumerary larval stage. The remaining insects pupated and adults emerged from these pupae. All control larvae developed normally into adults (Fig. 1A).
Figure 1.
Developmental responses of penultimate and final instar larvae of T. castaneum to different doses of methoprene (A) and hydroprene (B). The staged penultimate and final instar larvae were treated with JHA before 24-h AEFL and the observations were recorded 15 days after treatment. The number of supernumerary larvae, pupae, and adults was recorded in each treatment. Control represents the group of larvae treated with an equivalent amount of carrier (acetone) alone. The X-axes indicate the stage of treatment and dosages of JHA and Y-axes denote the percentage of each stage observed. Ten larvae were used in each treatment and the treatments were replicated three times. Mean±S.E. for the three independent experiments are shown.
The effect of hydroprene was similar to that of methoprene at a high dose of 10 ppm (Fig. 1B). At a 1-ppm dose, hydroprene blocked larval-pupal metamorphosis completely (100%) in larvae treated during the penultimate and final instar stages. Even at low dosages of 0.1 and 0.5 ppm, hydroprene blocked pupal metamorphosis in more than 80% of larvae irrespective of stage of treatment. The remaining 20% of insects that became pupae did not survive to adulthood (Fig. 1B). Hydroprene at 0.5 ppm blocked larval-pupal metamorphosis in more than 90% of final instar larvae. Hence, the above JHA, dose, and stage were used in subsequent experiments.
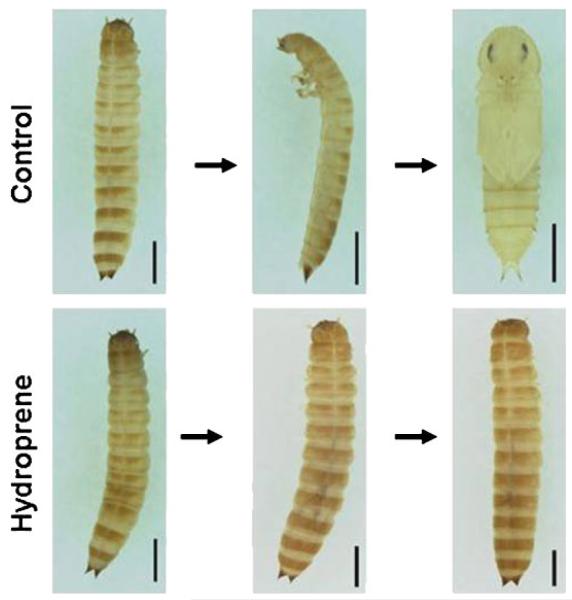
Continuous feeding of hydroprene at 0.5 ppm during the final instar larval stage prolonged the larval life-span by inducing supernumerary larval molts (Fig. 2). The final instar larvae molted into two subsequent larval stages at weekly intervals and died finally. The supernumerary larval molts produced giant larvae that showed a darker color integument than the integument of control final instar larvae. The control final instar larvae treated with acetone alone entered the quiescent stage and pupated within a week.
Figure 2.
Effect of hydroprene on the development of T. castaneum. The final instar larvae were treated with 0.5ppm of hydroprene before 24-h AEFL. The subsequent molt or metamorphic stages are shown. The control larvae treated with acetone alone entered the quiescent stage and pupated while hydroprene-treated larvae molted into supernumerary larvae twice, avoiding the quiescent stage. Scale bar = 1 mm.
Differences in Gene Expressions During Supernumerary Larval Molt and Larval-Pupal Molt
The relative mRNA levels were determined by real-time quantitative RT-PCR analysis (Fig. 3). Interestingly, the gene expression varied between the supernumerary larval molt and the larval-pupal molt. The mRNA levels of FTZ-F1 (Fuzhi-tarazu) were up-regulated significantly by 11.6-fold during the supernumerary larval molt when compared to the larval-pupal molt, while expression of EcRB (Ecdysone receptor), E75B (Ecdysone-inducible gene), E74, and HR3 (Hormone receptor) were up-regulated by 1.5–2.5-fold more in the supernumerary larval molt than in the larval to pupal molt. The mRNA levels of Broad were down-regulated by 2.3-fold in the supernumerary larval molt in comparison with the larval-pupal molt. However, the differences in gene expression of only Broad, FTZ-F1, and E74 alone were statistically significant and the expression of EcRA, E75A, and Met were not significantly different between the supernumerary larval molt and the larval-pupal molt.
