Protein energy wasting (PEW) is highly prevalent in the dialysis population (1, 2). Several studies have shown that PEW tends to occur concomitantly with inflammation and both conditions are strongly associated with higher mortality in dialysis patients (3-7). It has been proposed that the combination of PEW and inflammation in dialysis patients be designated as malnutrition-inflammation complex syndrome (MICS) (8-10) or malnutrition, inflammation and atherosclerosis (MIA) syndrome (11).
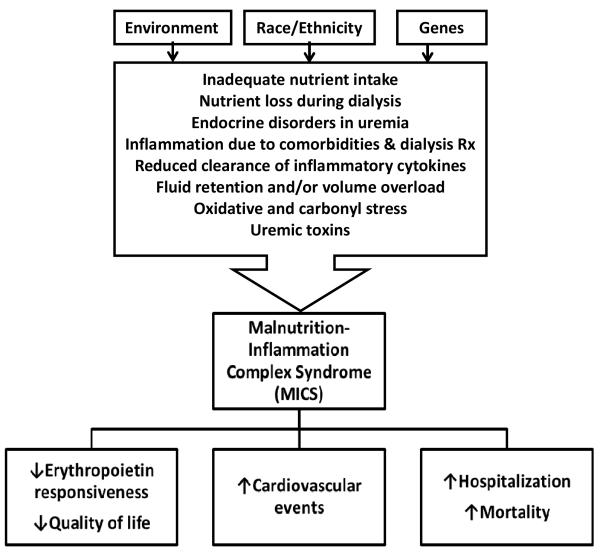
Conditions leading to PEW and inflammation appear to overlap. Causes of MICS include inadequate nutrient intake, nutrient loss during dialysis, inflammation caused by comorbid conditions and dialysis treatment, endocrine disorders of uremia, oxidative and carbonyl stress, uremic toxins, volume overload and reduced clearance of pro-inflammatory cytokines. The presence of MICS in dialysis patients is signaled by hypoalbuminemia, hypocholesterolemia, hypohomocysteinemia, increased levels of inflammatory markers and low body mass index, and its possible consequences include erythropoietin hyporesponsiveness, increased risks of atherosclerotic cardiovascular events, poor quality of life, and increased hospitalization and mortality rates in dialysis patients (Figure 1) (8).
Figure 1.
Possible causes and consequences of MICS (8).
Race-ethnicity and survival paradoxes in CKD and ESRD
The annual mortality rate for US dialysis patients is approximately 20%; the extremely low 5-year survival rate (<35%) is lower than that for many cancer patients (12, 13). It has been recognized that ESRD is more commonly encountered in certain races/ethnicities particularly in African Americans and Hispanics (14). In 2009, incidence rates of ESRD in the United States for African Americans and Hispanics were 3.5 and 1.5 times higher than that seen in non-Hispanic whites, respectively (15). Contributing factors to these racial/ethnicity discrepancies include, but are not limited to a genetic susceptibility to select glomerular diseases, increased rates of major risk factors such as diabetes mellitus, and hypertension, disparities in socioeconomic status affecting access to care, and cultural differences such as diet and lifestyle choices (14). Even though African Americans comprise only 12% of the general population in the United States (16), approximately 37% of the prevalent US dialysis patients are African American. Similarly, Hispanics compose 16% of the US dialysis population (15). Compared with white dialysis patients, black patients are less likely to be dialyzed adequately (17) and undergo AV fistula placement (18, 19). In the US general population, blacks have higher mortality rates than whites due to multiple factors, including but not limited to disparities in income, education, health, diet, lifestyles and co-morbidities (20, 21).
