Abstract
Background:
The differences between marginal gingiva and interdental papilla may be due to variation in the molecular composition of these two different anatomical structures. The aim of this study was to evaluate the staining intensity of fibronectin in human marginal gingiva and interdental papilla.
Materials and Methods:
In a prospective analytical study, 16 healthy subjects needing crown lengthening surgery were selected. All participants were medically healthy, non-smokers, with no medication intake, and a healthy periodontium. During surgery, facial/buccal marginal gingiva and interdental papilla were separately harvested. The specimens were subjected to hematoxylin and eosin, histochemical (Masson’ strichorom, reticulin, and elastic), and immunohistochemical staining for evaluation of morphology and inflammation; assessment of connective tissue fibers (collagen, reticulin, and elastic); and determination of fibronectin staining intensity. The data were analyzed by Spsssoftware, Wilcoxon, and Spearman tests. P<0.05 was considered to be statistically significant.
Results:
From a total of 32 specimens, 21 specimens were found to be normal or having mild inflammation, while the remaining specimens had moderate to severe inflammation in some parts. Collagen fibers were found to be dense in reticular connective tissue and degenerated in the region of inflammation. Reticulin fibers strongly stained near epithelium. Elastic fibers were sparsely found. Mean fibronectin staining intensity between marginal gingiva and interdental papilla was not statically significant (P=0.44). There is no statistically significant correlation between tissue inflammation and fibronectin staining intensity (P=0.76 for marginal gingival and P=0.20 for interdental papilla). Considering all specimens, fibronectin staining intensity of connective tissue adjacent to Sulcular/Junctional epithelium was higher than reticular connective tissue (P=0.003) and higher than connective tissue adjacent to oral epithelium (P<0.001).
Conclusion:
This study did not show any difference in interdental papilla and marginal gingival with respect to fibronectin composition. More studies in this context are needed.
Keywords: Fibronectin, gingiva, histochemistry, immunohistochemistry
INTRODUCTION
Loss of interdental papilla can lead to esthetic concerns, phonetic problems, and food impaction which promote periodontal disease.[1,2] When the interdental papilla is lost as a result of periodontal disease, surgery, or trauma, its regenerative capacity is also limited compared to marginal or attached gingiva.[3,4] Normal marginal gingiva forms around dental implants placed in edentulous areas, whereas the regeneration of interdental papilla remains limited.[5,6] Hereditary and drug induced gingival overgrowth seems to manifest first at the interdental papilla and then spread to other parts of the gingiva.[7]
In spite of other factors that seem to limit the regenerative capacity of the interdental papilla, it is also possible that the interdental papilla has unique functional characteristics that are due to distinct cellular or molecular properties. Walsh et al. have shown the presence of α5β1 integrin in interdental papilla of drug-induced gingival enlargement patients, which was neither found in oral epithelium of normal gingiva nor in periodontitis. They suggested that interdental region may have unique phenotype.[8]
Fibronectin is a multifunctional glycoprotein found in plasma and extracellular matrix of tissues. This glycoprotein plays a number of important roles including providing structural support and signaling cues for cell survival, migration, differentiation, gene expression, growth factor signaling, and cell contractility.[9] Fibronectin interacts with cellular receptors known as integrins through specific sites of the protein. The classic fibronectin receptor is α5β1 integrin.[10] In addition to its cell-binding properties, fibronectin also binds other glycoproteins, including components of complement and coagulation systems.[11,12]
In early wound, fibroblast migration seems to be more primarily mediated by fibronectin.[13] Fibronectin–fibrin meshwork undergoes covalent cross-linking to stabilize clot and allow reparative cell migration.[11,14]
Baum and Wright demonstrated the presence of fibronectin in human gingival fibroblasts using indirect immunoflorecence. Fibronectin derived from these cells possessed biological properties of agglutination and promotion of cell adhesion to a collagen substrate. They considered a role for fibronectin in organization of gingival tissues.[15]
Camargo et al. reported fibronectin to have a stabilizing or proliferative effect on gingival soft tissue by promoting less postoperative gingival recession.[16]
Csiszar et al. in 2007 found procollogen type I, fibronectin, and tenacin C to be higher in connective tissue of interdental papilla compared to that in marginal gingiva. They concluded that molecular composition of the interdental papilla is distinct from marginal gingiva.[17]
To assess molecular variations in different anatomical parts of gingiva, we conducted an investigation to evaluate the staining intensity of fibronectin in human marginal gingiva and interdental papilla by immunohistochemical staining.
MATERIALS AND METHODS
A prospective analytical experimental study was performed on 16 healthy subjects referred for crown lengthening surgery. All participants were medically healthy, non-smokers, with no medication intake, and a healthy periodontium. There were no clinical signs of inflammation at the sites of surgery.