Figure 3.
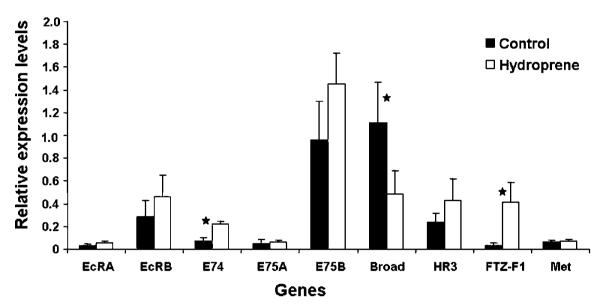
Comparison of relative gene expression levels at the end of the final instar larvae treated with hydroprene and acetone alone (control). Hydroprene-treated larvae undergo a larval-larval molt and control larvae undergo a larval-pupal molt. Hydroprene (0.5 ppm) was administered with diet before 24-h AEFL of final instar larvae. Total RNA was extracted 12 h before subsequent ecdysis. The head cover slippage (96-h AEFL) and appearance of a dorsal split of the thoracic cuticle (132-h AEFL) were used as markers for hydropren-treated and control insects, respectively. The mRNA levels were quantified using qRT-PCR analysis. The expression levels of each gene were normalized using the expression levels of internal control, ribosomal protein. Three insects were used per replication and each treatment was replicated three times with a total of nine insects. The significance of means was tested by a Two-tailed paired “t”-test using Statistix 8.0 software. The log transformation was done to stabilize the sample variance wherever needed. Stars: Significance at P<0.05. Mean±S.D. for the three independent experiments are shown.
Window of Sensitivity to Hydroprene
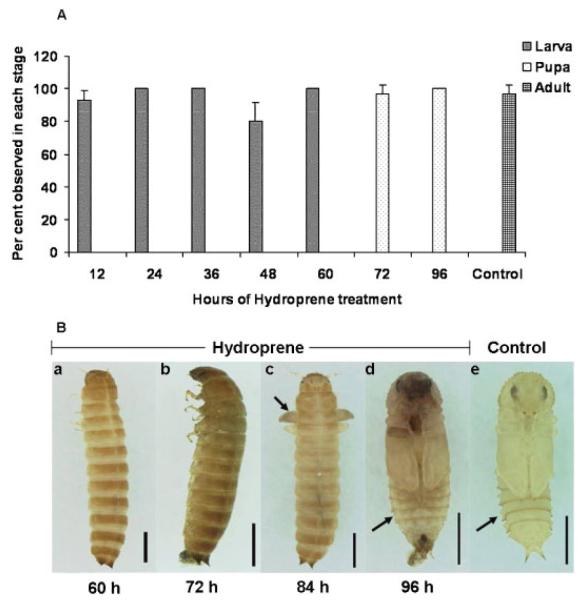
To determine the critical period of JH sensitivity, 0.5 ppm hydroprene was administered with diet at different time points during the final instar larval stage and the effect on the development was recorded (Fig. 4A). The final instar larvae were sensitive to hydroprene until 60-h AEFL. More than 90% of the treated insects remained as larvae by molting into the supernumerary larval stage. Administration of hydroprene at 72- and 96-h AEFL did not block larval-pupal metamorphosis since more than 90% of the treated larvae became pupae but the pupae eventually died and no adults emerged from these pupae. All the control larvae pupated and emerged as adults.
Figure 4.
A: Developmental responses of final instar larvae fed orally with 0.5 ppm of hydroprene at different time points after last ecdysis. The number of larvae, pupae, and adults was recorded after 15 days of treatment. The control group represents the average of each control at different time points that were exposed to acetone alone. The X-axis indicates the hours of final instar larvae when hydroprene treatment was given. The Y-axis represents the percentage of insects in each stage. Ten insects were used for each time point and replicated three times. Mean±S.D. of three independent experiments are shown. B: Effect of topical application of hydroprene on the metamorphosis. Hydroprene (1 mg) in acetone was applied topically at different time points of final instar larvae and the effects are shown (a–e). Application at 60-h AEFL resulted in all larvae undergoing supernumerary larval molt (a). When applied at 72-h AEFL, most of the larvae died during the quiescent stage unable to pupate (b). Administration at 84-h AEFL resulted in larvalpupal intermediaries (40%) with the development of wings (c, black arrow) and the remaining 60% remained as larvae. Application at 96-h AEFL did not block larval-pupal metamorphosis but the resultant pupae were malformed and gin-traps were not well developed (d, black arrow). All control larvae became pupae (e). Scale bar = 1 mm.
