Abstract
Background:
The aim of this research was to evaluate the effect of adipose derived stem cells on bone repair in through and through mandibular bone defects of canine.
Materials and Methods:
In this prospective comparative study, adipose-derived stem cells were isolated from subcutaneous fat of lateral thoracic area of 4 dogs. The isolated cells were cultured and expanded through 3 passages. The undifferentiated stem cells were seeded in Collatamp and transferred into mandibular bone through-and-through defects. Similar defects on control group were filled with cell-free Collatamp. After 6 weeks, biopsies were taken and histomorphometric analysis was performed. The percentage of new bone formation was measured in each case. The data were subject to statistical analysis using the Wilcoxon test. Differences at P≤0.05 were considered significant.
Results:
H and E staining of decalcified samples revealed more bone formation in the group, which stem cells were seeded. Cell-free collatamp group revealed an average bone regeneration of %41±13.21, while adipose derived stem cell-seeded collatamp group showed %49±8.24.
Conclusion:
The use of stem cell seeded collatamp scaffold in mandibular defects caused more bone regeneration.
Keywords: Bone regeneration, mesenchymal stem cells, scaffold
INTRODUCTION
One of the most important issues in oral and maxillofacial surgery is restoring the lost bone resulting from trauma, tumors, congenital defects or malunion of fractures.[1]
For many decades, autologous bone graft (iliac crest, rib…) has been the gold standard to treat bone defects. Because of the risk and morbidity[2] of such procedures many efforts have been done to find other ways to treat these lesions.[3,4]
Tissue engineering is a scientific scope which contains cell culture, extracellular matrix, biochemistry, and also has a close relation with molecular biology of cells, genetic and also basic sciences such as physics and chemistry.
A tissue–engineering approach capable of combining stem cells with an appropriate three dimensional bioabsorbable scaffold can stimulate bone regeneration and repair.[5]
Mesenchymal stem cells (MSCs) are multipotent cells that can give rise to cells with mesodermal origin such as bone, cartilage, tendon, muscle and so on.[6]
MSCs can be isolated from many sources such as bone marrow,[7] dermis,[8] adipose tissue,[9] dental pulp,[10] synovium,[11] and deciduous teeth.[12] Great efforts have been done in recent years in isolating and characterizing the MSCs.
Adipose tissue can be harvested in large amount with minimal morbidity compared to harvesting bone graft. It also contains several kinds of cells such as adipocytes, vascular endothelial cells and vascular smooth muscle cells.
In addition, it also contains cells with an ability to differentiate into several lineages[13,14] which are named as adipose-derived stem cells (ADSCs).
The later cells are also capable to express multiple growth factors including vascular endothelial and hepatocyte growth factors.[15] Applying fresh adipose-derived stem cells in a cranial defect has shown healing without side effects.[16]
About 5×105 stem cells could be obtained from 400 to 600 mg of adipose tissue.[17]
Appropriate scaffolds should be resorbable, biocompatible, and inert and also can promote angiogenesis and serve as a proper matrix for loaded cells.
Collagen scaffold is suitable for nesting ADSCs and acting as a three dimensional bone engineering framework, in vitro and in vivo.[18] Collagen is a significant component of extra cellular matrix. The scaffolds made of collagen had been used in a variety of purposes such as hemostasis, guided bone regeneration and so on. It has low antigenicity and good mechanical characteristics, also collagen scaffolds have been observed promoting cell and tissue attachment and growth.[19–20]
In a research accomplished by Kakudo et al.,[21] ASCs were cultured up to 14 days in a honeycomb scaffold and transplanted subcutaneously into nude mice and excised after 8 weeks. Bone formation was examined by H and E staining, which showed significant positive stains indicating bone regeneration in the samples.
This research was designed to compare the degree of ossification in canines mandibular through and through bone defects filled with cell-free collatamp[22] (a lyophilized collagen implant impregnated with gentamycin) with ADSCs-seeded collatamp.
