Abstract
Background:
Natural products are proved to play a good role as an alternative to synthetic chemicals in clinical conditions. Previous studies showed that Pelargonium graveolens has anti-inflammatory and antifungal activity against Candida albicans. The aim of this study was to evaluate the efficacy of essential oil of Pelargonium graveolens in the treatment of denture stomatitis.
Materials and Methods:
In this double-blind randomized clinical trial conducted in Isfahan (Iran), 80 (51 females and 29 males) eligible wearers of complete denture were included. According to the patients’ profiles number, they randomly divided to 2 groups of 40 patients’ case and 40 patients control treated with Pelargonium 1% gel or placebo, respectively. They were recommended to apply the gel twice daily for a 14-day course. All data were analyzed using SPSS® for windows (v.18). We have used the χ2 test for analyzing qualitative and Student t-test for quantitative data considering as P<0.05 as significant.
Results:
According to mycological data and clinical observation after treatment in the case group, 34% of patients had been improved completely, 56% partially and 10% had no improvement. In the control group, 5% of patients had complete recovery, 25% partial recovery, and 70% no improvement. A significant reduction in fungal growth was observed in case group rather than the control group (P value<0.0001).
Conclusion:
It seems that the application of a 1% Geranium oil topical gel formulation is more effective than placebo in the treatment of denture stomatitis.
Keywords: Candida albicans, denture stomatitis, Pelargonium graveolens
INTRODUCTION
Denture stomatitis, more commonly known as denture sore mouth, is an erythematous condition involving the mucosa of the hard palate in complete denture wearers.[1] Its clinical features include a diffuse or patchy redness of the palate under a denture and confined to the area covered by the denture.[2] This condition is usually asymptomatic, but can be associated with burning, bleeding, and unpleasant taste or halitosis.[3]
The involvement of Candida spp. as a potential causative agent in denture stomatitis is previously described and Candida albicans remains the most frequent isolated yeast in the oral cavity. Candida-associated denture stomatitis is observed in approximately 11-67% of denture wearers.[4]
Several factors which are considered influent in the initiation of denture stomatitis are: local trauma from ill-fitting denture, poor denture hygiene, continuous denture wear, bacterial and fungal infection, malnutrition, hormonal imbalance, and use of antibiotics.[5] Formation of a dental plaque by the normal flora is facilitated by the presence of debris, irregularities in the acrylic resin, negative pressure in the acrylic resin-mucosa interface and therefore follows the action of microorganisms to result in varying grades of denture stomatitis.[6]
Common drugs used for controlling denture stomatitis are Imidazole compounds, polyenic derivatives (nystatin), and amphotericin B.[7,8] However, toxicity and resistance to these antifungal drugs are major concern to which variable results have been observed and recurrence rate is high.[9,10] Despite the availability of many commercial denture-cleansing products, less than 60% of the wearers make use of any of them.[11]
Some natural products have been considered to play a good role as an alternative to synthetic chemicals in this clinical condition.[10] A wide variety of plants extracts have been reported to have antifungal activity against Candida albicans. Among such plants, the essential oil of Pelargonium graveolens in several studies has been investigated for its anti-inflammatory and antifungal activity especially against C. albicans.[12] Pelargonium extracts have profound anti-oxidant and antibacterial effects that can be used in food and cosmetic industries.[13] Several species of Pelargonium have immunomodulatory and cytoprotective effects which enable them for use in the treatment of acute bronchitis.[14] Another characterization of geranium oil is insecticidal activity, mainly because of high amount of phenolic compounds such as geraniol.[15] In one study, synergistic effects of geranium oil and ketoconazole were evaluated and a potent inhibitory activity was observed against Trichophyton spp. This effect was explained for the high active and stable primary alcohols citronellol and geraniol.[16] The main constituent of “Geranium oil” are “beta-citronellol” and “geranoil”.[17] Due to remarkable inhibitory effects of geranium oil on growth of Candida, as well as its wound-healing properties, it could be beneficial in the treatment of denture-induced stomatitis.[13]
This study was conducted to evaluate the probable clinical usefulness of a 1% gel formulation of Pelargonium graveolens essential oil in the treatment of denture stomatitis.