The final instar larvae slowly reduced food consumption and stopped feeding between 72–96 h AEFL upon gaining the critical weight and also as a mark of prepupal behavior. The effect of hydroprene by oral feeding after 72-h AEFL, as observed above, may not represent the actual response of the final instar larvae. Hence, topical application on the integument was performed starting at 60-h AEFL at 12-h intervals. Topical application at 60-h AEFL served as a positive control (Fig. 4B, a). Surprisingly, the topical application of hydroprene showed various phenotypes. Hydroprene blocked larval-pupal metamorphosis when applied at 72-h AEFL and all the larvae died during the quiescent stage (Fig. 4B, b). Application of hydroprene at 84-h AEFL resulted in larval-pupal intermediaries. The ecdysis was completed and the resultant stage had underdeveloped wings (Fig. 4B, c). Hydroprene did not interfere with larval-pupal metamorphosis when applied at 96-h AEFL; however, the pupae were malformed (Fig. 4B, d) and died subsequently. All the control larvae pupated.
JHA Blocks Midgut Remodeling and Interferes With 20E-Induced Gene Expression
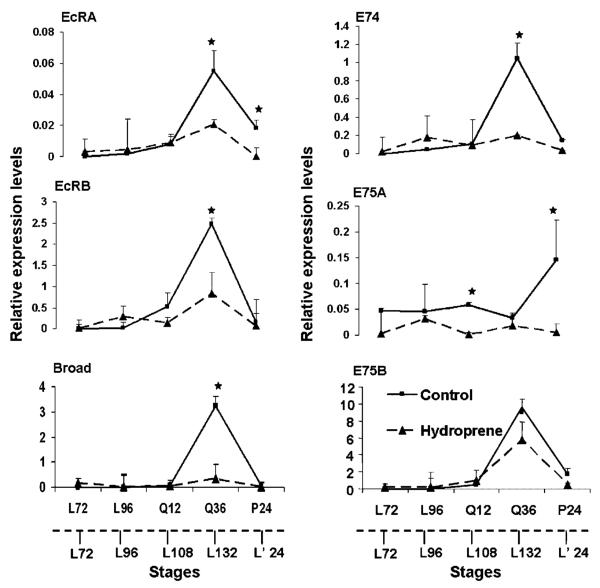
We examined the morphology of the gut epithelium by nuclear staining of the cross-sections of midgut dissected from hydroprene-treated and control insects (Fig. 5). Application of hydroprene at the beginning of the final instar larvae blocked midgut remodeling. In the treated insects, the midgut epithelium consisted of large larval cells with a few imaginal cells on the periphery at the end of the final instar larval stage (Fig. 5, a). The larval cells had intact nuclei with no signs of PCD indicated by fragmented nuclei. In contrast, in the control insects, the larval cells moved into the gut lumen with fragmented nuclei and the small imaginal cells differentiated to form the pupal midgut epithelium (Fig. 5, b). The cross-sections of midguts dissected from supernumerary larvae treated with hydroprene during the final instar larval stage resembled the morphology of midgut epithelium of the early stage final instar larvae except for the number of imaginal cells on the periphery (Fig. 5, c and d). The mRNA levels of genes such as EcRA, EcRB, Broad, E74, E75A, and E75B were compared in midguts dissected from hydroprene-treated and control insects using qRT-PCR (Fig. 6). The expression levels were monitored at different time points during the final instar larval stage including the quiescent stages and after pupation in the control larvae and the corresponding time points in the hydroprene-treated larvae. In the control midguts, the expression of EcRA, EcRB, Broad, E74, E75A, and E75B showed a peak at the end of the quiescent stage before pupation. The expression levels of all these genes were low after pupation. In hydroprene-treated larval midguts, the expression of all these genes was suppressed. At the end of the quiescent stage, the mRNA levels of EcRA were 6-fold less in midguts dissected from hydroprene-treated insects when compared to the levels in midguts dissected from acetone-treated insects. Similarly, at this stage the EcRB mRNA levels were 2.5-fold less in midguts dissected from hydroprene-treated insects when compared to the levels in midguts dissected from acetone-treated insects. Broad mRNA levels remained low throughout the period of observation. E74 had an expression pattern similar to EcRB. E75A expression levels were relatively low when compared to control. The mRNA levels of E75B were similar to control until 12 h after entering the quiescent stage, but the peak expression level at 36 h after entering the quiescent stage was only 1.5-fold less than in the control.