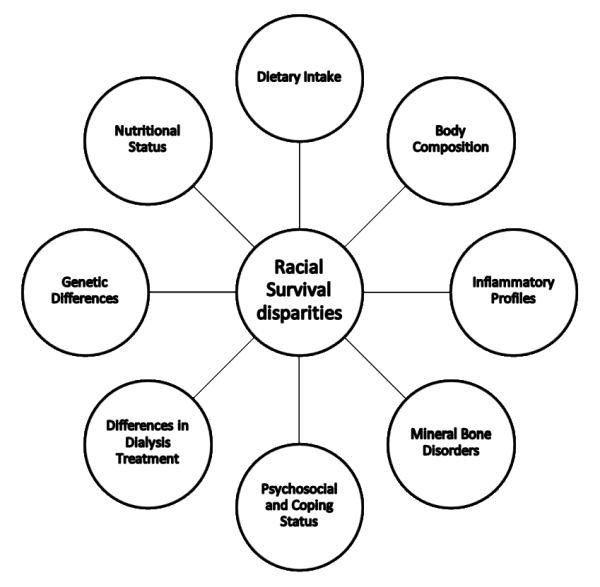
In spite of racial disparities in receipt of ESRD quality care indicators and a shorter life expectancy for blacks compared with that among whites in the general population, African American and Hispanic CKD and dialysis patients have greater survival than non-Hispanic white patients even after adjusting for such traditional risk factors as demographics, socioeconomic status, dialysis vintage, dialysis dose, co-morbid conditions and the presence of residual kidney function (22, 23). The racial/ethnic survival disparities of CKD and dialysis patients have been cited as a survival paradox for minorities including African Americans and Hispanics (24). Efforts have been made to discover the factors responsible for survival advantages of African American and Hispanic dialysis population. Better understanding the potential causes of racial/ethnic survival disparities in dialysis patients might improve outcomes in patients with chronic kidney diseases, other chronic disease states and even the general population. Possible contributing factors to racial survival disparities in dialysis patients include differences in nutritional status, dietary intake, body composition, inflammatory profiles, mineral bone disorders, psychosocial status, mental coping mechanisms, dialysis treatments and genetic polymorphisms (21, 23, 25-34) (Figure 2).
Figure 2.
Possible contributing factors to racial survival disparities among the dialysis population (21, 23, 25-34).
Nutritional axis of racial survival disparities of dialysis population
Survival disparities across various racial/ethnic groups in the dialysis population can be attributed in part to differences in nutritional status, dietary intakes and body composition. A prospective 6 year cohort study of 799 hemodialysis patients by Noori et al. (32) reported that African Americans had better nutritional status including higher body mass index, lean body mass, serum pre-albumin, creatinine and homocysteine concentrations compared with white patients. This study also observed that African Americans had significantly higher dietary energy and both unsaturated and saturated fat intake but lower dietary intake of fibers than white dialysis patients. In this study, higher serum levels of albumin, pre-albumin and creatinine correlated with greater survival in all racial/ethnic groups, though this association was not statistically significant for serum creatinine concentrations in African Americans (32).
Similarly, Streja et al. (23) studied association of race/ethnicity with 5-year survival in 124,029 US hemodialysis patients including African Americans (35%), non-Hispanic Whites (49%) and Hispanics (16%) and found that African Americans and Hispanics had greater survival rates than non-Hispanic Whites in spite of controlling for demographics, socioeconomic status, diabetes, dialysis vintage, dialysis dose and presence of residual renal function. However, after adjustment for surrogate markers of nutritional and inflammatory status, African Americans had even higher mortality rates than Whites, while Hispanics had similar survival rates compared to White patients (23), suggesting a powerful role for the influence of nutrition and inflammation on mortality.
Several cohort studies observed that higher body mass index (BMI) correlated with better survival in the dialysis population (35-37). In the U.S. ESRD population, the prevalence of obesity was higher in blacks compared to non-Hispanic Whites (38). A 6 year cohort study of 109,605 hemodialysis patients by Ricks et al. (33) investigated the association of increased body mass index with mortality among different races/ethnicities. This study showed that higher BMI was associated with lower mortality in all race groups with various degrees of correlation. A 1 kg/m2 higher BMI correlated with 2%, 2.5% and 1% lower risk of death for non-Hispanic Whites, Blacks and Hispanics, respectively.
