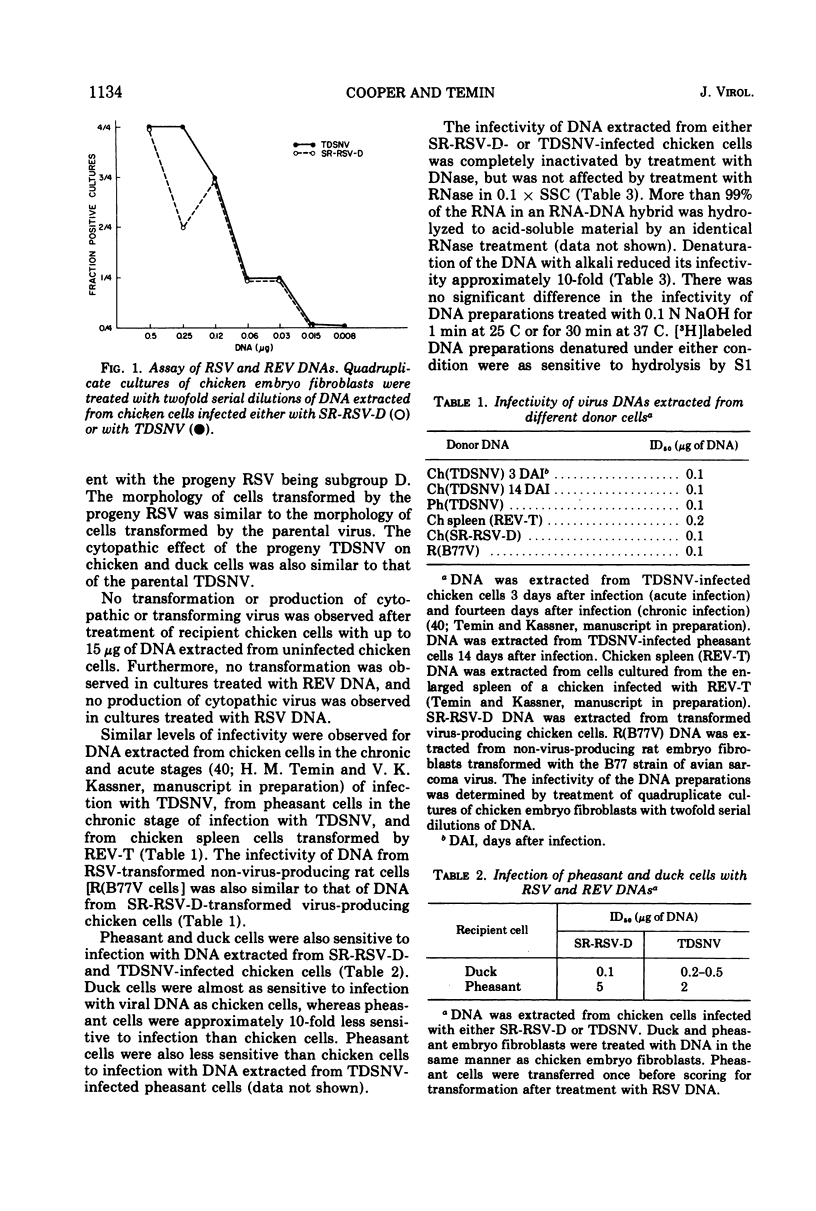
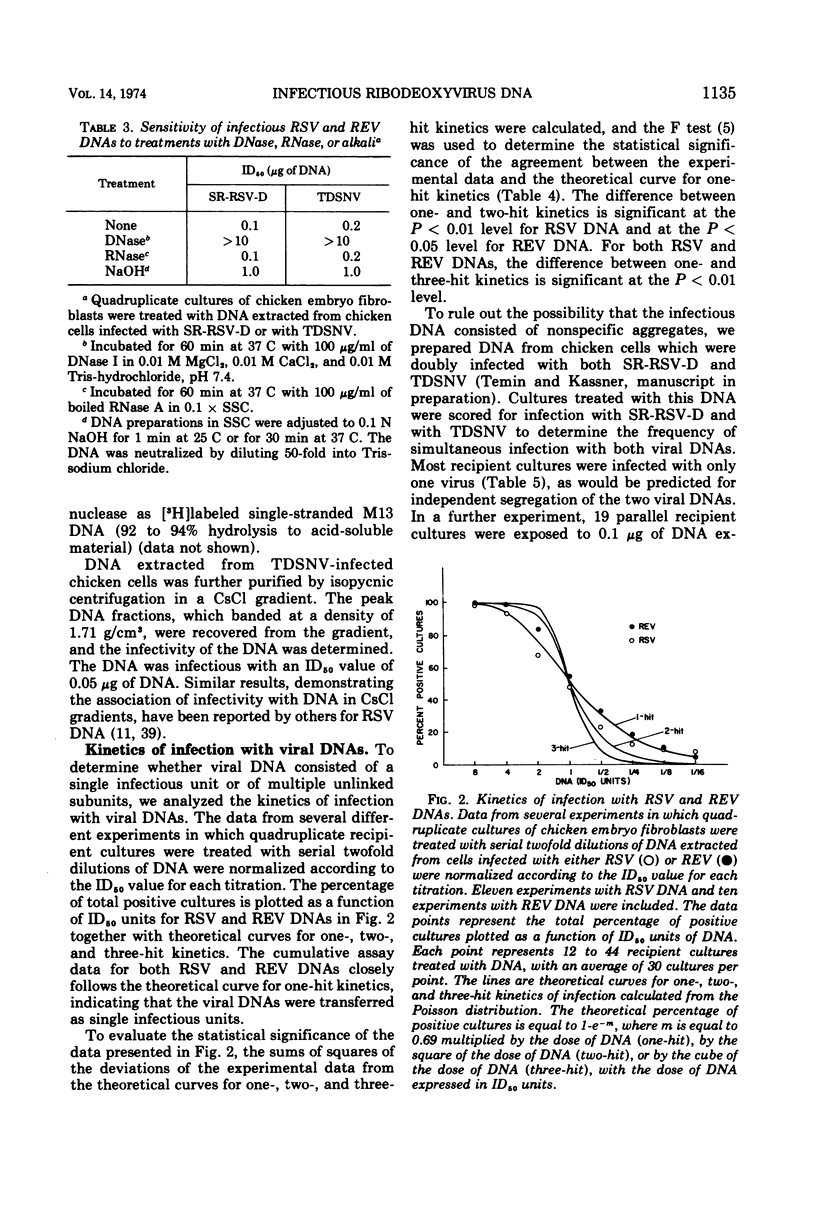
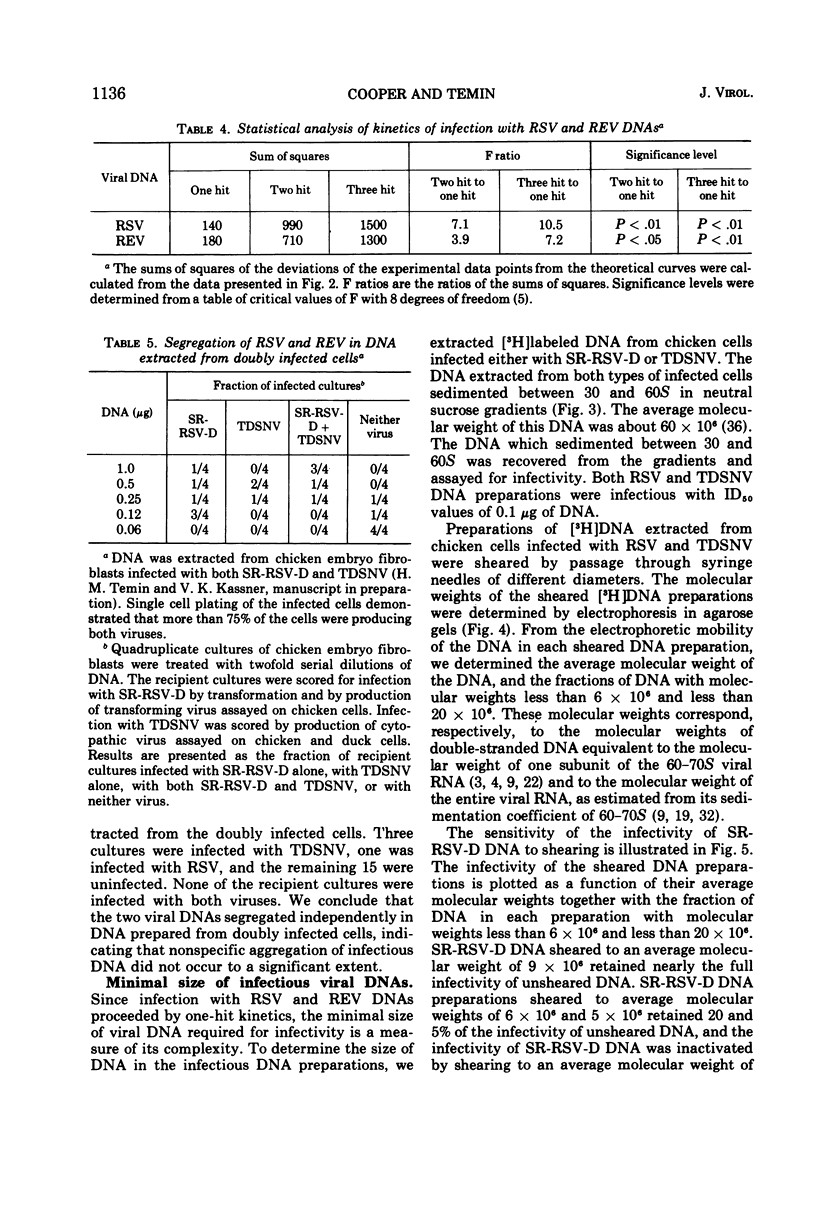
Abstract
An efficient and quantitative assay for infectious Rous sarcoma virus and reticuloendotheliosis virus DNAs is described. The specific infectivities of viral DNA corresponded to one infectious unit per 105 to 106 viral DNA molecules. Infection with viral DNA followed one-hit kinetics. The minimal size of infectious Rous sarcoma virus DNA was approximately 6 million daltons, whereas the minimal size of infectious reticuloendotheliosis virus DNA was larger, 10 to 20 million daltons.
Full text
PDF

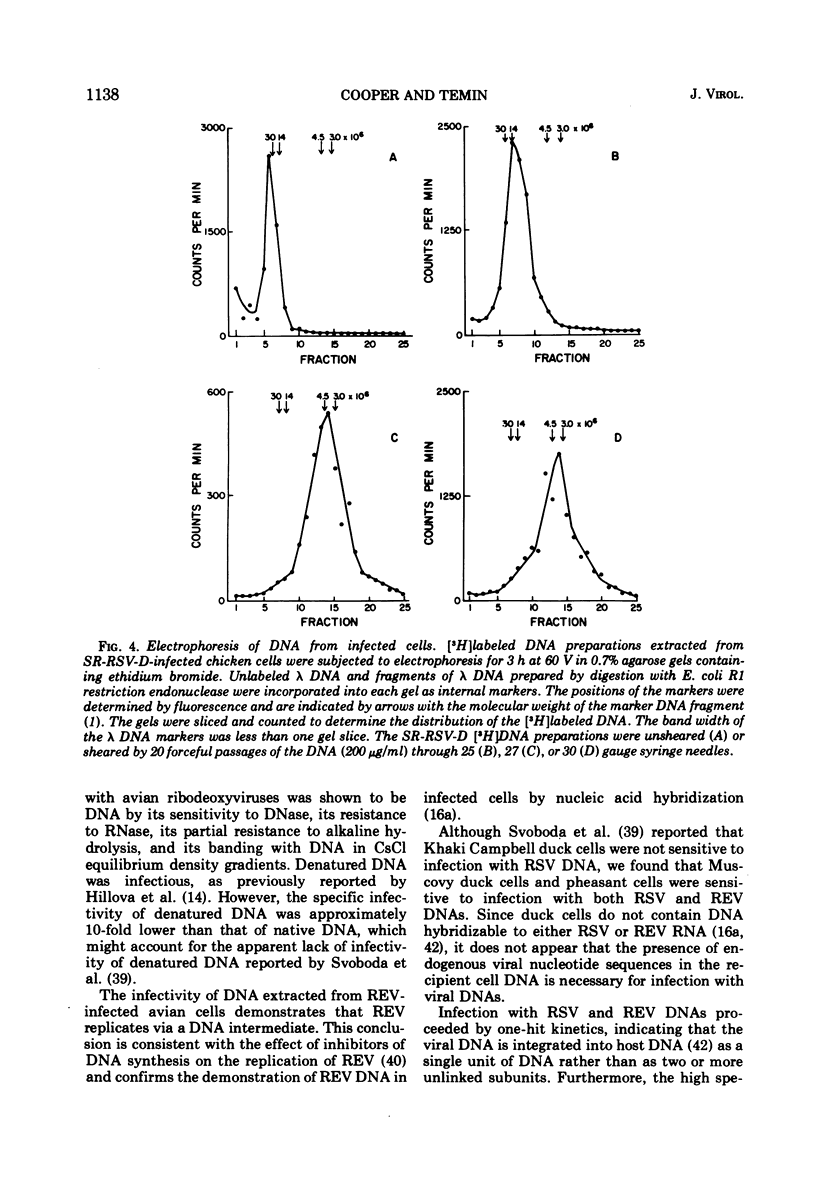
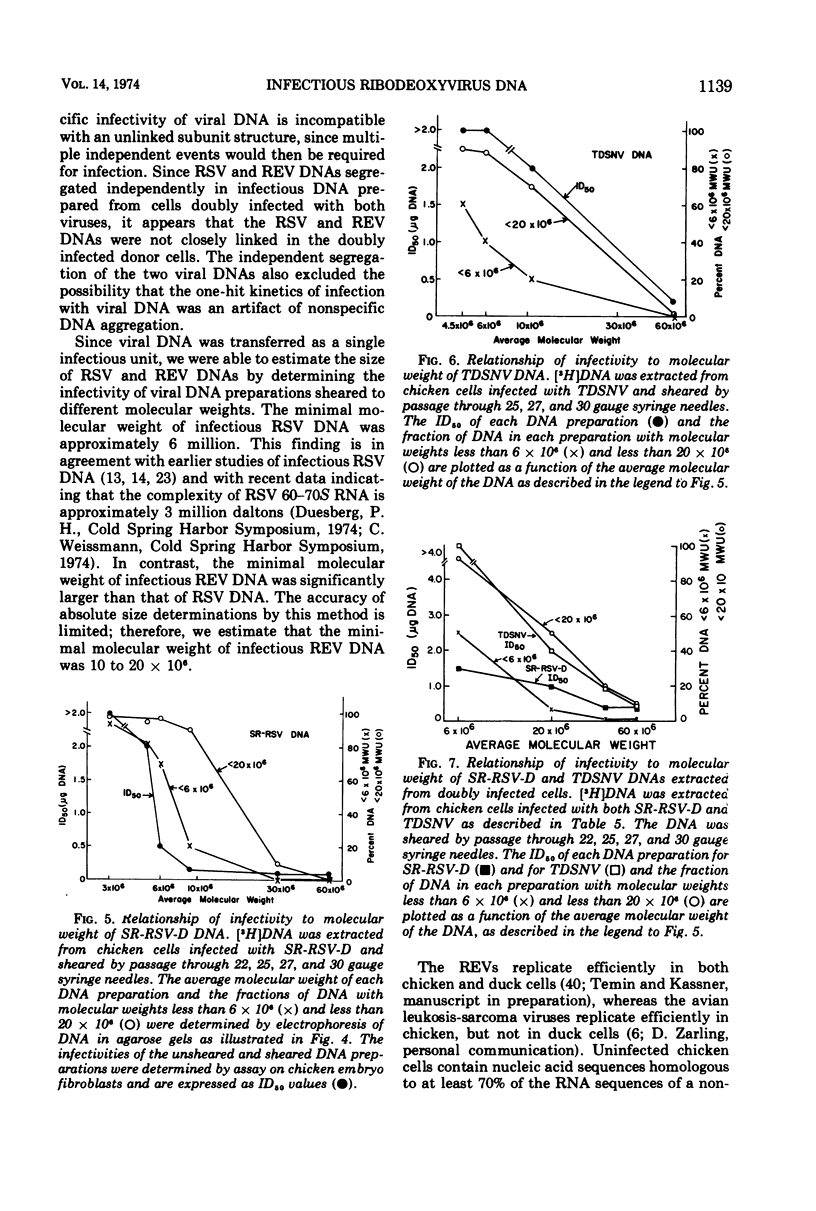


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Jeppesen P. G., Katagiri K. J., Delius H. Mapping the DNA fragments produced by cleavage by lambda DNA with endonuclease RI. Nature. 1973 Jan 12;241(5385):120–123. doi: 10.1038/241120a0. [DOI] [PubMed] [Google Scholar]