Figure 5.
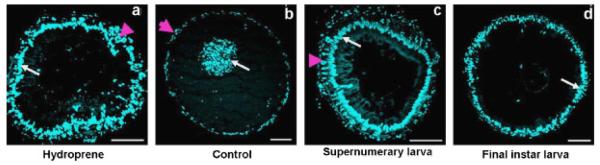
Cross-sections of midguts of hydroprene-treated and control larvae. CS (10-μm-thick) of midguts were nuclear stained with DAPI. White arrows indicate the larval cells and pink arrowhead denote the imaginary cells. In hydroprene-treated insects, the midgut epithelium consisted of mostly larval cells with a few imaginary cells. a: Note the nuclei of larval cells were intact. b: In the control insects, the midgut epithelium consisted of differentiated imaginal cells and the larval cells undergoing PCD with fragmented nuclei moved into the lumen. The midgut epithelium of supernumerary larvae (c) resembled that of final instar larvae (d) except for a greater number of imaginary cells. Scale bar = 50 μm.
Figure 6.
Relative expression levels of 20E-induced genes in the midguts of hydroprene-treated and control insects. The mRNA levels were normalized using the ribosomal protein levels and quantified using qRT-PCR analysis. The solid lines represent the control insects and the dotted line represents the hydroprene-treated insects. The solid X-axes represent the stages of control insects (L72, L96; hours after ecdysis into final instar larvae; Q12, Q36: hours after entering the quiescent stage; P24: hours after ecdysis into pupal stage). The dotted X-axes represent the stages of hydroprene-treated insects (L72-L132: hours after ecdysis into final instar larvae, L’24: hours after supernumerary larval molt). The significance of means at each time-point between control and hydroprene-treated was tested by the Two-tailed paired “t”-test using Statistix 8.0 software. Stars: Significance at P<0.05. Mean±S.D. of three replications for each time point are shown.
DISCUSSION
Numerous analogs of juvenile hormones have been synthesized and a few are being used for insect pest control (Dhadialla et al., 1998). The discovery that exogenously applied juvenile hormone could interfere with metamorphosis in insects (Wiggles-worth, 1965; Williams, 1961) has also been useful in understanding the mechanisms by which hormones control metamorphosis (Riddiford, 1996). There are several reports of JHA action in insects belonging to different orders (Zhou et al., 1998a; Wu et al., 2006; Parthasarathy and Palli, 2007). Though the action of JHA as a potential insecticide has been demonstrated in several coleopteran stored grain insect pests (Kostyukovsky et al., 2000; Arthur, 2001; Toews et al., 2005), the molecular mechanisms of JH action remains unknown. Here we used JHA in T. castaneum to mimic JH action and studied its role in metamorphosis.
In this study, the sensitivity of T. castaneum to JHA such as methoprene and hydroprene varied, hydroprene being more effective than methoprene. Riddiford and Ashburner (1991) showed that pyripoxyfen was a more powerful JH agonist than methoprene in D. melanogaster. The side chains (methyl, ethyl, isopropyl) of these compounds make them resistant to metabolism in insects and determine the half-life/persistence in the treated insects (Hammock and Quistad, 1981). It is also possible that the proteins involved in JH action recognize and respond to these molecules at different efficiencies resulting in differential action. Unlike in dipteran insects such as D. melanogaster and Ae. aegypti (Wilson, 2004; Wu et al., 2006), the presence of JHA during the penultimate or final instar larvae of T. castaneum blocked larval-pupal metamorphosis and induced supernumerary larval molts. This action of JHA in coleopteran insects is similar to that in lepidopteran insects (Zhou et al., 1998a; Parthasarathy and Palli, 2007).