MATERIALS AND METHODS
In this prospective comparative study, four 3-year-old healthy and vaccinated dogs with weight range of 25 to 35 kgs were used. Dog is a good biomedical model. It has an anatomy nearly similar to human and also is suitable to evaluate the results of experimental therapies such as those employing stem cells.[23] Our study (research No: 389334) was performed in Torabinejad Research Center at the School of Dentistry, Isfahan University of Medical Sciences (IUMS), Isfahan, Iran. The animals were housed for 10 days to become familiar to housing and diet.
Harvesting subcutaneous fat
In the first phase of the experiment 10 to 15 g fat was harvested from subcutaneous tissue of lateral thoracic area under general anesthesia.
To anesthetize the dogs, premeditation composed of intramuscular injection of acepromazine %1 (0.02 ml/ kg) (Aveco Co Inc, Fort Dodge, IA) and subcutaneous injection of atropine sulfate (0.05 mg/ kg) (Atropine 0.5,Daroupakhsh Pharmaceutical Mfg Co, Tehran, Iran) were given. Induction of general anesthesia started with intravenous injection of ketamine (15 mg/ kg) (Alfasan, Holland) and followed by respiratory halothane and N2O intra-tracheal tube.
Lateral thoracic area was cleaned, shaved, prepared and draped.
After injecting local anesthetic (Lidocaine %2 plus Epinephrine 1:80000) an incision with a length of 5 cm was performed. After dissection, about 10 to 15 gram subcutaneous fat was harvested and while maintaining in phosphate-buffered saline (PBS) (Sigma) transferred to cell culture laboratory.
Wound was irrigated with sufficient amount of saline and sutured layer by layer.
Animals were sent to recovery room and housed again after intramuscular injection of antibiotics (1 vial of penicillin 6.3.3).
Adipose-derived stem cell isolation and cultivation
In the cell culture lab, ADSCs were isolated according to the protocol of Zuk et al.[24] but with some modifications. Processing of fat consisted of irrigating with PBS. adding 1 mg/ gr collagenase type I (Sigma) in order to perform enzymatic digestion, neutralizing it with Dulbecco modified eagle medium (DMEM; Gibco-BRL, Life Technologies, Grand island, NY) supplemented with fetal bovine serum and after that in final steps irrigating with PBS and centrifuging (1400 rpm, 10 min) it. The upper layer of fluid was thrown away and the remainder of suspension was transferred to the flask containing medium consisted of Dulbecco modified eagle medium enriched with fetal bovine serum %10 (FBS; Dainippon pharmaceutical, Osaka, Japan) and penicillin-streptomycin %0.5 (Gibco-BRL, Life Technologies).
For each passage the medium was removed, irrigation was performed with PBS, Trypsin-EDTA was added for 3 min and after that neutralized with Medium. After centrifuging, the fluid on top was thrown away and the remainder of suspension transferred to a new flask.
Non-adherent cells were removed from culture by washing with PBS. The adherent cells were expanded as monolayer culture in a medium consisted of Dulbecco modified eagle medium enriched with fetal bovine serum %10 and penicillin-streptomycin %0.5 at 5% Co2 at a temperature of 37°C. This was done to achieve a total viable cell density of 5-6×106 cell [Figures 1 and 2].
Figure 1.
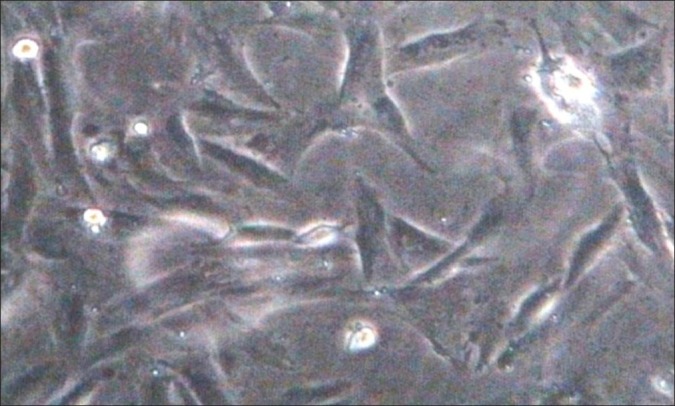
Undifferentiated canine adipose-derived stem cells
Figure 2.