- Altaner C., Temin H. M. Carcinogenesis by RNA sarcoma viruses. XII. A quantitative study of infection of rat cells in vitro by avian sarcoma viruses. Virology. 1970 Jan;40(1):118–134. doi: 10.1016/0042-6822(70)90384-3. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Genetic interactions of avian RNA tumor viruses and their host cells. Am J Clin Pathol. 1973 Jul;60(1):25–30. doi: 10.1093/ajcp/60.1.25. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Veldhuisen G., Wilkie N. M. Infectious herpesvirus DNA. Nat New Biol. 1973 Oct 31;245(148):265–266. doi: 10.1038/newbio245265a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Wade E., Rucker E., Baxter-Gabbard K. L., Levine A. S., Friis R. R. A study of the relationship of reticuloendotheliosis virus to the avian leukosis-sarcoma complex of viruses. Virology. 1973 Jun;53(2):287–299. doi: 10.1016/0042-6822(73)90206-7. [DOI] [PubMed] [Google Scholar]
- Hill M., Hillova J. RNA and DNA forms of the genetic material of C-type viruses and the integrated state of the DNA form in the cellular chromosome. Biochim Biophys Acta. 1974 Apr 29;355(1):7–48. doi: 10.1016/0304-419x(74)90006-7. [DOI] [PubMed] [Google Scholar]