MATERIALS AND METHODS
In this double-blind randomized clinical trial, which was conducted in dental clinics affiliated to the Isfahan University of Medical Sciences Isfahan, Iran, from July 2010 until May 2011, 80 (51 females and 29 males) eligible wearers of complete upper denture, with the age range of 38 to 78 years (61.39±9.038) were included. Patients, who were carrying denture for more than 1 year, were included in this study. However, patients who had diabetes or systemic diseases, had used antibiotic, corticosteroids, or antifungal drugs in the past 1 month as well as during the treatment course, and had not followed medical procedure during treatment was excluded from the study. Demographic data and full medical and dental history for all patients were recorded and all clinical examinations were done by a registered prosthodontics specialist. The presence of C. albicans was confirmed using culture medium. Palatal swabs were taken from both patients’ upper denture and mucosa bearing denture for assessment of the presence of C. albicans. According to clinical examination and mycological tests, patients who have developed generalized erythema and edema of denture bearing area from moderate to severe, defined as type II and III denture stomatitis. According to the patients’ profiles number and the random Figures tables, they were randomly divided into two groups of 40 patients, cases and controls, treated with Pelargonium gel and placebo, respectively. We considered complete clinical improvement, if the lesion was completely eliminated and no colony was observed. In the case of reduced inflammation of palatal mucosa, it was reported partial improvement.
Essential oil of Pelargonium graveolens was provided from Barij Essence Company (Kashan, Iran) and the process of preparing the Orabase Gel was designed and formulated at the school of Pharmacy, Isfahan University of Medical science. We have formulated a carboxy methyl cellulose, Carbomer 934 (Merck®, Germany) and Plastibase (JaberebneHayyan®, Tehran, Iran) gel base as placebo and the same gel base containing 1% of Geranium essence (Barij® Essence Company, Kashan, Iran) for the use of patients in this study.
Patients were examined for denture-wearing habits, at the time of admission (day 0) and on days 7 and 14. At each visit, mycological samples were taken from the palatal mucosa and fitting surface and then they were inoculated on Sabouraud's dextrose agar plates and incubated at 37°C and after 48 h, the colonies were counted. Remission of lesion was recorded by the specialized dentist. At baseline, all patients were instructed for denture hygiene and to remove dentures during the night. They were recommended to apply the gel (placebo or drug) twice daily (morning and night) for a complete 14-day course.
The study protocol was approved by the board ethics for human research at Isfahan University of Medical Sciences. All patients have completed informed consent form prior to the study and no patient was deprived from the normal ordinary treatment if he/she refused for participation in the study.
All data were analyzed using SPSS® for windows (v.18). We have used the χ2 test for analyzing qualitative data and Student t-test for quantitative data considering as P<0.05 as significant.
RESULTS
All patients were compared in terms of age, gender, disease history, duration of denture use, and detection of C. albicans on a palatal sample [Table 1]. No statistically significant difference was observed between the intervention (cases) and the control (placebo) group of patients in terms of age, gender, and denture use duration.
Table 1.
Baseline characteristics and the outcome of treatment with Geranium essential oil in the study patients
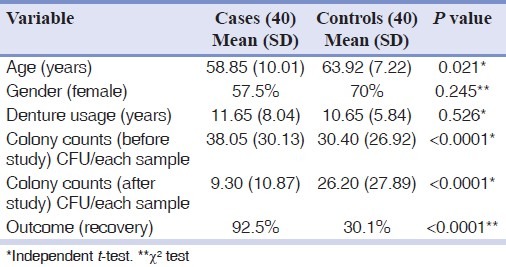
According to mycological culture data and clinical observation before and after treatment in the case group, 34% of patients had been improved completely and 56% of them were reported with partial recovery. Candida colony counts were dramatically decreased in treatment group. In 10% of patients, improvement was not observed. In the control group, 5% of patients had complete recovery, 25% had partial recovery, and the rest of 70% had no considerable improvement.
Efficacy of Pelargonium essence gel in the remission of erythema was seen dramatically in the patients receiving Pelargonium gel compared with the placebo group. A significant reduction in fungal load and erythematous extent in the oral cavity was observed (P<0.0001).
