Abstract
Background
Despite being a highly prevalent disorder and substantial cause of disability, migraine is understudied in Africa. Moreover, no previous study has investigated the effects of stress and unipolar psychiatric comorbidities on migraine in a sub-Saharan African cohort.
Objective
To evaluate the prevalence of migraine and its association with stress and unipolar psychiatric comorbidities among a cohort of African adults.
Methods
This was a cross-sectional epidemiologic study evaluating 2,151 employed adults in sub-Saharan Africa. A standardized questionnaire was used to identify socio-demographic, headache, and lifestyle characteristics of participants. Migraine classification was based on the International Classification of Headache Disorders (ICHD)-2 diagnostic criteria. Depressive, anxiety and stress symptoms were ascertained with the Patient Health Questionnaire (PHQ-9) and the Depression Anxiety Stress Scale (DASS-21) respectively. Multivariable logistic regression models were used to estimate adjusted odds ratio (OR) and 95% confidence intervals (CI).
Results
A total of 9.8% (n=212) of study participants fulfilled criteria for migraine (9.8%; 95%CI: 8.6, 11.1) with a higher frequency among women (14.3%; 95%CI: 11.9, 16.6) than men (6.9%; 95%CI: 5.5, 8.3). Similar to predominantly Caucasian migraine cohorts, sub-Saharan African migraineurs were more likely to be younger, have a lower education and more likely to report a poor health status than non-migraineurs. However, in contrast to historical reports in predominantly Caucasian migraine cohorts, sub-Saharan African migraineurs were less likely to report smoking than non-migraineurs. Participants with moderately severe depressive symptoms had over a 3-fold increased odds of migraine (OR=3.36; 95% CI 1.30,8.70), compared with those classified as having minimal or no depressive symptoms; and the odds of migraine increased with increasing severity of depressive symptoms (p-trend <0.001). Similarly those with mild, moderate and severe anxiety symptoms had increased odds of migraine (OR=2.28; 95%CI 1.24, 4.21; OR=1.77; 95%CI 0.93, 3.35, and OR=5.39; 95%CI 2.19, 13.24, respectively). Finally, those with severe stress had a 3.57-fold increased odds of migraine (OR=3.57; 95%CI 1.35, 9.46).
Conclusion
Although historically it has been reported that migraine prevalence is greater in Caucasians than African Americans, our study demonstrates a high migraine prevalence among urban dwelling Ethiopian adults (9.9%) that is comparable to what is typically reported in predominantly Caucasian cohorts. Further, among employed sub-Saharan African adults, and similar to predominantly Caucasian populations, migraine is strongly associated with stress and unipolar psychiatric symptoms. The high burden of migraine and its association with stress and unipolar psychiatric symptoms in our study of well-educated and urban dwelling African adults has important clinical and public health implications pending confirmation in other African populations.
Keywords: Migraine, Depression, Anxiety, Stress, Comorbidities, Sub-Saharan Africa
Introduction
Migraine is a neurological disorder marked by episodic attacks of disabling headaches1. These attacks are often characterized by episodes of unilateral, severe throbbing, pulsatile headache associated with nausea, vomiting, photophobia, phonophobia, and aversion to physical activity1, 2. Globally, migraine is a major cause of disability 3. Although there is a strong body of research on migraine and its impact on lost productivity costs in high income countries, the epidemiology of migraine in sub-Saharan Africa is not well documented 4, 5. In the past two decades, very few studies have evaluated the burden of migraine in sub-Saharan Africa 6-10 using the International Classification of Headache Disorder (ICHD) criteria11. The results have been inconsistent. For instance Dent et al 7 in Tanzania reported an overall 5% prevalence of migraine among rural residents. However, Adoukonou and colleagues 12 in their study among college students found an 11.3% prevalence of migraine. Similarly, Ofovwe et al 13 reported a 13.5% prevalence of migraine among secondary school students 13 To date, there are only two published studies documenting the prevalence of migraine among adults in Ethiopia using the ICHD criteria. The first, conducted in the early 1990s in rural Ethiopia, reported a 1 year period prevalence of 3% 9. The second study was conducted in 2007 among textile mill workers in Addis Ababa, and reported a one-year period prevalence of 6.2%. 10
There is a substantial and growing body of epidemiologic data demonstrating the cooccurrence of stress and unipolar psychiatric disorders with migraine14, 15,16. Little is known, however, about the association and impact of stress and unipolar psychiatric symptoms among migraineurs in sub-Saharan African populations. Given the high prevalence of migraine and psychiatric disorders, it is important to evaluate their co-occurrence as the presence of one may impact the treatment and prognosis of the other.
Studies have shown that migraine headache is a common cause of absenteeism and reduced on-the-job productivity17. However, most of the previously published African studies evaluated community residents, hospital patients or college students6-9. Given the scarcity of epidemiologic studies evaluating the magnitude and impact of migraine among the African workforce, we conducted this study to estimate the prevalence of migraine and its association with co-occurring stress and unipolar psychiatric symptoms in relation to migraine among an occupational cohort of Ethiopian adults.
Materials and Methods
Study Design and Population
This study was conducted in Addis Ababa, Ethiopia between December 2009 and January 2010.18, 19. Participants were employees of the Commercial Bank of Ethiopia and teachers from public schools in Addis Ababa. Workplaces were selected based on their high stability of workforce and willingness to participate in the study. Multistage sampling by means of probability proportional to size (PPS) procedures were used to select participants20. This approach was performed for both institutions, and all individuals at selected locations were invited to participate. Approximately 93% of individuals who were invited to participate in the study elected to do so.