- Hill M., Hillova J. Recovery of the temperature-sensitive mutant of Rous sarcoma virus from chicken cells exposed to DNA extracted from hamster cells transformed by the mutant. Virology. 1972 Jul;49(1):309–313. doi: 10.1016/s0042-6822(72)80034-5. [DOI] [PubMed] [Google Scholar]
- Hill M., Hillova J. Virus recovery in chicken cells tested with Rous sarcoma cell DNA. Nat New Biol. 1972 May 10;237(71):35–39. doi: 10.1038/newbio237035a0. [DOI] [PubMed] [Google Scholar]
- Hill M., Hillová J. Production virale dans les fibroblastes de poule traités par l'acide désoxyribonucléique de cellulex XC de rat transformées par le virus de Rous. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jun 14;272(24):3094–3097. [PubMed] [Google Scholar]
- Hillova J., Goubin G., Hill M. Transfection des fibroblastes de poule par l'acide désoxyribonucléique dénaturé de cellules transformées de Rous. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 27;274(13):1970–1973. [PubMed] [Google Scholar]
- Hlozánek I., Svoboda J. [Characterization of viruses obtained after cell fusion or transfection of chicken cells with DNA from virogenic mammalian rous sarcoma cells]. J Gen Virol. 1972 Oct;17(1):55–59. doi: 10.1099/0022-1317-17-1-55. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpas A., Milstein C. Recovery of the genome of murine sarcoma virus (MSV) after infection of cells with nuclear DNA from MSV transformed non-virus producing cells. Eur J Cancer. 1973 Apr;9(4):295–299. doi: 10.1016/0014-2964(73)90097-2. [DOI] [PubMed] [Google Scholar]
- Lacour F., Fourcade A., Merlin E., Huynh T. Détection de virus de la myéloblastose aviaire dans des cultures de fibroblastes de poule traités par de l'ADN de cellules leucémiques productrices de virus. C R Acad Sci Hebd Seances Acad Sci D. 1972 Apr 10;274(15):2253–2255. [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R. Relationship of reticuloendotheliosis virus to the avian tumor viruses: nucleic acid and polypeptide composition. J Virol. 1973 May;11(5):741–747. doi: 10.1128/jvi.11.5.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Lack of serological relationship among DNA polymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken cells. J Virol. 1973 Sep;12(3):440–448. doi: 10.1128/jvi.12.3.440-448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnier L., Goldé A., Vigier P. A possible subunit structure of Rous sarcoma virus RNA. J Gen Virol. 1969 Apr;4(3):449–452. doi: 10.1099/0022-1317-4-3-449. [DOI] [PubMed] [Google Scholar]
- Montagnier L., Vigier P. Un intermediaire ADN infectieux et transformant du virus du sarcome de Rous dans le cellules de poule transformees par ce virus. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 27;274(13):1977–1980. [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P. E. Rous sarcoma virus nucleotide sequences in cellular DNA: measurement by RNA-DNA hybridization. Science. 1972 Nov 17;178(4062):750–753. doi: 10.1126/science.178.4062.750. [DOI] [PubMed] [Google Scholar]
- Nicolson M. O., McAllister R. M. Infectivity of human adenovirus-1 DNA. Virology. 1972 Apr;48(1):14–21. doi: 10.1016/0042-6822(72)90109-2. [DOI] [PubMed] [Google Scholar]
- Peterson D. A., Baxter-Gabbard K. L., Levine A. S. Avian reticuloendotheliosis virus (strain T): V. DNA polymerase. Virology. 1972 Jan;47(1):251–254. doi: 10.1016/0042-6822(72)90259-0. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Ludford C., Nazerian K., Cox H. W. A new group of oncogenic viruses: reticuloendotheliosis, chick syncytial, duck infectious anemia, and spleen necrosis viruses. J Natl Cancer Inst. 1973 Aug;51(2):489–499. [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Laithier M., Lando D., Ryhiner M. L. Infectious DNA from herpes simplex virus: infectivity of double-stranded and single-stranded molecules. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3621–3625. doi: 10.1073/pnas.70.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Svoboda J., Hlozánek I., Mach O. Detection of chicken sarcoma virus after transfection of chicken fibroblasts with DNA isolated from mammalian cells transformed with Rous Virus. Folia Biol (Praha) 1972;18(2):149–153. [PubMed] [Google Scholar]
- Svoboda J., Hlozánek I., Mach O., Michlová A., Rïman J., Urbánková M. Transfection of chicken fibroblasts with single exposure to DNA from virogenic mammalian cells. J Gen Virol. 1973 Oct;21:47–55. doi: 10.1099/0022-1317-21-1-47. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D., Thorne H. V. The infectivity of polyoma virus DNA for mouse embryo cells in the presence of diethylaminoethyl-dextran. J Gen Virol. 1968 Dec;3(3):371–377. doi: 10.1099/0022-1317-3-3-371. [DOI] [PubMed] [Google Scholar]