DISCUSSION
Phytomedicine is increasingly becoming popular all over the world. Denture stomatitis presents as an inflammatory under maxillary prosthesis affecting about 25-65% of subjects, with C. albicans being the principal etiological agent.[9] Toxicity and resistance to normally used antifungal drugs are a complicated challenge.[10] To take into consideration are the expanded source of medicinal plants in Iran and immense anti-inflammatory and anti-microbial activity of them in the treatment of skin disorders. A wide variety of plant extracts have been reported to have dramatic inhibitory effects on C. albicans. In recent studies, some plants such as Zatariamultiflora, Origanumvulgare, Melaleucaalternifolia, and P. graveolens have been proved to have inhibitory effects on C. albicans which were assessed in synergism with Amphotericin B.[12] Several compounds derived from the oil of P. graveolens have been reported to have acaricidal and insect-repellent activity which can be used for preservative agent in food and cosmetic preparation.[18,19] Essential oil of some species of Geraniaceae have antibacterial and immunomodulatory activities and are used in respiratory and mucosal infection.[14] Among such plants, essential oil of P. graveolens has great antifungal activity as well as anti-inflammatory and repairing effects. According to classical references “Geranium oil” has been discussed as being useful in healing wounds, abscesses, fever, colic, cold, and sore throats.[13]
On the other hand, the diluent and cleansing effect of saliva may reduce the level of antimicrobial agents below their therapeutic concentration.[8] Application of Orabase gel which adhere to buccal mucosa to have long term effects, would be much more preferable.[20]
In this study, all patients of the two study groups were matched in terms of age, gender, colony count of C. albicans on palatal sample, duration of denture wearing, removal of denture during at night, and Newton's type of denture stomatitis [Table 1].
Patients in the intervention arm of the study (cases) who applied 1% gel for two consecutive weeks had a statistically significant improvement in colony count of Candida [Table 1] and the erythema of palatal mucosa [Figure 1].
Figure 1.
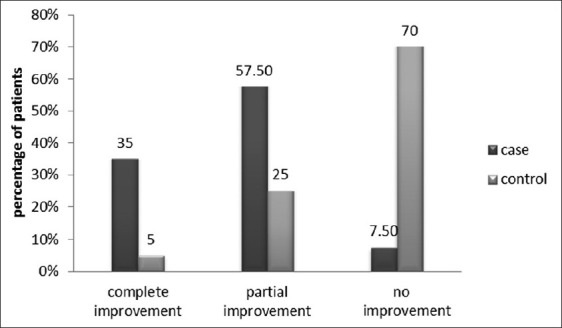
Inflammation changes of palatal mucosa in cases and control groups
According to the results of present study, it seems that topical formulation of P. graveolens may have some beneficial therapeutic effects in the treatment of denture stomatitis. It is important to mention that we have not observed any reportable side effects in patients receiving Geranium gels. Further clinical studies with larger number of patients are recommended.
CONCLUSION
Application of a 1% Geranium oil in the form of Orabase gel is more effective than placebo in the treatment of denture stomatitis. It seems that use of formulated gel following proper oral and denture hygiene, may decrease Candida infection and reduces the local inflammation.
ACKNOWLEDGEMENT
This research was the result of a doctor of pharmacy thesis project which was supported financially by the vice-chancellery of research at the Isfahan University of Medical Sciences. It was designed and conducted by the Isfahan Clinical Toxicology Research Centre (http://ctrc.mui.ac.ir). The authors would like to thank the academic faculty members of the school of Dentistry at the Isfahan University of Medical Sciences and all of the colleagues and the staff of the Professor Torabinejad Dental Research Center for their valuable endeavors and support.
Footnotes
Source of Support: This project was financially supported by the Vice-chancellery for Research and Technology of the Isfahan University of Medical Sciences, Isfahan, Iran.
Conflict of Interest: Authors had no conflict of interest, including personal or financial relationships with organizations that might inappropriately influence, or be perceived to influence the work.
