Abstract
Background:
Some studies have shown that casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) and acidulated phosphate fluoride (APF) gel can protect teeth against erosion. The aim of this study was to assess whether CPP-ACP and fluoride could reduce enamel wear rates under erosive conditions simulating abrasion and acidic diet regimen.
Materials and Methods:
Enamel specimens consisted of 3 experimental groups (receiving CPP-ACP, APF or both) and a control group. Specimens were subjected to 5,000 wear cycles at a load of 30 N and a pH of 3 in a tooth wear machine. The amount of wear was determined by stereomicroscope. Data were analyzed using one-way analysis of variance and Tukey post hoc tests (α = 0.05).
Results:
Mean wear rate (mean±SD) was 194.6±49.2 micrometers in CPP-ACP group, 197.6±39.5 in APF group, 134.6±44.7 in combination group and 266.2± 22.7 in the control group. Statistical analysis indicated significantly higher wear rate in the control group than the experimental groups and also in the CPP-ACP and APF group than the combination group (P<0.05).
Conclusions:
We concluded that although either CPP-ACP or APF can protect enamel against wear, their combination provides significant enamel wear reduction. These findings would lead to new strategies for the clinical management of tooth wear.
Keywords: Casein phosphopeptide-amorphous calcium phosphate, enamel, fluoride, wear
INTRODUCTION
Tooth wear has a multi-factorial aetiology involving the interplay of attrition, erosion and abrasion. Dental erosion is defined as tooth wear due to chemical dissolution of dental hard tissues by acids or chelators in the absence of micro organisms.[1] Under certain circumstances and in some individuals tooth wear over time may occur excessively and thereby pathological.[2]
Epidemiological studies have shown an increase in the prevalence of tooth erosion.[3,4] Children and adolescents are the most involved age groups.[4–6] The erosion-causing acids can be distinguished by their origin into extrinsic or intrinsic factors. Acidic diet is the most prevalent extrinsic aetiological factor for erosion in these groups.[7] A combination of erosion with attrition or abrasion, referred to as erosive tooth wear, result in more destructive wear than if these processes occur independently.[8] It has been suggested that clinical control of tooth wear should be based on early diagnosis and prevention, rather than complex treatment modalities.[9] Previous studies have indicated that topical fluoride can protect enamel and dentine against erosion[10–13] and a combination of erosion and abrasion,[14–17] but not against attritional wear between opposing enamel and dentine.[18] Recently Tooth Mousse (Gc Corporation, Tokyo, Japan) has been suggested to control tooth erosion, with its anticariogenic remineralizing agent in the form of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) nanocomplex.[19] For example, it has been shown that CPP-ACP can reduce tooth erosion by citric acid,[20] white wine[21] and acidic sport drinks.[22] When applied topically in an in vitro study, Tooth Mousse containing 0.2% w/v CPP-ACP was reported to reduce enamel erosive lesions significantly.[23] This agent has also been reported to reduce enamel wear due to heavy attrition (at pH. 1.2).[24] Ranjitkar et al.[25] reported that attritional wear of dentine was almost eliminated in vitro with continuous application and reduced with intermittent application of CPP-ACP in both acidic and near neutral environments.
However, recent studies reported that CPP-ACP can reduce erosive tooth wear involving toothbrush abrasion.[17,26] A current study by Wegehaupt et al.[17] showed significant protective effect of daily application of fluoride gels, regardless of the fluoride compound against erosive/abrasive tooth wear, although the application of CPP-ACP-containing mousse was less effective. Notwithstanding, this effect was reproducible in other studies dealing with CPP-ACP containing products.[27,28]
The aim of this study was to determine the effect of CPP-ACP and acidulated phosphate fluoride (APF) gel on enamel wear resulted from combination of erosion (using citric acid) and abrasion (tooth cusp against ceramic disks) in vitro. It was hypothesized that application of CPP-ACP and APF gel would reduce enamel wear.