A professional scaling and root planning (SRP) was performed and oral hygiene instructions were given. The procedure was explained and a written consent was obtained from patients. After 3 weeks, crown lengthening surgery was performed and facial/buccal marginal gingiva and interdental papilla were harvested separately. The specimens were immersed in normal saline and after several minutes in 10% formalin. At least one section of 5-μm thickness and four sections of 4-μm thickness were made from each specimen.
Histological evaluation
The 5-μm section was stained with hematoxylin and eosin to assess the morphology of tissues and the presence of inflammation.
According to the signs of inflammation,[1] the specimens were categorized in four groups: Normal (without any sign of inflammation), mild (initial), moderate (early), and severe (established).
Histochemical staining
In histochemical staining, the presence of collagen fibers with fibroblasts was considered as normal tissue, degeneration of collagen fibers as acute inflammation, and the presence of collagen fibers with fibrocytes was considered as fibrosis. Staining intensity was scored as 0=without staining intensity; 1=weak; 2=moderate; 3=sever staining intensity.
Reticulin and elastic staining was performed (Merk, Darmstadt, Germany) to evaluate the status of reticulin and elastic fibers, and determine their relation to tissue inflammation.
Immunohistochemical staining
Following deparaffinization, the sections were heated at 60°C for 30 min incubated for 5 min in clean xylene and thereafter incubated for 5 min in ethanol 70%, 96%, and 100%, respectively. Primary anti-fibronectin (Novocastra™, Leica Microsystems, United Kingdom) and Proteinaz K (P.K) enzyme were used for immunostaining and antigen preparation, respectively. The slides were then washed with distilled water.
Relative staining intensity of the immunohistochemical specimens was scored as follows: 0=no immunoreactivate staining intensity; 1=weak, but visible; 2=moderately; and 3=strong staining intensity. The histological procedures were supervised by two pathologists.
Statistical analysis
Statistical tests were done using Spss0 (Version 13, SPSS INC) software. The Wilcoxon and Spearman tests were used for comparison of staining intensity and to evaluate the correlation between inflammation and fibronectin staining intensity respectively. P<0.05 was considered to be statistically significant.
RESULTS
Histological finding
With respect to inflammation, of the total 32 interdental papilla and marginal gingiva tissues tested, 6 specimens were found to be normal, 15 specimens with mild inflammation, and the remaining 11 specimens had moderate to severe inflammation in some parts.
Lymphocytes were found to be the predominant cell infiltrate in most of the specimens while a 50% Polymorphonuclear and 50% lymphocyte infiltration was observed in two specimens.
Histochemical finding
In Masson's Trichrome staining, collagen fibers appeared blue. Tissue epithelium was not stained (score 0), collagen fibers showed weak staining in the region of inflammation (score 1), collagen fibers of papillary connective tissue were moderately stained (score 2), and collagen fibers of reticular connective tissue were intensely stained (score 3).[18]
In reticulin staining, reticulin fibers appeared black, dense and strongly stained near both oral and sulcular/junctionalepithelium (S/JE), particularly in the basement membrane. In elastic staining, elastic fibers were black and mostly found in inflamed tissues.
Immunohistochemical findings
The immunohistochemistry test detected fibronectin in the connective tissue. Due to different staining intensities in different parts of tissue, each section was divided into three areas [Tables 1 and 2].
Table 1.
Frequency distribution of fibronectin staining intensity in different areas of marginal gingiva
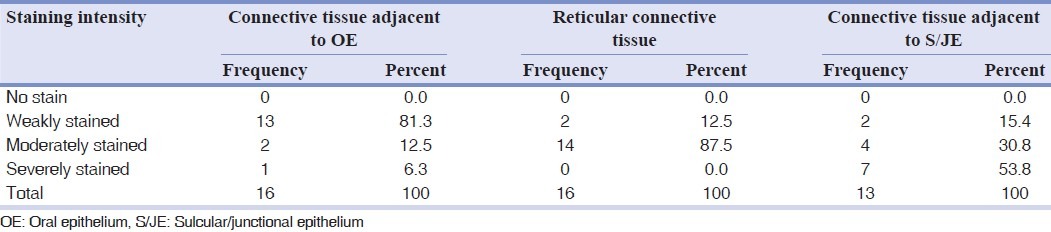
Table 2.