REFERENCES
- 1.Renner RP, Lee M, Andors L, McNamara TF, Brook S. The role of C. albicans in denture stomatitis. Oral Surg Oral Med Oral Pathol. 1979;47:323–8. doi: 10.1016/0030-4220(79)90254-8. [DOI] [PubMed] [Google Scholar]
- 2.Nairn RI. Nystatin and amphotericin B in the treatment of denture-related candidiasis. Oral Surg Oral Med Oral Pathol. 1975;40:68–75. doi: 10.1016/0030-4220(75)90348-5. [DOI] [PubMed] [Google Scholar]
- 3.Redding S, Bhatt B, Rawls HR, Siegel G, Scott K, Lopez-Ribot J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:669–72. doi: 10.1016/j.tripleo.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Amanlou M, Beitollahi JM, Abdollahzadeh SH, Tohidast-Ekrad Z. Miconazole Gel compared with Zataria multiflora Boiss. Gel in the treatment of denture stomatitis. Phytother Res. 2006;20:966–9. doi: 10.1002/ptr.1986. [DOI] [PubMed] [Google Scholar]
- 5.Ritchie GM, Fletcher AM, Main DM, Prophet AS. The etiology, exfoliative cytology, and treatment of denture stomatitis. J Prosthet Dent. 1969;22:185–200. doi: 10.1016/0022-3913(69)90246-7. [DOI] [PubMed] [Google Scholar]
- 6.Catalan A, Herrera R, Martinez A. Denture plaque and palatal mucosa in denture stomatitis: Scanning electron microscopic and microbiologic study. J Prosthet Dent. 1987;57:581–6. doi: 10.1016/0022-3913(87)90341-6. [DOI] [PubMed] [Google Scholar]
- 7.Cross LJ, Bagg J, Wray D, Aitchison T. A comparison of fluconazole and itraconazole in the management of denture stomatitis: A pilot study. J Dent. 1998;26:657–64. doi: 10.1016/s0300-5712(97)00047-x. [DOI] [PubMed] [Google Scholar]
- 8.Ellepola AN, Samaranayake LP. Adhesion of oral Candida albicans isolates to denture acrylic following limited exposure to antifungal agents. Arch Oral Biol. 1998;43:999–1007. doi: 10.1016/s0003-9969(98)00075-2. [DOI] [PubMed] [Google Scholar]
- 9.Salerno C, Pascale M, Contaldo M, Esposito V, Busciolano M, Milillo L, et al. Candida-associated denture stomatitis. Med Oral Patol Oral Cir Bucal. 2011;16:e139–43. doi: 10.4317/medoral.16.e139. [DOI] [PubMed] [Google Scholar]
- 10.Casaroto AR, Lara VS. Phytomedicines for Candida-associated denture stomatitis. Fitoterapia. 2010;81:323–8. doi: 10.1016/j.fitote.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Pinto TM, Neves AC, Leao MV, Jorge AO. Vinegar as an antimicrobial agent for control of Candida spp. in complete denture wearers. Appl Oral Sci. 2008;16:385–90. doi: 10.1590/S1678-77572008000600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosato A, Vitali C, Gallo D, Balenzano L, Mallamaci R. The inhibition of Candida species by selected essential oils and their synergism with amphotericin B. Phytomedicine. 2008;15:635–8. doi: 10.1016/j.phymed.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Lalli JYY, Van Zyl RL, Van Vuuren SF, Viljoen AM. In vitro biological activities of South African Pelargonium (Geraniaceae) species. S Afr J Bot. 2008;74:153–7. [Google Scholar]
- 14.Michaelis M, Doerr HW, Cinatl J., Jr Investigation of the influence of EPs(R) 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine. 2011;18:384–6. doi: 10.1016/j.phymed.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo SM, Kim J, Lee SG, Shin CH, Shin SC, Park IK. Fumigant antitermitic activity of plant essential oils and components from Ajowan (Trachyspermumammi), Allspice (Pimentadioica), caraway (Carumcarvi), dill (Anethumgraveolens), Geranium (Pelargonium graveolens), and Litsea (Litseacubeba) oils against Japanese termite (Reticulitermessperatus Kolbe) J Agric Food Chem. 2009;57:6596–602. doi: 10.1021/jf9015416. [DOI] [PubMed] [Google Scholar]
- 16.Shin S, Lim S. Antifungal effects of herbal essential oils alone and in combination with ketoconazole against Trichophyton spp. J Appl Microbiol. 2004;97:1289–96. doi: 10.1111/j.1365-2672.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- 17.Hajhashemi V, Rabbani M, Ghanadi A, Davari E. Evaluation of antianxiety and sedative effects of essential oil of Ducrosia anethifolia in mice. Clinics (Sao Paulo) 2010;65:1037–42. doi: 10.1590/S1807-59322010001000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pohlit AM, Lopes NP, Gama RA, Tadei WP, Neto VF. Patent literature on mosquito repellent inventions which contain plant essential oils–a review. Planta Med. 2011;77:598–617. doi: 10.1055/s-0030-1270723. [DOI] [PubMed] [Google Scholar]
- 19.Jeon JH, Lee CH, Lee HS. Food protective effect of geraniol and its congeners against stored food mites. J Food Prot. 2009;72:1468–71. doi: 10.4315/0362-028x-72.7.1468. [DOI] [PubMed] [Google Scholar]
- 20.Santos VR, Gomes RT, de Mesquita RA, de Moura MD, Franca EC, de Aguiar EG, et al. Efficacy of Brazilian propolis gel for the management of denture stomatitis: A pilot study. Phytother Res. 2008;22:1544–7. doi: 10.1002/ptr.2541. [DOI] [PubMed] [Google Scholar]