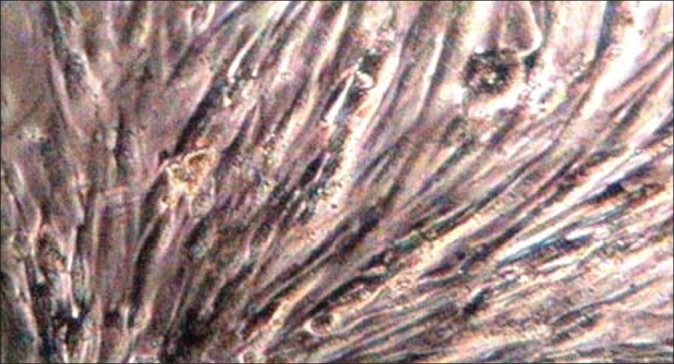
Undifferentiated canine adipose-derived stem cells at the third passage
The obtained stem cells were counted and trypsinized. About 5×106 cells were suspended in a little amount of medium (100 μl). The suspension was transferred with a sampler to a 9×5 mm cylindrical collatamp (syntacoll, GmbH, D-93340 saal/D.Germany) scaffold and incubated for 2 h. After that medium was added and incubated again for 48 h before implanting in mandibular bone defects.
Implantation of scaffolds
Three weeks after initial surgery the second surgery was performed. In this phase, animals were premedicated and anesthetized. Submandibular area was shaved, prepared, and draped. A submandibular incision was made in each side and layered dissection was performed toward mandibular bone.
One 9 mm in diameter cylindrical through and through defect [Figure 3] was created in each side by trephine bur. (Meisinger, Dusseldorf, Germany).
Figure 3.
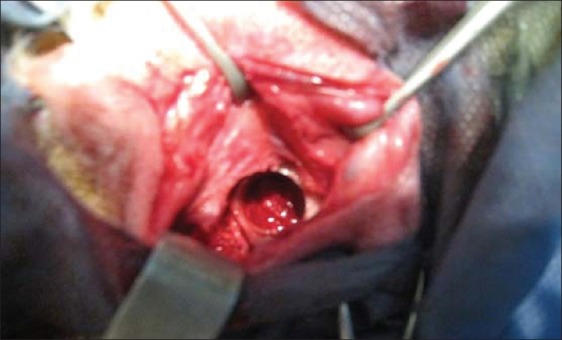
Through-and-through defects caused by trephine bur in dog mandible
The right mandibular defect was filled with ADSCs-seeded collatamp and the left side with cell-free collatamp [Figure 4].
Figure 4.
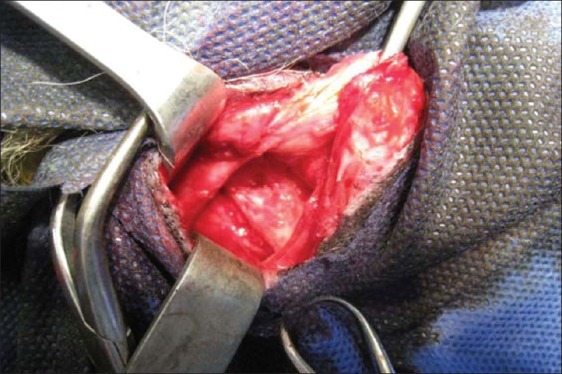
Filling surgical defect with scaffold
The wound was closed in layered fashion. (Vicryl 3.0, nylon 4.0, Ethicon Gmbh and Co. KG. Norderstedt, Germany).
Animals received ceftriaxone 1 gr I.V (Loghman pharmaceutical and Hygienic co, Iran) once a day for 5 days.
Biopsy
After 6 weeks in the third phase of this study, biopsies were taken by a larger trephine bur (13.0 mm). Biopsies were numbered, placed in neutral buffered formalin %10 and transferred to histology laboratory to determine the degree of ossification [Figure 5].
Figure 5.