MATERIALS AND METHODS
Sample preparation
Thirty-six intact, non-carious, non-hypoplastic, not fractured and not malformed freshly human premolar teeth extracted for orthodontic reasons were selected. The protocol was approved ethically by Isfahan School of Dentistry Research board (Grant no: 387081). Teeth were wiped free of debris and rinsed with normal saline. Each tooth was explored for any cracks using a stereomicroscope (SF-100B, Lomo, Russia) under ×4 magnification. Teeth without cracks were stored in 0.2% thymol solution for 24 h. All specimens were kept in distilled water at room temperature during and after preparation for the test to prevent micro-crack formation. Each tooth was sectioned in a buccolingual direction parallel to its longitudinal axis through buccal cusp tip using a double-sided diamond disc with a thickness of 0.15 mm no. 943, Miniflex diamond disc [Brasseler, Lemgo, Germany] under water cooling. Sections were photographed under stereomicroscope using the camera provided by the manufacturer, and the angle between slopes of the cusps was determined using Motic Image Plus 2.0 software (Ted Pella, Redding, CA). Specimens with an angle of 90±1 degrees between the slopes were selected [Figure 1a]. The root of each half tooth specimen was embedded in the upper specimen holder using transparent auto polymerizing acrylic resin (Flash Acrylic, Yates Motloid, Chicago, IL, USA) [Figure 2].
Figure 1.
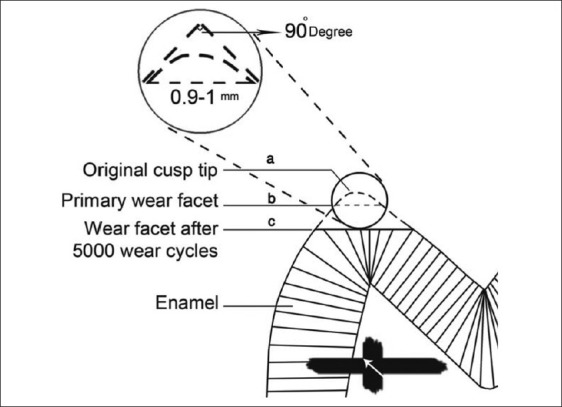
(a) Selection of Specimens with an angle of 90±1 degrees between the slopes (b) Primary wear facet length after 200 cycles (c) Wear facet after 5000 cycles
Figure 2.

Wear machine
Specimens were placed in the wear machine under a load of 200 g for 10 to 30 cycles depending on the geometry of the cusp[29] against ceramic samples described later to produce facets with a buccolingual diameter of 0.9 to 1.0 millimetre (mm) determined under the stereomicroscope [Figure 1b]. This procedure produced a uniform contact area for all specimens and removed the aprismatic layer, which is a variable structure at the surface of permanent teeth tending to make variations in enamel hardness between individuals.[29,30]
A horizontal line was traced on the sectioned surface of the tooth 4 mm from the cusp facet parallel to it using a silicon carbide ultra thin end cutter disk (Dedeco International, Long Eddy, NY, USA) mounted on a milling machine (F1, Degussa, Frankfurt, Germany) as a reference line. By employing a special jig, the tooth specimen could be placed repeatedly in the same position on the stereomicroscope stage. The tooth position was adjusted so that the traced line coincided with the x-axis of the microscope. The traced line was thus a reproducible reference line from which the farthest point of the tooth enamel could be measured. Tooth specimen was measured every 1,000 cycles until 5,000 cycles. The cusp reduction (enamel wear) was determined by subtraction of the distance of the worn cusp from the initial baseline [Figure 1c].
Thirty six flat disks of pressable ceramic material (IPS e.max Press, Ivoclar Vivadent, Liechtenstein) were fabricated using prefabricated wax patterns of 10 mm diameter and 2 mm thickness which were invested, removed by heat and press filled with the pressable material at a temperature of 925°C.[31] These one layer glazed ceramic discs, were mounted in clear acrylic resin to be placed against tooth specimens.