The latest larval stage at which application of exogenous JH results in the delay or blockage of pupation is known as the JH-sensitive period and this varies among insects (Webb and Riddiford, 1988; Lan and Grier, 2004). In the case of T. castaneum, based on the topical application studies, the critical period of JH sensitivity appeared to occur between 72- and 96-h AEFL. Treatment of hydroprene until 60-h AEFL resulted in supernumerary larval molt. Application of hydroprene at 72-h AEFL resulted in a block in pupation and application of hydroprene at 84- and 96-h AEFL led to larval-pupal intermediaries and malformed pupa. There are small peaks of ecdysteroids in the final instar larvae of T. castaneum around 60- and 84-h AEFL (Parthasarathy et al., 2008a). Hence, it is likely that pupal commitment in T. castaneum occurs between 72- and 96-h AEFL.
To identify the molecular mechanisms that underlie the larval-pupal metamorphosis, we compared gene expression in hydroprene-treated larvae that undergo supernumerary larval molt and control larva that metamorphose into pupa. Interestingly, the pupal-specific gene, Broad, was down-regulated in the hydroprene-treated larvae undergoing larval-larval molt. Broad mRNA transcripts appeared in the epidermis of M. sexta and Bombyx mori larvae only during the later stages of the final instar larvae coinciding with the pupal stage (Zhou et al., 1998a; Ijiro et al., 2004). In this study, FTZ-F1 was up-regulated significantly in the larval-larval molt in the hydroprene-treated larvae when compared to the larval-pupal molt in the control larvae. Woodard et al. (1994) showed that FTZ-F1 repressed its own expression and expressed only for a brief period in mid pre-pupae of D. melanogaster. The expression of FTZ-F1 during the larval-larval molt has not been addressed so far. Further studies are needed to identify the specific role of FTZ-F1. In this study, E74, but not E75 isoforms, expression was up-regulated during the larval-larval molt in hydroprene-treated larvae. The expression of E74, E75A, and E75B was observed during both the larval-larval and larval-pupal molts in M. sexta (Zhou et al., 1998b; Stilwell et al., 2003). Beckstead et al. (2007) showed that E74B was induced by JH III while E74A and E75A showed no response to JH in larval organ cultures of Drosophila. In vitro experiments with wing discs of M. sexta revealed that E75A was not upregulated by pyripoxyfen treatment (Keshan et al., 2006). From these studies, it is clear that expression of isoforms of 20E-induced transcription factors and their response to JH varies between molts and also among insects. Recent studies showed that isoforms of EcR play distinct roles in T. castaneum development (Tan and Palli, 2008b), it is likely that JH is involved in isoform-specific action of EcR and other genes involved in 20E signal transduction.
Also, the presence of JHA during the final instar larvae blocked midgut remodeling. Midgut remodeling occurs during the larval-pupal metamorphosis wherein the larval midgut cells undergo PCD, move into the gut lumen, and get eliminated from the gut. Simultaneously, the imaginal cells adjoining the basement membrane proliferate and differentiate into the pupal gut epithelium and replace the larval gut. These events occur during the quiescent stage (Parthasarathy and Palli, 2008). In the present study, due to the presence of JHA during the later stages of the final instar larvae, the larval gut epithelium failed to undergo PCD and the proliferation and differentiation of the imaginal cells were impaired. The morphology of the gut epithelium is maintained in the supernumerary larval molt except for an increase in the number of imaginal cells, which appear to proliferate during the final instar larval stage. Similar observations were made when methoprene was administered in the final instar larval stages of mosquitoes (Nishiura et al., 2003; Wu et al., 2006) and Heliothis virescens (Parthasarathy and Palli, 2007). The comparison of gene expression in the midguts of hydroprene-treated and control larvae revealed that the presence of JHA suppressed 20E-induced gene expression as observed in other insects (Nishuira et al., 2005; Wu et al., 2006). Thus, the role of JHA in regulating midgut metamorphosis appears to be conserved in most of the holometabolous insects. Taken together, this study affords an opportunity to understand the molecular mechanisms of hormonal regulation of beetle metamorphosis.