Frequency distribution of fibronectin staining intensity in different areas of interdental papilla
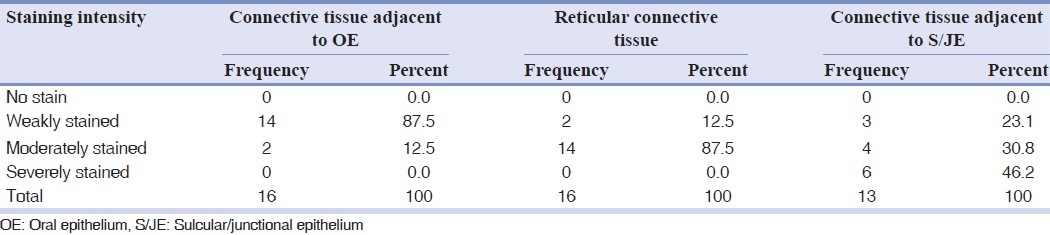
The Wilcoxon test showed fibronectin staining intensities not to be significantly different between marginal gingiva and interdental papilla in connective tissue adjacent to oral epithelium (OE), reticular connective tissue, and connective tissue adjacent to S/ JE. Mean staining intensity between marginal gingiva and interdental papilla was not statically significant.
Inflammation in interdental papilla and marginal gingiva was not significantly different but slight differences were observed at borderline.
The Spearman test revealed no statistically significant correlation between tissue inflammation and fibronectin staining intensity.
Significant correlation was found between inflammation and interdental papilla connective tissue adjacent to OE. Considering other findings, it must be because of type 1 error (in this study type 1 error rate or α was set by statistical consultant as 0.05). Fibronectin showed more intensity of staining in the connective tissue adjacent to S/JE compared to reticular connective tissue, and likewise more intense in reticular connective tissue than the connective tissue adjacent to OE. These differences were statistically significant.
DISCUSSION
Knowledge of the molecular composition of interdental papilla as a unique anatomical location may help in developing a procedure to regenerate this part of the gingiva.
This study was undertaken to determine the relationship between fibronectin staining intensity and tissue inflammation on a histological level. But results of our study showed there is no correlation between inflammation and fibronectin staining intensity.
The interdental papilla exhibited a higher level of inflammation than the marginal gingiva. This difference although not statistically significant was at borderline (P=0.058) and may prove to be significant with a larger sample size. Low daily use of interdental cleaning tools and difficulty in establishment of flossing habit for patients probably accounts for the higher level of inflammation found in the interdental papilla compared to that in the marginal gingiva.
Masson's trichrome staining showed dense collagen fibers in laminapropria of specimens which degenerated in inflamed regions. Fibrosis was observed only in one of the tissues with sever inflammation. The destruction of collagen from collagenase and other enzymes released by infiltrating and transmigrating neutrophils was shown in other studies.[1,19]
Reticulin histochemical staining showed higher staining intensity subjacent to both oral and S/J epithelium, especially in the basement membrane than in reticular connective tissue. This finding was similar in all specimens either normal or inflamed tissues and was in accordance with Lorencini et al.[20]
In our study, only small amount of elastic fibers were found mainly in the inflamed tissues which may be a sign of early fibrosis. Similar findings were reported by Bourke et al.[21]
Unlike the findings of Csiszar et al., our study did not demonstrate any difference in fibronectin staining intensity between interdental papilla and marginal gingiva.[17] They found fibronectin levels to be significantly higher in the connective tissue of interdental papilla. The staining of fibronectin was evaluated in three zones of connective tissue (basement membrane zone, subepithelial connective tissue, and deep connective tissue) but no comparison was made between these areas.
The present study revealed statistically significant fibronectin staining intensity in the connective tissue adjacent to S/J epithelium [Tables 1 and 2] which may be due to the unique characteristics of junctional epithelium. The latter has a high rate of cell turnover, 50-100 times higher than oral epithelium.[22] Cell proliferation of junctional epithelium is localized to the basal layer adjacent to the gingival connective tissue.[23]
Since fibronectin is responsible for cell differentiation and growth factor signaling,[9] its high concentration near a region with high turnover rate may be explainable.
The high concentration of fibronectin in junctional epithelium is due to the presence of fibronectin receptors (integrins) in this tissue.
CONCLUSION
Fibronectin composition was found to be similar both in interdental papilla and marginal gingiva. Studies in this context were limited and further investigations are needed to clarify whether interdental papilla possess unique composition.
Footnotes
Source of Support: This report is based on a thesis which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran, in partial fulfillment of the requirements for the MSc degree in periodontics (#38939). The study was approved by the Medical Ethics and Research Office at the Isfahan University of Medical Sciences and financially supported by this University.
Conflict of Interest: None declared.