Wear machine properties
The wear machine used in this study consisted of two rotating discs, an adjustable stationary specimen holder and a 750-watt DC electric motor configured to operate at variable speeds. The specimen holder could hold two specimens to wear simultaneously [Figure 3]. The holder could be adjusted to hold the specimen against different radii of the rotating disc, and was able to support weights to control the load. The upper section of machine was fixed and the lower section was mobile. The electric motor powered a 12:1 reduction gearbox which, along with the motor spin control drive, could reduce the motor speed to 10 to 120 cycles per minute.
Figure 3.

Two specimens were simultaneously
Wear test procedure
Tooth and ceramic specimens were worn against each other in the mentioned electromechanical wear machine in a circular path with a diameter of 3 mm at a rate of 80 cycles per min under a constant load of 3 kg for 5000 cycles. Citric acid with a pH of 3.0 (titratable acidity=0.001 M) was used as lubricant to simulate acidic diet. Tooth specimens were mounted in the upper stationery holder which had a slot to ensure that the insertion of the tooth specimen was made in the same axis of the specimen holder, perpendicular to the ceramic discs on the lower rotating holder of the machine. The tooth cusp tip was brought into contact with the ceramic specimen before the machine was activated. At the beginning of every 1,000 cycles, 3 ml of citric acid was placed over the ceramic discs so that the cusp was immersed in acid while being worn. Each ceramic disc was discarded after 5,000 cycles.
The specimens were tested in 4 experimental groups as follows. Nine specimens were allocated randomly to each group. In group 1 fresh, tooth mousse (TM) paste was placed in contact with the specimens using a micro brush as a 1 mm thick layer for 4 min. The specimens were then rinsed gently with water for 2 min, dried with air for 15 seconds and subjected to wear for 1,000 cycles. The machine was stopped every 1,000 cycles and the specimens were rinsed for 30 seconds and dried for 15 seconds, and the above protocol was repeated every 1,000 cycles up to 5,000 cycles. A similar protocol was carried out in group 2 replacing tooth mousse with 1.23% APF gel (Sultan, Topex, NJ, USA) having a pH of 3.5. In group 3, enamel was first treated with 1.23% APF gel for 4 min, rinsed, dried and then treated with tooth mousse paste for 4 min followed by similar protocol in groups 1 and 2. In the control group, the specimens were subjected to same wear protocol without any prior treatment.
Enamel wear quantification
A stereomicroscope was used to determine the amount of wear of the tooth specimens by measuring the reduction in the tooth cusp height at the end of 5,000 cycles. The cusp reduction (enamel wear) was determined by measuring the decrease in the distance of the worn cusp from the reference line using stereomicroscope under ×16 magnification (±0.1 micrometers). All measurements were performed by the same investigator using Motic Image plus 2.0 software. The software was calibrated using the photograph of a standard grid provided by the manufacturer before performing measurement on every specimen.
Statistical analysis
Normality of the data distribution was tested by Kolmogrov-Smirnov test. Data were analyzed using one-way analysis of variance (ANOVA) and Tukey post-hoc tests. Data analyses were carried out by IBM SPSS version 11.5 software (SPSS, Inc, Chicago, IL) and P<0.05 was considered as the significance threshold.
RESULTS
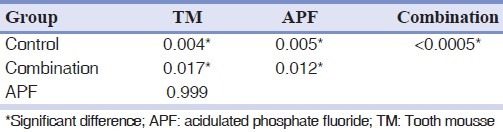
The amount of enamel wear represented as reduction of the distance from cusp tip facet to the reference line after 5,000 cycles is summarized in Table 1. Kolmogrov-Smirnov test indicated normality of the data (P>0.05). One-way ANOVA test showed a significant difference between enamel wear in 4 groups (P<0.05). Pair-wise comparisons by Tukey post-hoc test showed significantly higher wear of enamel in control group than 3 experimental groups (P<0.05), and the lowest amount of enamel wear in group 3 (TM and APF) (P<0.05), but there was no significant difference found between groups 1 and 2 (P>0.05) [Table 2].