ACKNOWLEDGMENTS
This work was supported by National Science Foundation (IBN-0421856) and National Institutes of Health (GM 070559). This is contribution number 08-08-126 from the Kentucky Agricultural Experimental Station. We thank Tribolium genome consortium members for sharing sequence information and other resources. We also thank Dr. Richard Beeman of GMPRC, USDA-ARS, and Dr. Yoonseong Park of Kansas State University for sharing Tribolium stock. We thank Dr. Sheryn Perry, Dr. Michael Goodin, and Dr. Michael Sharkey from the University of Kentucky for use of their histology facilities and microscopes.
LITERATURE CITED
- Arakane Y, Muthukrishnan S, Kramer KJ, Specht CA, Tomoyasu Y, Lorenzen MD, Kanost M, Beeman RW. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol Biol. 2005;14:453–463. doi: 10.1111/j.1365-2583.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- Arthur FH. Susceptibility of last instar red flour beetles and confused flour beetles (Coleoptera: Tenebrionidae) to hydroprene. J Econ Entomol. 2001;94:772–779. doi: 10.1603/0022-0493-94.3.772. [DOI] [PubMed] [Google Scholar]
- Beckstead RB, Lam G, Thummel CS. Specific transcriptional responses to juvenile hormone and ecdysone in Drosophila. Insect Biochem Mol Biol. 2007;37:570–578. doi: 10.1016/j.ibmb.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhadialla TS, Carlson GR, Le DP. New insecticides with ecdysteroidal and juvenile hormone activity. Annu Rev Entomol. 1998;43:545–569. doi: 10.1146/annurev.ento.43.1.545. [DOI] [PubMed] [Google Scholar]
- Hammock BD, Quistad GB. Metabolism and mode of action of juvenile hormone, juvenoids, and other insect growth regulators. In: Hutson DH, Roberts TR, editors. Progress in pesticide biochemistry. Wiley; New York: 1981. pp. 1–83. [Google Scholar]
- Ijiro T, Urakawa H, Yasukochi Y, Takeda M, Fujiwara Y. cDNA cloning, gene structure, and expression of Broad-complex (BR-C) genes in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2004;34:963–969. doi: 10.1016/j.ibmb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Keshan B, Hiruma K, Riddiford LM. Developmental expression and hormonal regulation of different isoforms of the transcription factor E75 in the tobacco hornworm Manduca sexta. Dev Biol. 2006;295:623–632. doi: 10.1016/j.ydbio.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Kostyukovsky M, Chen B, Atsmi S, Shaaya E. Biological activity of two juvenoids and two ecdysteroids against three stored product insects. Insect Biochem Mol Biol. 2000;30:891–897. doi: 10.1016/s0965-1748(00)00063-1. [DOI] [PubMed] [Google Scholar]
- Lan Q, Grier CA. Critical period for pupal commitment in the yellow fever mosquito, Aedes aegypti. J Insect Physiol. 2004;50:667–676. doi: 10.1016/j.jinsphys.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Nishiura JT, Ho P, Ray K. Methoprene interferes with mosquito midgut remodeling during metamorphosis. J Med Entomol. 2003;40:498–507. doi: 10.1603/0022-2585-40.4.498. [DOI] [PubMed] [Google Scholar]
- Nishiura JT, Ray K, Murray J. Expression of nuclear receptor-transcription factor genes during Aedes aegypti midgut metamorphosis and the effect of methoprene on expression. Insect Biochem Mol Biol. 2005;35:561–573. doi: 10.1016/j.ibmb.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Palli SR. Developmental and hormonal regulation of midgut remodeling in a lepidopteran insect, Heliothis virescens. Mech Dev. 2007;124:23–34. doi: 10.1016/j.mod.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Palli SR. Proliferation and differentiation of instestinal stem cells during metamorphosis of the red flour beetle, Tribolium castaneum. Dev Dyn. 2008;237:893–908. doi: 10.1002/dvdy.21475. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Tan A, Bai H, Palli SR. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech Dev. 2008a;125:299–314. doi: 10.1016/j.mod.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy R, Tan A, Palli SR. bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval-pupal metamorphosis. Mech Dev. 2008b;125:601–616. doi: 10.1016/j.mod.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford LM. Molecular aspects of juvenile hormone action in insect metamorphosis. In: Gilbert LI, Tata JR, Atkinson BG, editors. Metamorphosis: postembryonic reprogramming of gene expression in amphibian and insect cells. Academic Press; San Diego: 1996. pp. 223–251. [Google Scholar]