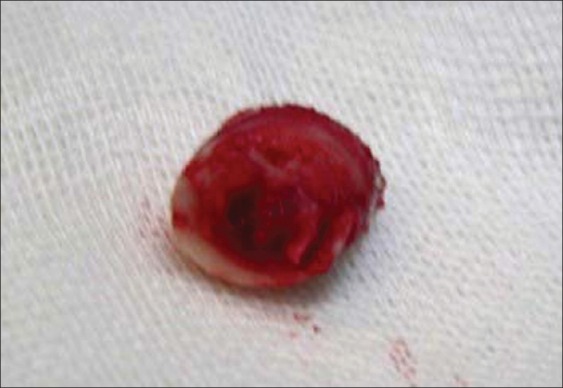
Trephined bone as a biopsy
Histological method
The specimens were kept in formalin for an extra 24 h, and then decalcified by maintaining in formic acid 10% for 7 days. The specimens were washed with water, dehydrated with ethyl alcohol, cleared with xylene, infiltrated with paraffin, and casted. The casts were placed in a cool place then in freezer. Six sections with 3 to 4 μm of thickness were prepared from each biopsy using a microtome. Sections were warmed up and incubated for 30 min at 65°C then processed in descending ethyl alcohol. (100, 100, 95, 85, 75, distilled water).
Afterwards, they were stained with hematoxylin for 5 min; excess dye was washed out with acid alcohol (0.3%). The sections were placed under tap water for 2 min.
Sections were stained with eosin at this time and processed in ascending alcohol (75, 85, and 95, 100, 100).
Finally slides were processed and histological evaluation including the amount of woven bone formation, bone marrow, and osteoblasts using a light microscope was performed (Zeiss Microscopy, LLC, New York). Totally 48 microscopic fields were studied.
Statistical analysis
The data were subject to statistical analysis using the Wilcoxon test.
Differences at P≤0.05 were considered significant in this study.
RESULTS
Histological evaluation
In our study, all the animals were healthy and no significant problem occurred at the interval between fat harvesting and scaffolds implanting. Healing period was normal in all animals.
Neither foreign body reaction nor inflammation or infection was seen in animals.
In light microscopic evaluation, each section was studied under magnification of ×40 and ×100. The newly formed tissue was in varying sizes consisted of islands of woven bone and large marrow spaces with fat cells and hematopoietic cells. Osteoblast-like cells was found in ADSCs-seeded group [Figures 6–9].
Figure 6.
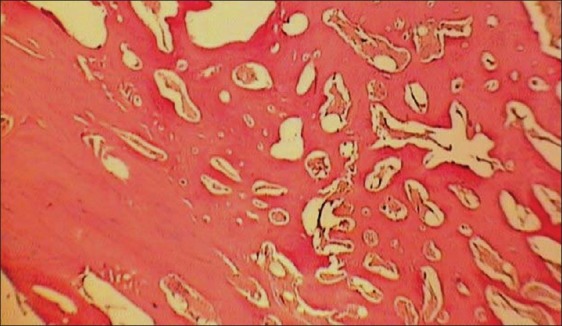
Histologic view of ADSCs -loaded collatamp defect (×40)
Figure 9.
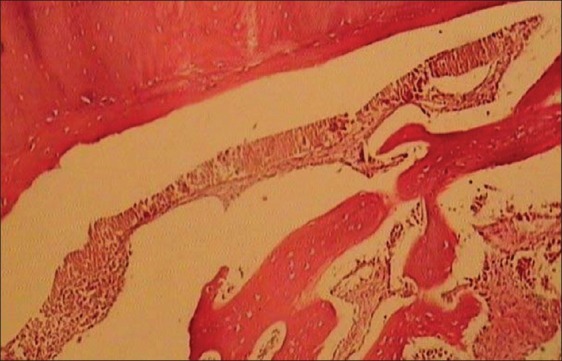
Histologic view of cell free collatamp defect (×100)
Figure 7.