Table 1.
Cusp height reduction (mean±SD) (μm) following wear procedure
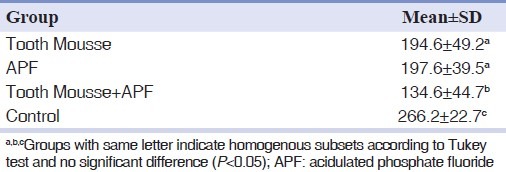
Table 2.
P values for multiple comparisons between experimental and control groups (Tukey test)
DISCUSSION
This, in vitro study was designed to investigate wear of enamel against ceramic disks under acidic conditions. The present method, was designed to mimic the chewing cycle (30 N, 80 cycles per min) and acidic diet (citric acid media, pH=3.0). However, exact simulation by a machine may never be possible.[32] Hence, this point should always be kept in mind when interpreting the results of any similar in vitro study. Citric acid was used as it is the most common type of acid in soft drinks and fruits. The time of exposure to the lubricant was limited to 1 h, so that the direct effects of acid were minimized and were not enough to bias the measurement of tooth loss under dynamic load.[33]
In the current study, a load of 30 N was used according to limitations of the wear machine, which falls in the range of masticatory forces.[34]
Eighty cycles per min has been reported to be a reasonable estimation for the chewing cycle rate.[18]
Ceramic discs were used in the current study against tooth cusps instead of flat enamel specimens, because enamel composition and crystalline structure is different among teeth or different areas within an individual tooth.[35] Application of a uniform material against all tooth specimens may give more reliable results.
Limited evidences are available regarding the effect of remineralizing agents on prevention of abrasion combined with erosion. Several studies have shown that, CPP-ACP can reduces the erosive wear of enamel[19–23] and dentine.[21] But, there are few studies assessing the effect of CPP-ACP on erosive tooth wear combined with attrition[24,25] and abrasion.[17,26]
In the present study, CPP-ACP was observed to reduce abrasive enamel wear under erosive condition. Which is in accordance with the results of Ranjitkar et al.[26] who reported that GC tooth mousse reduced erosive enamel and dentine wear involving toothbrush abrasion. But, it is in contrast to the findings of Wegehaupt et al.,[17] who showed no protective effect of CPP-ACP-containing mousse on erosive/abrasive tooth wear.
In another study, Ranjitkar et al.,[24] have reported that CPP-ACP can reduce enamel wear under heavy attritional forces (10,000 cycles and 100 N) and severe erosive conditions (pH 1.2).
The mechanism by which CPP-ACP reduces tooth wear is not fully understood,[22,25] however Ranjitkar et al.,[25] stated that this may be due to both lubricating and remineralizing effects of CPP-ACP. Previous studies have shown that the anticariogenic effect of CPP-ACP may be responsible for the prevention of enamel erosion.[22,36] Moreover, it is well documented that CPP-ACP can increase microhardness of enamel and reduce erosion caused by cola drinks.[36] This evidences, mostly, point toward the ability of CPP-ACP in remineralizing eroded lesions. The process of remineralisation of eroded lesions is unclear, but it is likely to involve a repair process by deposition of minerals into the porous zone rather than crystal regrowth.[37]
Ranjitkar et al.,[25] suggested that lubricating potential of TM appears to be more pronounced than its remineralizing effect since in their experience, dentine wear was almost eliminated with continuous application of TM, and the wear rate was lower at pH. 6.1 than at pH. 3.0 with intermittent application of TM. It is hypothesized that CPP-ACP nanocomplexes and other ingredients of the TM formulation (e.g., glycerol) provide lubrication at the wear interface, thus producing very smooth, polished wear facets.