REFERENCES
- 1.Newman MG, Takei HH, Klokkevold PR. Carranza's clinical periodontology. 10th ed. California: Saunders; 2006. p. 17. [Google Scholar]
- 2.Tarnow DP, Magner AW, Fletcher P. The effect of the distance from the contact point to the crest of bone on the presence or absence of the interproximal dental papilla. J Periodontol. 1992;63:995–6. doi: 10.1902/jop.1992.63.12.995. [DOI] [PubMed] [Google Scholar]
- 3.Cardaropoli D, Re S, Corrente G, Abundo R. Reconstruction of the maxillary midline papilla following a combined orthodontic-periodontic treatment in adult periodontal patients. J Clin Periodontol. 2004;31:79–84. doi: 10.1111/j.0303-6979.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 4.Cortellini P, Tonetti MS. Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. J Clin Periodontol. 2009;36:157–63. doi: 10.1111/j.1600-051X.2008.01352.x. [DOI] [PubMed] [Google Scholar]
- 5.Schropp L, Isidor F, Kostopoulos L, Wenzel A. Interproximal papilla levels following early versus delayed placement of single-tooth implants: A controlled clinical trial. Int J Oral Maxillofac Implants. 2005;20:753–61. [PubMed] [Google Scholar]
- 6.Meijndert L, Raghoebar GM, Meijer HJA, Vissink A. Clinical and radiographic characteristics of single tooth replacements preceded by local ridge augmentation: A prospective randomized-clinical trial. Clin Oral Impl Res. 2008;19:1295–303. doi: 10.1111/j.1600-0501.2008.01523.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindhe J, Lang NP, Karring T. Clinical periodontology and implant dentistry. 5th ed. Oxford, Ames, lowa: Blackwell Munksgaard; 2008. [Google Scholar]
- 8.Walsh P, Häkkinen L, Pernu H, Knuuttile M, Larjava H. Expression of fibronectin-binding integrins in gingival epithelium in drug-induced gingival overgrowth. J Periodontal Res. 2007;42:144–51. doi: 10.1111/j.1600-0765.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 9.Faralli JA, Schwinn MK, Gonzalez JM, Jr, Filla MS, Peters DM. Functional properties of fibronectin in the trabecular meshwork. Exp Eye Res. 2009;88:689–93. doi: 10.1016/j.exer.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cáceres M, Hidalgo R, Sanz A, Martínez R, Riera P, Smith PC. Effect of platelet rich plasma on cell adhesion, cell migration, and myofibroblastic differentiation in human gingival fibroblasts. J Periodontol. 2008;79:714–20. doi: 10.1902/jop.2008.070395. [DOI] [PubMed] [Google Scholar]
- 11.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–63. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 13.Häkkinen L, Uitto V, Larjava H. Cell biology of gingival wound healing. Periodontol 2000. 2000;24:127–52. [PubMed] [Google Scholar]
- 14.Baum BJ, Wright WE. Demonstration of fibronectin as a major extracellular protein of human gingival fibroblasts. J Dent Res. 1980;59:631–7. doi: 10.1177/00220345800590031301. [DOI] [PubMed] [Google Scholar]
- 15.Pender N, Heaney TG, Pycock D, West CR. Progenitor connective tissue cell populations in the gingival papilla of the rat. J Periodont Res. 1988;23:175–81. doi: 10.1111/j.1600-0765.1988.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 16.Christgau M, Caffesse RG, Schmalz G, D’Souza RN. Extracellular matrix expression and periodontal wound-healing dynamics following guided tissue regeneration therapy in canine furcation defects. J Clin Periodontol. 2007;34:691–708. doi: 10.1111/j.1600-051X.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 17.Csiszar A, Wiebe C, Larjava H, Häkkinen L. Distinctive molecular composition of human gingival interdental papilla. J Periodontol. 2007;78:304–14. doi: 10.1902/jop.2007.060165. [DOI] [PubMed] [Google Scholar]
- 18.Bancroft JD. Theory and practice of histological techniques. 5th ed. London: Churchill Living Stone; 2002. p. 153. [Google Scholar]
- 19.Stryer L. Biochemistry. 3rd ed. Newyork: Freeman; 1988. p. 772. [Google Scholar]
- 20.Lorencini M, Silva JAF, Almedia CA, Bruni-Cardoso A, Carvalho HF, Stach-Machado DR. A new paradigm in the periodontal disease progression: Gingival connective tissue remodeling with simultaneous collagen degradation and fibers thickening. Tissue Cell. 2009;41:43–50. doi: 10.1016/j.tice.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Bourke KA, Haase H, Li H, Daley T, Bartold M. Distribution and synthesis of elastin in porcine gingiva and alveolar mucosa. J Periodont Res. 2000;35:361–8. doi: 10.1034/j.1600-0765.2000.035006361.x. [DOI] [PubMed] [Google Scholar]
- 22.Bartold PM, Walsh LJ, Sampath N. Molecular biology and cell biology of the gingiva. Periodontol 2000. 2000;24:28–55. doi: 10.1034/j.1600-0757.2000.2240103.x. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama S, Yaegashi T, Oikawa Y, Fujiwara H, Mikami T, Takeda Y, et al. Expression pattern of adhesion molecules in junctional epithelium differs from that in other gingival epithelia. J Periodontal Res. 2006;41:322–8. doi: 10.1111/j.1600-0765.2006.00875.x. [DOI] [PubMed] [Google Scholar]