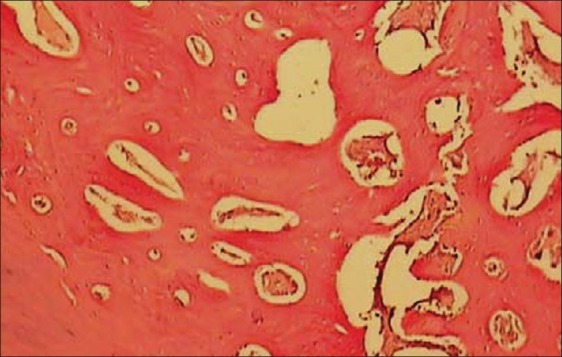
Histologic view of ADSCs loaded collatamp defect (×100)
Figure 8.
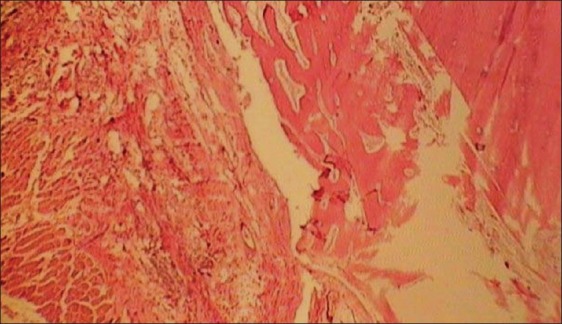
Histologic view of cell free collatamp defect (×40)
Histomorphometric analysis
Results of the histomorphometric analysis are shown in Table 1. Area of new bone fill (BF) is reported in percentage of the whole area of defect excluding the primary cortical bone margin.
Table 1.
Mean percentage of new bone fill in mandibular bone defects in both groups

Density of bone, length of residual defect, and area of new (BF), was measured histomorphometrically.[25,26] Defect sites exhibited varying degrees of bone formation ranging from limited to 60%. The mean percentage of new bone formation in ADSCs-seeded collatamp group was %49±8.24, while in control group (cell-free collatamp filled defect) was %41±13.21 [Figure 10].
Figure 10.
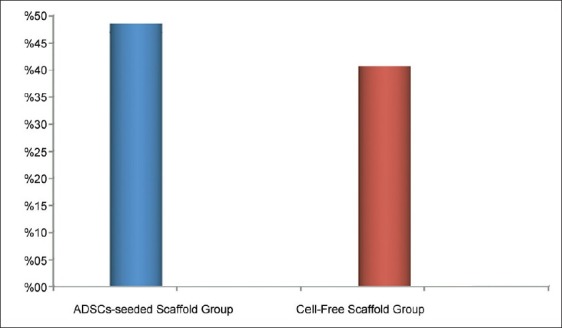
Histomorphometric data of percentage of new bone fill (BF) in ADSCs-seeded scaffold group and cell-free scaffold group revealed higher percentage of BF in ADSCs-seeded group
Our results indicated no significant differences in bone formation between ADSCs-seeded and cell-free collatamp groups (P value = 0.102).
DISCUSSION
Today, one of the apprehensions of an oral and maxillofacial surgeon is reconstructing bony defects and enhancing bone regeneration with avoidance of harvesting autologous bone grafts.
A method to achieve this goal is regenerating bone through tissue engineering. In the present study, adipose-derived stem cells were loaded into scaffolds and placed in bony defects to enhance bone regeneration.[27]
Schliephake et al.[28] designed a research in which human trabecular bone cells were isolated and seeded in three different types of scaffolds (porous tricalcium phosphate, mineralized collagen and calcium carbonate). Stem cell seeded scaffolds were implanted in rat mandibular defects and after 6 weeks the presence of human cells was assessed. They reported that seeding human cells or culturing technique did not increase the amount of early bone formation.
In a research performed by Abukawa et al.,[29] autologous porcine stem cells were seeded in D, L-lactic-co-glycolic acid and transferred to mandibular defects. They reported that the repaired bone in the defects treated with stem cell seeded scaffolds was indistinct radiographically from native bone, but the defects of control group were remained radiolucent. In histological evaluation of defects that were treated by tissue-engineering method, hard tissue resembling to bone was formed but at the control group with unseeded scaffold ossification was limited to the periphery of defect.