- Riddiford LM, Ashburner M. Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol. 1991;82:172–183. doi: 10.1016/0016-6480(91)90181-5. [DOI] [PubMed] [Google Scholar]
- Stilwell GE, Nelson CA, Weller J, Cui H, Hiruma K, Truman JW, Riddiford LM. E74 exhibits stage-specific hormonal regulation in the epidermis of the tobacco hormworm, Manduca sexta. Dev Biol. 2003;258:76–90. doi: 10.1016/s0012-1606(03)00105-2. [DOI] [PubMed] [Google Scholar]
- Tan A, Palli SR. Identification and characterization of nuclear receptors from the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 2008a;38:430–439. doi: 10.1016/j.ibmb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Tan A, Palli SR. Edysone receptor isoforms play distinct roles in controlling molting and metamorphosis in the red flour beetle, Tribolium castaneum. Mol Cell Endocrinol. 2008b;291:42–49. doi: 10.1016/j.mce.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews MD, Campbell JF, Arthur FH, West M. Monitoring Tribolium castaneum (Coleoptera: Tenebrionidae) in pilot-scale warehouses treated with residual applications of (S)-hydroprene and cyfluthrin. J Econ Entomol. 2005;98:1391–1398. doi: 10.1603/0022-0493-98.4.1391. [DOI] [PubMed] [Google Scholar]
- Tomoyasu Y, Denell RE. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol. 2004;214:575–578. doi: 10.1007/s00427-004-0434-0. [DOI] [PubMed] [Google Scholar]
- Tribolium Genome Sequencing Consortium The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–955. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- Truman JW, Riddiford LM. Endocrine insights into the evolution of metamorphosis in insects. Annu Rev Entomol. 2002;47:467–500. doi: 10.1146/annurev.ento.47.091201.145230. [DOI] [PubMed] [Google Scholar]
- Webb BA, Riddiford LM. Synthesis of two storage proteins during larval development of the tobacco hornworm, Manduca sexta. Dev Biol. 1998;130:671–681. doi: 10.1016/0012-1606(88)90359-4. [DOI] [PubMed] [Google Scholar]
- Wigglesworth VB. Hormones controlling growth and development in insects. Biochem Soc Symp. 1965;25:79–82. [PubMed] [Google Scholar]
- Williams CM. The juvenile hormone.I. Its role in the endocrine control of molting, pupation, and adult development of the cecropia silkworm. Biol Bull. 1961;121:572–585. [Google Scholar]
- Wilson TG. The molecular site of action of juvenile hormone and juvenile hormone insecticides during metamorphosis: how these compounds kill insects. J Insect Physiol. 2004;50:111–121. doi: 10.1016/j.jinsphys.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Woodard CT, Baehrecke EH, Thummel CS. A molecular mechanism for the stage specificity of the Drosophila prepupal genetic response to ecdysone. Cell. 1994;79:607–615. doi: 10.1016/0092-8674(94)90546-0. [DOI] [PubMed] [Google Scholar]
- Wu Y, Parthasarathy R, Bai H, Palli SR. Mechanisms of midgut remodeling: juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action. Mech Dev. 2006;123:530–547. doi: 10.1016/j.mod.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zhou B, Hiruma K, Shinoda T, Riddiford LM. Juvenile hormone prevents ecdysteroid-induced expression of broad complex RNAs in the epidermis of the tobacco hornworm, Manduca sexta. Dev Biol. 1998a;203:233–244. doi: 10.1006/dbio.1998.9059. [DOI] [PubMed] [Google Scholar]
- Zhou B, Hiruma K, Jindra M, Shinoda T, Segraves WA, Malone F, Riddiford LM. Regulation of the transcription factor E75 by 20-hydroxyecdysone and juvenile hormone in the epidermis of the tobacco hornworm, Manduca sexta, during larval molting and metamorphosis. Dev Biol. 1998b;193:127–138. doi: 10.1006/dbio.1997.8798. [DOI] [PubMed] [Google Scholar]
- Zhou X, Riddiford LM. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development. 2002;129:2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]