Inflammatory axis and racial/ethnic survival disparities of dialysis patients
In addition to differences in nutritional status, racial/ethnic differences in inflammation have been postulated as potential contributors to racial/ethnic survival paradox of the dialysis population. Crews et al. (25) investigated the association of race and inflammation with mortality in 816 incident hemodialysis patients including 554 Caucasians and 262 African Americans with a median follow-up of 3 years. This study (25) supported a significant lower mortality rate in African Americans compared to that seen in Caucasians after adjusting for demographics and traditional risk factors. Nevertheless, the survival advantage of African American dialysis patients was only seen in the presence of the highest levels of inflammatory markers including C-reactive protein (CRP) and interleukin-6 (IL-6), whereas survival disparities between African Americans and Caucasians did not exist in dialysis patients with low-level inflammation. In the setting of elevated inflammation levels the survival advantage of African Americans over Caucasians was more pronounced in patients aged over 60 years, men and individuals with diabetes (25).
Similarly, Noori et al. (32) reported an association of higher levels of CRP and IL-6 with increased mortality risks in African American and white dialysis patients. Interestingly, the highest (vs. the lowest) quartile of IL-6 correlated with 2.4 and 4.1 times higher risks of death in African Americans and whites, respectively suggesting that African Americans might be more resilient when confronting the deleterious effects of inflammation. Moreover, the study by Streja et al. cited above (23) showed that African Americans tended to have lower white blood cell counts and higher percentages of lymphocytes reflecting a lower level of inflammation or an attenuated inflammatory response. This study also found that African American and Hispanic dialysis patients had better survival than whites; however, after controlling for surrogates of MICS, African Americans actually had lower survival than whites, whereas the survival rates between Hispanics and whites were similar. The authors concluded that more favorable nutritional and inflammatory status could be a major cause of the survival advantage seen among minority dialysis population (23).
A potential mechanism of action explaining the association of MICS with adverse outcomes is its effect on thrombocytosis and platelet activation, predisposing to thromboembolic events and death. A cohort study of 40,787 hemodialysis patients by Molnar et al. (39) reported that higher platelet count correlated with markers of MICS including lower serum levels of albumin, creatinine, hemoglobin, the percentages of lymphocyte counts, normalized protein nitrogen appearance and Kt/V. Higher platelet count was also associated with higher all-cause and cardiovascular mortality; however, the association of platelet count with all-cause and cardiovascular mortality did not exist after controlling for surrogates of MICS. The investigators hypothesized that MICS increased the mortality risk of dialysis patients in part via elevated platelet count and activity (39). Megakaryopoiesis is regulated by inflammatory factors including IL-6, IL-11 and leukemia inhibitor factor, which involves megakaryocyte maturation. The elevated levels and actions of inflammatory cytokines such as IL-6 observed among dialysis patients could result in reactive thrombocytosis (40, 41).
African American race-ethnicity may thus be an important effect-modifier for both the development of inflammation and for the deleterious effects caused by inflammation once it is established. This could be caused by genetic polymorphisms or environmentally induced epigenetic changes, which may provide permissive effects of genotype on the mortality risk of inflammation (25, 29, 30). Further exploration of these phenomena could aid in the better identification of populations at highest risk for adverse outcomes. A better understanding of the genetic susceptibility to the development of inflammation, and to the deleterious effects of established inflammation could also further the development of targeted interventions against inflammation and its harmful effects.
Conclusion
In summary, racial/ethnic differences in nutritional status and the level of inflammation including host inflammatory response among dialysis patients appear to be substantial contributors to the survival disparities of dialysis patients. Studying the basis for these associations could shed light on novel mechanisms of action of inflammation, and could spawn the development of targeted screening and intervention strategies in the most vulnerable populations exposed to the effects of inflammation.
Acknowledgement
Dr. Kovesdy is an employee of the US Department of Veterans Affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily reflect the opinions of the Department of Veterans Affairs.
Funding Source: KN is supported by NIH grants U54MD007598 (formerly U54RR26138 and P20MD000182).
Footnotes
Potential Conflict of Interest: None declared.