In this study to enhance the efficacy of stem cells, the selected scaffold was collatamp. It has been proved that collagen scaffold is excellent for stem cell differentiation and proliferation.[30,31]
In our study, ADSCs were not treated with osteogenic medium before implantation in animal model. They were used as undifferentiated cells. It was thought that osteogenesis in implantation site was influenced by bone microenvironment and action of the materials.
Analysis of results in this study showed a little difference in bone regeneration between cell-free scaffold and ADSCs-seeded scaffold. This little and statistically not significant difference may be due to insufficient healing time before doing biopsies. In a research performed by Haghighat et al.,[32] it was stated that bone healing in mandibular third molar sockets needs 4 months to be relatively completed. So, if we had done biopsies at a time later than 4 months it could have revealed the differences better. The reason is that undifferentiated cells need some time to differentiate into osteoblasts and since in this research biopsies were taken at the 6th week, these cells had not had sufficient time to become osteoblast and show their efficacy in osteogenesis. On the other hand, the size of defects may affect the result. In this research, the diameter of defects was 9.0 mm. At this size, spontaneous healing may occur. Therefore observing no difference between test and control groups could be related to the size of defects. So, it is necessary to investigate more in this scientific subject.
CONCLUSION
Our results revealed no significant difference between in ADSCs-seeded and cell-free collatamp groups in terms of the percentage of BF.
ACKNOWLEDGMENT
The authors would like to acknowledge the truthfully cooperation of the staff of Torabinejad Dental Research Center in all stages of this research.
Footnotes
Source of Support: This report is based on a thesis which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran, in partial fulfillment of the requirements for the MSc degree in Oral and Maxillofacial Surgery (#389214). The study was approved by the Medical Ethics and Research Office at the Isfahan University of Medical Sciences and financially supported by this University.
Conflict of Interest: None declared.
REFERENCES
- 1.Arrigoni E, Lopa S, de Girolamo L, Stanco D, Brini AT. Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: From small to large animal models. Cell Tissue Res. 2009;338:401–11. doi: 10.1007/s00441-009-0883-x. [DOI] [PubMed] [Google Scholar]
- 2.Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–9. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous Iliac Crest Bone Graft. Complications and Functional Assessment. Clin Orthop Relat Res. 1997;339:76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Herford AS, Boyne PJ. reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2) J Oral Maxillofac Surg. 2008;66:616–24. doi: 10.1016/j.joms.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Jafarian M, Eslaminejad MB, Khojasteh A, Mashhadi Abbas F, Dehghan MM, Hassanizadeh R, et al. Marrow-derived mesenchymal stem cells-directed bone regeneration in the dog mandible: A comparison between biphasic calcium phosphate and natural bone mineral. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e14–24. doi: 10.1016/j.tripleo.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Shanti RM, Li WJ, Nesti LJ, Wang X, Tuan RS. Adult mesenchymal stem cells: Biological Properties, Characteristics, and Applications in Maxillofacial Surgery. J Oral Maxillofac Surg. 2007;65:1640–7. doi: 10.1016/j.joms.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Pontikoglou C, Deschaseaux F, Sensebe L, Papadaki HA. Bone Marrow mesenchymal stem cpells: Biological Properties and Their Role in Hematopoiesis and Hematopoietic Stem Cell Transplantation. Stem Cell Rev. 2011;7:569–89. doi: 10.1007/s12015-011-9228-8. [DOI] [PubMed] [Google Scholar]
- 8.Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, et al. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009;5:378–86. doi: 10.1007/s12015-009-9094-9. [DOI] [PubMed] [Google Scholar]