This study has shown that (12,300 ppm and pH. 3.5), can reduce abrasive enamel wear under erosive condition. This is in accordance with the results of other studies that had shown that highly fluoridated acidic gel up to 12,500 ppm is able to protect enamel from erosion and tooth brushing abrasion.[14,15,17,38,39] This may be due to high concentration of fluoride along with acidic pH of the gel that results in thicker calcium fluoride-like layer and hence more effective protection of enamel.[40] Yet Li et al.,[18] found that pretreatment of the samples with high concentration fluoride varnish (22,600 ppm, PH=3.9) increased dentin attritional wear.
In our experience, enamel wear was found to be significantly lower in the group of CPP-ACP combined with APF gel than in groups of either CPP-ACP or APF alone. This is in sharp contrast with the Wang et al.[41] results. They found that tooth erosion is not preventable or mendable by novel agents, such as Pure Gc Tooth Mousse or combined with 900 ppm fluoride (MI paste plus). Our results were in accordance with the findings of Srinivasan et al.,[42] (CPP-ACP with 900 ppm fluoride), Lussi[43] (CPP-ACP with 900 ppm fluoride) and Kumar et al.,[44] (250-500 ppm fluoride dentifrice and CPP-ACP). These results suggest that, if CPP-ACP is used in combination with fluoride, the benefits of both will be promoted. This synergistic effect of CPP-ACP and fluoride may be due to the ability of CPP-ACP to interact with fluoride ions to form a stabilized amorphous calcium fluoride phosphate phase.[45]
On the other hand, a recent study by Lata et al.,[46] has shown different results. In their study, the combination of fluoride and CPP-ACP did not provide any additive remineralisation potential compared to fluoride alone, because most of the GC Tooth Mousse cream was lost after washing in distilled water as suggested by the author of that article. Another finding of our study is the almost equivalent effect of CPP-ACP and fluoride on reduction of enamel wear. This is in contrast to Wegehaupt et al.,[17] study in which the erosive/abrasive tooth wear was reduced significantly by fluoride gels (both neutral and acidic pH), while the CPP-ACP-containing mousse was less effective. However, it might be postulated that the application frequency and duration of CPP-ACP-containing mousse must be lesser in their study than the present study.
Further studies are needed to elucidate the protective effect of CPP-ACP and fluoride against enamel wear due to heavy chewing or bruxing forces as well as dynamic forces, since the current study used a relatively low static force to produce wear. In situ studies and clinical trials may further enlighten the efficacy of combination of CPP-ACP and fluoride against enamel wear and determine the true potential of CPP-ACP in prevention of erosive tooth wear. It might be suggested to apply some precise methods like measuring the reduction in volume of enamel specimens instead of height reduction and using a 3D profilometer to achieve reliable results.
CONCLUSION
We conclude that, CPP-ACP and fluoride are both able to reduce the enamel wear caused by the combination of abrasion and erosion. Moreover, their concurrent use is more effective than using either of them alone.
ACKNOWLEDGMENT
This paper was based on a thesis submitted to the School of Dentistry, Isfahan University of Medical Sciences, in partial fulfilment of the requirement for MSc degree. This study was supported by Isfahan University of Medical Sciences (Grant No. 387081).
Footnotes
Source of Support: This report is based on a thesis which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran, in partial fulfillment of the requirements for the MSc degree in Pediatric dentistry (#387081). The study was approved by the Medical Ethics and Research Office at the Isfahan University of Medical Sciences and financially supported by this University.
Conflict of Interest: None declared.