References
- 1.Aparicio M, Cano N, Chauveau P, Azar R, Canaud B, Flory A, Laville M, Leverve X. Nutritional status of haemodialysis patients: a French national cooperative study. French Study Group for Nutrition in Dialysis. Nephrol Dial Transplant. 1999;14:1679–1686. doi: 10.1093/ndt/14.7.1679. [DOI] [PubMed] [Google Scholar]
- 2.Rocco MV, Paranandi L, Burrowes JD, Cockram DB, Dwyer JT, Kusek JW, Leung J, Makoff R, Maroni B, Poole D. Nutritional status in the HEMO Study cohort at baseline. Hemodialysis. Am J Kidney Dis. 2002;39:245–256. doi: 10.1053/ajkd.2002.30543. [DOI] [PubMed] [Google Scholar]
- 3.Combe C, Chauveau P, Laville M, Fouque D, Azar R, Cano N, Canaud B, Roth H, Leverve X, Aparicio M. Influence of nutritional factors and hemodialysis adequacy on the survival of 1,610 French patients. Am J Kidney Dis. 2001;37:S81–88. doi: 10.1053/ajkd.2001.20756. [DOI] [PubMed] [Google Scholar]
- 4.Dwyer JT, Larive B, Leung J, Rocco MV, Greene T, Burrowes J, Chertow GM, Cockram DB, Chumlea WC, Daugirdas J, Frydrych A, Kusek JW. Are nutritional status indicators associated with mortality in the Hemodialysis (HEMO) Study? Kidney Int. 2005;68:1766–1776. doi: 10.1111/j.1523-1755.2005.00593.x. [DOI] [PubMed] [Google Scholar]
- 5.Fung F, Sherrard DJ, Gillen DL, Wong C, Kestenbaum B, Seliger S, Ball A, Stehman-Breen C. Increased risk for cardiovascular mortality among malnourished end-stage renal disease patients. Am J Kidney Dis. 2002;40:307–314. doi: 10.1053/ajkd.2002.34509. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AR, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimburger O, Lindholm B, Bergstrom J. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol. 2002;13(Suppl 1):S28–36. [PubMed] [Google Scholar]
- 7.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42:864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38:1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 11.Pecoits-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome -- the heart of the matter. Nephrol Dial Transplant. 2002;17(Suppl 11):28–31. doi: 10.1093/ndt/17.suppl_11.28. [DOI] [PubMed] [Google Scholar]
- 12.Excerpts from the USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. American journal of kidney diseases. 2006;47:S1–286. [Google Scholar]
- 13.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 14.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68:914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 15.Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States . USRDS 2011 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Dieseases; 2011. [Google Scholar]
- 16.U.S. Census Bureau [Accessed June 18, 2012];Census Results. 2010 Available at http://2010.census.gov/2010census/data.
- 17.Owen WF, Jr., Chertow GM, Lazarus JM, Lowrie EG. Dose of hemodialysis and survival: differences by race and sex. Jama. 1998;280:1764–1768. doi: 10.1001/jama.280.20.1764. [DOI] [PubMed] [Google Scholar]
- 18.Hopson S, Frankenfield D, Rocco M, McClellan W. Variability in reasons for hemodialysis catheter use by race, sex, and geography: findings from the ESRD Clinical Performance Measures Project. Am J Kidney Dis. 2008;52:753–760. doi: 10.1053/j.ajkd.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Reddan D, Klassen P, Frankenfield DL, Szczech L, Schwab S, Coladonato J, Rocco M, Lowrie EG, Owen WF., Jr. National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol. 2002;13:2117–2124. doi: 10.1097/01.asn.0000022422.79790.a8. [DOI] [PubMed] [Google Scholar]
- 20.Davey Smith G, Neaton JD, Wentworth D, Stamler R, Stamler J. Mortality differences between black and white men in the USA: contribution of income and other risk factors among men screened for the MRFIT. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Lancet. 1998;351:934–939. doi: 10.1016/s0140-6736(00)80010-0. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Golan E, Shohat T, Streja E, Norris KC, Kopple JD. Survival disparities within American and Israeli dialysis populations: learning from similarities and distinctions across race and ethnicity. Semin Dial. 2010;23:586–594. doi: 10.1111/j.1525-139X.2010.