- 9.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, Schouten TE, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166–77. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 10.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 11.Fan J, Varshney RR, Ren L, Cai D, Wang DA. Synovium-derived mesenchymal stem cells: A new cell source for musculoskeletal regeneration. Tissue Eng Part B Rev. 2009;15:75–86. doi: 10.1089/ten.teb.2008.0586. [DOI] [PubMed] [Google Scholar]
- 12.Barry FP, Murphy JM. Mesenchymal stem cells: Clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Farre-Guasch E, Marti-Page C, Hernadez-Alfaro F, Klein-Nulend J, Casals N. Buccal fat pad, an oral access source of human adipose stem cells with potential for osteochondral tissue engineering: An in vitro study. Tissue Eng Part C Methods. 2010;16:1083–94. doi: 10.1089/ten.TEC.2009.0487. [DOI] [PubMed] [Google Scholar]
- 14.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods. 2008;45:115–20. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 16.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–4. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–75. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 18.Harley BA, Gibson LJ. In vivo and in vitro applications of collagen-GAG scaffolds. Chem Eng J. 2008;137:102–21. [Google Scholar]
- 19.O’Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25:1077–86. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–41. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 21.Kakudo N, Shimotsuma A, Miyake S, Kushida S, Kusumoto K. Bone tissue engineering using human adipose-derived stem cells and honeycomb collagen scaffold. J Biomed Mater Res A. 2008;84:191–7. doi: 10.1002/jbm.a.31311. [DOI] [PubMed] [Google Scholar]
- 22.McKegney M, Taggart I, Grant MH. The influence of crosslinking agents and diamines on the pore size, morphology and the biological stability of collagen sponges and their effect on cell penetration through the sponge matrix. J Sci Mater Med. 2001;12:833–44. doi: 10.1023/a:1017989305873. [DOI] [PubMed] [Google Scholar]
- 23.Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008;14:1007–15. doi: 10.1089/ten.tea.2007.0207. [DOI] [PubMed] [Google Scholar]
- 24.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorustovich A, Veinsten F, Costa OR, Guglielmotti MB. Histomorphometric evaluation of the effect of bovine collagen granules on bone healing. An experimental study in rats. Acta Odontol Latinoam. 2004;17:9–13. [PubMed] [Google Scholar]
- 26.Schwarz F, Herten M, Ferrari D, Wieland M, Schmitz L, Engelhardt E, et al. Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite + beta tricalcium phosphate (Bone Ceramic) or a collagen-coated natural bone mineral (BioOss Collagen): An immunohistochemical study in dogs. Int J Oral Maxillofac Surg. 2007;36:1198–206. doi: 10.1016/j.ijom.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Cui L, Liu GP, Cao YL, Zhu JT, Cao Y. [Tissue-engineering bone with ADSCs and coral scaffold for repairing of cranial bone defect in canine] Zhonghua Zheng Xing Wai Ke Za Zhi. 2009;25:204–8. [PubMed] [Google Scholar]
- 28.Schliephake H, Zghoul N, Jager V, van Griensven M, Zeichen J, Gelinsky M, et al. Bone formation in trabecular bone cell seeded scaffolds used for reconstruction of the rat mandible. Int J Oral Maxillofac Surg. 2009;38:166–72. doi: 10.1016/j.ijom.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Abukawa H, Shin M, Williams WB, Vacanti JP, Kaban LB, Troulis MJ. Reconstruction of mandibular defects with autologous tissue-engineered bone. J Oral Maxillofac Surg. 2004;62:601–6. doi: 10.1016/j.joms.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 30.George J, Kuboki Y, Miyata T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol Bioeng. 2006;95:404–11. doi: 10.1002/bit.20939. [DOI] [PubMed] [Google Scholar]
- 31.Sumanasinghe RD, Osborne JA, Loboa EG. Mesenchymal stem cell-seeded collagen matrices for bone repair: Effects of cyclic tensile strain, cell density, and media conditions on matrix contraction in vitro. J Biomed Mater Res A. 2009;88:778–86. doi: 10.1002/jbm.a.31913. [DOI] [PubMed] [Google Scholar]
- 32.Haghighat A, Hekmatian E, Abdinian M, Sadeghkhani E. Radiographic Evaluation of Bone Formation and Density Changes after Mandibular Third Molar Extraction: A 6 Month Follow up. Dent Res J. 2011;8:1–5. [PMC free article] [PubMed] [Google Scholar]