REFERENCES
- 1.Zipkin I, Mc Clure FJ. Salivary citrate and dental erosion: Procedure for determining citric acid in saliva, dental erosion and citric acid in saliva. J Dent Res. 1949;28:613–26. doi: 10.1177/00220345490280061301. [DOI] [PubMed] [Google Scholar]
- 2.Kelleher M, Bishop K. The aetiology and clinical appearance of tooth wear. Eur J Prosthodont Restor Dent. 1997;5:157–60. [PubMed] [Google Scholar]
- 3.Nunn JH. Prevalence of dental erosion and the implications for oral health. Eur J Oral Sci. 1996;104:156–61. doi: 10.1111/j.1600-0722.1996.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 4.Lussi A, Schaffner M, Hotz P, Suter P. Dental erosion in a population of Swiss adults. Community Dent Oral Epidemiol. 1991;19:286–90. doi: 10.1111/j.1600-0528.1991.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 5.Millward A, Shaw L, Smith A. Dental erosion in four-year-old children from differing socio-economic backgrounds. J Dent Child. 1994;61:263–6. [PubMed] [Google Scholar]
- 6.Bartlett DW, Coward PY, Nikkah C, Wilson RF. The prevalence of tooth wear in a cluster sample of adolescent school children and its relationship with potential explanatory factors. Br Dent J. 1998;184:125–9. doi: 10.1038/sj.bdj.4809560. [DOI] [PubMed] [Google Scholar]
- 7.Zero DT, Lussi A. Erosion-chemical and biological factors of importance to the dental practitioner. Int Dent J. 2005;55:285–90. doi: 10.1111/j.1875-595x.2005.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 8.Meurman JH, Sorvari R. Interply of erosion and abrasion in tooth wear and possible approaches to prevention. In: Addy M, Embery G, Edgar W, Orchardson R, editors. Tooth wear and sensitivity: Clinical advances in restorative dentistry. 1st ed. London: Martin Dunitz Ltd; 2000. pp. 171–80. [Google Scholar]
- 9.Lussi A. Erosive tooth wear - a multifactorial condition of growing concern and increasing knowledge. Monogr Oral Sci. 2006;20:1–8. doi: 10.1159/000093343. [DOI] [PubMed] [Google Scholar]
- 10.Ganss C, Klimek J, Brune V, Schurmann A. Effects of two fluoridationmeasures on erosion progression in human enamel anddentine in situ. Caries Res. 2004;38:561–6. doi: 10.1159/000080587. [DOI] [PubMed] [Google Scholar]
- 11.Hove L, Holme B, Øgaard B, Willumsen T, Tveit AB. The protective effect of TiF4, SnF2 and NaF on erosion of enamel by hydrochloric acid in vitro measured by white light interferometry. Caries Res. 2006;40:440–3. doi: 10.1159/000094291. [DOI] [PubMed] [Google Scholar]
- 12.Mok TB, McIntyre J, Hunt D. Dental erosion: In vitro model of wine assessor's erosion. Aust Dent J. 2001;46:263–8. doi: 10.1111/j.1834-7819.2001.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones L, Lekkas D, Hunt D, McIntyre J, Rafir W. Studies on dental erosion: An in vivo-in vitro model of endogenous dental erosion–its application to testing protection by fluoride gel application. Aust Dent J. 2002;47:304–8. doi: 10.1111/j.1834-7819.2002.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 14.Attin T, Zirkel C, Hellwig E. Brushing abrasion of eroded dentin after application of sodium fluoride solutions. Caries Res. 1998;32:344–50. doi: 10.1159/000016470. [DOI] [PubMed] [Google Scholar]
- 15.Attin T, Deifuss H, Hellwig E. Influence of acidified fluoride gelon abrasion resistance of eroded enamel. Caries Res. 1999;33:135–9. doi: 10.1159/000016507. [DOI] [PubMed] [Google Scholar]