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Anderson JE, Derose SF, Kalantar-Zadeh K. Outcomes associated with race in males with nondialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:973–978. doi: 10.2215/CJN.06031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streja E, Kovesdy CP, Molnar MZ, Norris KC, Greenland S, Nissenson AR, Kopple JD, Kalantar-Zadeh K. Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis. 2011;57:883–893. doi: 10.1053/j.ajkd.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kovesdy CP, Norris KC. Racial Survival Paradox of Dialysis Patients: Robust and Resilient. Am J Kidney Dis. 2012 doi: 10.1053/j.ajkd.2012.02.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crews DC, Sozio SM, Liu Y, Coresh J, Powe NR. Inflammation and the paradox of racial differences in dialysis survival. J Am Soc Nephrol. 2011;22:2279–2286. doi: 10.1681/ASN.2011030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feroze U, Martin D, Reina-Patton A, Kalantar-Zadeh K, Kopple JD. Mental health, depression, and anxiety in patients on maintenance dialysis. Iran J Kidney Dis. 2010;4:173–180. [PubMed] [Google Scholar]
- 27.Feroze U, Noori N, Kovesdy CP, Molnar MZ, Martin DJ, Reina-Patton A, Benner D, Bross R, Norris KC, Kopple JD, Kalantar-Zadeh K. Quality-of-life and mortality in hemodialysis patients: roles of race and nutritional status. Clin J Am Soc Nephrol. 2011;6:1100–1111. doi: 10.2215/CJN.07690910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, Miller JE, Kovesdy CP, Mehrotra R, Lukowsky LR, Streja E, Ricks J, Jing J, Nissenson AR, Greenland S, Norris KC. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25:2724–2734. doi: 10.1002/jbmr.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Norris KC. Is the malnutrition-inflammation complex the secret behind greater survival of African-American dialysis patients? J Am Soc Nephrol. 2011;22:2150–2152. doi: 10.1681/ASN.2011101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovesdy CP, Kalantar-Zadeh K. Do genes allow inflammation to kill or not to kill? J Am Soc Nephrol. 2009;20:1429–1431. doi: 10.1681/ASN.2009050510. [DOI] [PubMed] [Google Scholar]
- 31.Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Van Wyck D, Greenland S, Kalantar-Zadeh K. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis. 2010;55:100–112. doi: 10.1053/j.ajkd.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noori N, Kovesdy CP, Dukkipati R, Feroze U, Molnar MZ, Bross R, Nissenson AR, Kopple JD, Norris KC, Kalantar-Zadeh K. Racial and ethnic differences in mortality of hemodialysis patients: role of dietary and nutritional status and inflammation. Am J Nephrol. 2011;33:157–167. doi: 10.1159/000323972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricks J, Molnar MZ, Kovesdy CP, Kopple JD, Norris KC, Mehrotra R, Nissenson AR, Arah OA, Greenland S, Kalantar-Zadeh K. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis. 2011;58:574–582. doi: 10.1053/j.ajkd.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf M, Betancourt J, Chang Y, Shah A, Teng M, Tamez H, Gutierrez O, Camargo CA, Jr., Melamed M, Norris K, Stampfer MJ, Powe NR, Thadhani R. Impact of activated vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol. 2008;19:1379–1388. doi: 10.1681/ASN.2007091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46:489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, Block G, Kopple JD. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83:202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 37.Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 38.Glanton CW, Hypolite IO, Hshieh PB, Agodoa LY, Yuan CM, Abbott KC. Factors associated with improved short term survival in obese end stage renal disease patients. Ann Epidemiol. 2003;13:136–143. doi: 10.1016/s1047-2797(02)00251-x. [DOI] [PubMed] [Google Scholar]
- 39.Molnar MZ, Streja E, Kovesdy CP, Budoff MJ, Nissenson AR, Krishnan M, Anker SD, Norris KC, Fonarow GC, Kalantar-Zadeh K. High platelet count as a link between renal cachexia and cardiovascular mortality in end-stage renal disease patients. Am J Clin Nutr. 2011;94:945–954. doi: 10.3945/ajcn.111.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, Forstrom JW, Buddle MM, Oort PJ, Hagen FS, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- 41.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, Atkins MB, Mier JW, Tilg H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]