- 16.Attin T, Siegel S, Buchalla W, Lennon AM, Hannig C, Becker K. Brushing abrasion of softened and remineralised dentin: An in situ study. Caries Res. 2004;38:62–6. doi: 10.1159/000073922. [DOI] [PubMed] [Google Scholar]
- 17.Wegehaupt FJ, Attin T. The role of Fluoride and Casein phosphopeptide/Amorphous Calcium Phosphate in the prevention of Erosive/Abrasive Wear in an in vitro model using Hydrochloric acid. Caries Res. 2010;44:358–63. doi: 10.1159/000316542. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Watson TF, Sherriff M, Curtis R, Bartlett DW. The influence of fluoride varnish on the attrition of dentine. Caries Res. 2007;41:219–22. doi: 10.1159/000099322. [DOI] [PubMed] [Google Scholar]
- 19.Smales RJ, Yip K HK. Prevention and control of tooth erosion. In: Yip K HK, Smales RJ, Kaidonis JA, editors. Tooth erosion: Prevention and treatment. 1st ed. New Delhi: Jaypee Brothers, Medical Publishers (P) Ltd; 2006. pp. 33–46. [Google Scholar]
- 20.Rees J, Loyn T, Chadwick B. Pronamel and Tooth Mousse: An initial assessment of erosion prevention in vitro. J Dent. 2007;35:355–7. doi: 10.1016/j.jdent.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Piekarz C, Ranjitkar S, Hunt D, McIntyre J. An in vitro assessment of the role of Tooth Mousse in preventing wine erosion. Aust Dent J. 2008;53:22–5. doi: 10.1111/j.1834-7819.2007.00003.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramalingam L, Messer LB, Reynolds EC. Adding caseinphosphopeptide-amorphous calcium phosphate to sports drinks eliminate in vitro erosion. Pediatric Dent. 2005;27:61–7. [PubMed] [Google Scholar]
- 23.Manton DJ, Cai F, Yuan Y, Walker GD, Cochrane NJ, Reynolds C, et al. Effect of casein phosphopeptide-amorphous calcium phosphate added to acidic beverages on enamel erosion in vitro. Aust Dent J. 2010;55:275–79. doi: 10.1111/j.1834-7819.2010.01234.x. [DOI] [PubMed] [Google Scholar]
- 24.Ranjitkar S, Kaidonis J, Richards L, Townsend G. The effect of CPP-ACP on enamel wear under severe erosive conditions. Arch Oral Biol. 2009;54:527–32. doi: 10.1016/j.archoralbio.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Ranjitkar S, Narayana T, Kaidonis JA, Hughes TE, Richards LC, Townsend GC. The effect of casein phosphopeptide-amorphous calcium phosphate on erosive dentine wear. Aust Dent J. 2009;54:101–7. doi: 10.1111/j.1834-7819.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- 26.Ranjitkar S, Rodriguez JM, Kadionis JA. The effect of casein phosphopeptide amorphous calcium phosphate on erosive enamel and dentine wear by toothbrush abrasion. J Dent. 2009;37:250–4. doi: 10.1016/j.jdent.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Lennon AM, Pfeffer M, Buchalla W, Becker K, Lennon S, Attin T. Effect of a casein/calcium phosphate containing tooth cream and fluoride on enamel erosion in vitro. Caries Res. 2006;40:154–8. doi: 10.1159/000091063. [DOI] [PubMed] [Google Scholar]
- 28.Willershausen B, Schulz-Dobrick B, Gleissner C. In vitro evaluation of enamel remineralisation by a casein phosphopeptide-amorphous calcium phosphate paste. Oral Health Prev Dent. 2009;7:13–21. [PubMed] [Google Scholar]
- 29.Eisenburger M, Addy M. Erosion and attrition of human enamel in vitro part I: Interaction effects. J Dent. 2002;30:341–7. doi: 10.1016/s0300-5712(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 30.Macpherson LM, Damato FA, MacFarlane TW, Strang R, Stephen KW. Variation in the susceptibility of enamel to an in-vitro demineralization system. Caries Res. 1991;25:143–5. doi: 10.1159/000261357. [DOI] [PubMed] [Google Scholar]
- 31.Heintze S, Cavalleri A, Forjanic M, Zellweger G, Rousson V. Wear of ceramic and antagonist–A systematic evaluation of influencing factors in vitro. Dent Mater J. 2008;24:433–49. doi: 10.1016/j.dental.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Douglas WH, Sakaguchi R, DeLong R. Frictional effects between natural teeth in an artificial mouth. Dent Mater. 1985;1:115–9. doi: 10.1016/S0109-5641(85)80040-3. [DOI] [PubMed] [Google Scholar]
- 33.Kaidonis JA, Richards LC, Townsend GC, Tansley GD. Wear of human enamel: A quantitative in vitro assessment. J Dent Res. 1998;77:1983–90. doi: 10.1177/00220345980770120601. [DOI] [PubMed] [Google Scholar]
- 34.Eisenburger M, Addy M. Erosion and attrition of human enamel in vitro part II: Influence of time and loading. J Dent. 2002;30:349–52. doi: 10.1016/s0300-5712(02)00049-0. [DOI] [PubMed] [Google Scholar]
- 35.Kohyama K, Hatakeyama E, Sasaki T, Dan H, Azuma T, Karita K. Effects of sample hardness on human chewing force: A model study using silicone rubber. Arch Oral Biol. 2004;49:805–16. doi: 10.1016/j.archoralbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Tantbirojn D, Huang A, Ericson MD, Poolthong S. Change in surface hardness of enamel by cola drink and a CPP-ACP paste. J Dent. 2008;36:74–9. doi: 10.1016/j.jdent.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Eisenburger M, Addy M, Hughes JA, Shellis RP. Effect of time on the remineralization of enamel by synthetic saliva after citric acid erosion. Caries Res. 2001;35:211–5. doi: 10.1159/000047458. [DOI] [PubMed] [Google Scholar]
- 38.Magalhaes AC, Rios D. Influence of fluoride dentifrice on brushing abrasion of eroded human enamel. Caries Res. 2007;41:77–9. doi: 10.1159/000096110. [DOI] [PubMed] [Google Scholar]
- 39.Lagerweij MD, Buchalla W, Kohnke S, Becker K, Lennon AM, Attin T. Prevention of erosion and abrasion by a high fluoride concentration gel applied at high frequencies. Caries Res. 2006;40:148–53. doi: 10.1159/000091062. [DOI] [PubMed] [Google Scholar]
- 40.Donly KT. Topical fluoride therapy. In: Garcia-Godoy F, Harris N, editors. Primary Preventive Dentistry. 6th ed. New Jersey: Prentice Hall; 2000. pp. 262–4. [Google Scholar]
- 41.Wang X, Megert B, Hellwig E, Neuhaus KW, Lussi A. Preventing erosion with novel agents. J Dent. 2011;39:163–70. doi: 10.1016/j.jdent.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan N, Kavitha M, Loganathan SC. Comparison of the remineralization potential of CPP-ACP and CPP-ACP with 900 ppm fluoride on eroded human enamel. Arch Oral Biol. 2010;55:541–4. doi: 10.1016/j.archoralbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Lussi A. Dental erosion-novel remineralising agents in prevention or repair. Adv Dent Res. 2009;21:13–6. doi: 10.1177/0895937409335592. [DOI] [PubMed] [Google Scholar]
- 44.Kumar VL, Itthagarun A, King NM. The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: An in vitro study. Aust Dent J. 2008;53:34–40. doi: 10.1111/j.1834-7819.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, et al. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2008;87:344–8. doi: 10.1177/154405910808700420. [DOI] [PubMed] [Google Scholar]
- 46.Lata S, Varghese NO, Varughese JM. Remineralization potential of fluoride and amorphous calcium phosphate-casein phosphopeptide on enamel lesions. J Conserv Dent. 2010;13:42–6. doi: 10.4103/0972-0707.62634. [DOI] [PMC free article] [PubMed] [Google Scholar]