The primary objective of the parent study was to evaluate the prevalence and risk factors associated with non-communicable diseases among employed Ethiopian adults and was designed in accordance with the WHO's Stepwise (STEPs) approach for non-communicable disease (NCD) surveillance21. The approach had three levels: (1) questionnaire to gather demographic and behavioral information, (2) simple anthropometric measurements, and (3) biochemical tests. Each participant was interviewed face-to-face by a trained interviewer using the WHO STEPs structured questionnaire. The STEPs questionnaire was supplemented with additional questions to reflect the context of Ethiopia. The questionnaire ascertained demographic information including age, sex, and education level. Questions were also included regarding behavioral risk factors such as tobacco, alcohol, and khat consumption. Khat is an evergreen plant with amphetamine-like effects commonly used as a mild stimulant for social recreation and to improve work performance in Ethiopia22, 23. The modified questionnaire was originally written in English, translated into Amharic, and back to English by experts, and was tested prior to use. Prior to the start of the study, research interviewers and experienced research nurses were trained for five days on the contents of the questionnaire, ethical conduct of human subjects research, and data collection techniques. All study participants provided informed consent and all research protocols were approved by the Institutional Review Boards of Addis Continental Institute of Public Health, Addis Ababa, Ethiopia and the Human Subjects Division at the University of Washington, USA.
Migraine Classification
A previously validated questionnaire with a high degree of reliability and validity developed by Henry et al was used in the survey24. Participants were queried if they suffered four to five headache attacks during their life time. Affirmative responses were followed with questions to ascertain headache frequency and differentiate if participants experienced episodic or daily headaches. Headache classification was subsequently based on the International Classification of Headache Disorders (ICHD)-2 criteria.25 Migraine was defined by at least five lifetime headache attacks lasting 4–72 hours, with at least two of the qualifying pain characteristics (unilateral location, pulsating quality, moderate or severe pain intensity), at least one of the associated symptoms (nausea and/or vomiting, or photo and phonophobia). Aggravation by routine physical exertion was not included.
Stress and Unipolar Psychiatric Symptom Classification
The Patient Health Questionaire-9 (PHQ-9)
All participants were evaluated for major depression symptoms using the PHQ-9. The PHQ-9 queries participants about the frequency of nine depressive symptoms experienced. The questions are based on the 9 Diagnostic and Statistical Manual of Mental Disorders (DSM) IV criteria for major depressive disorder criteria. Scores for each question range from 0 (“not at all”) to 3 (“nearly every day”). The PHQ-9 total score is the sum of scores for the nine items for each participant, and ranges from 0-27. A score of ≥ 10 on the PHQ-9 is associated with 88 % sensitivity and 88% specificity in diagnosing “major depressive disorder” using the DSM-IV criteria 26. Therefore, we defined major depression as a score ≥10 on PHQ-9 in participants. Additionally, we categorized participants as exhibiting minimal (PHQ-9 score 0-4), mild (PHQ-9 score 5-9), moderate (PHQ-9 score 10-14), and moderately severe/severe (PHQ-9 score ≥15) depressive symptoms26. Multiple studies have demonstrated that the PHQ-9 can be used in African populations as a valid and useful instrument in screening depression27-29.
Depression Anxiety Stress Scales (DASS-21)
Depressive, anxiety and stress symptoms were also assessed using the DASS-21 instrument30, 31. The DASS-21 is a 21-item instrument designed to measure the 3 negative affective states of depression, anxiety, and stress. The depression scale assessed dysphoria, hopelessness, devaluation of life, self-deprecation, lack of interest or involvement, anhedonia, and inertia. Although the PHQ-9 focuses more on the cognitive and somatic symptoms of depression, the DASS depression scale puts similar weight on the three cognitive, somatic and emotion domains of depression and includes an item on interpersonal symptoms32. The anxiety scale assessed autonomic arousal, situational anxiety, and subjective experience of anxious affect. The stress scale assessed difficulty relaxing, nervous arousal, and being easily upset or agitated, irritable, or over-reactive and impatience30, 31. Using previously suggested cutoff scores30, 31, participants were categorized as exhibiting normal (DASS score <9), mild (DASS score 10-13), moderate (DASS score 14-20), and severe (DASS score ≥21) depressive symptoms. Participants were categorized as exhibiting normal (DASS score <7), mild (DASS score 8-9), moderate (DASS score 10-14), and severe (DASS score ≥15) anxiety symptoms. The corresponding cutoff score for symptoms of stress were as follows: normal (DASS score <14), mild (DASS score 15-18), moderate (DASS score 19-25), and severe (DASS score ≥26). Since investigators have reported the importance of using factor structure (construct validity) when a screening instrument is applied in new context or cultural background 33, we used Exploratory Factor Analysis (EFA) to assess the construct validity of DASS21 as used in the present study population. The results of our EFA showed the unidimensional nature of the depression, anxiety and stress symptom questions. The item loadings ranged from 0.54 to 0.67 for depression, 0.54 to 0.68 for stress, and 0.34 to 0.64 for anxiety 33. These values are regarded as being compatible with an instrument having good construct validity.
Covariates
Alcohol consumption was classified into low (< 1 alcoholic beverage a week), moderate (1–21 alcoholic beverages a week), and high to excessive consumption (> 21 alcoholic beverages a week) according to the World Health Organization (WHO) classification 34. Other variables were categorized as follows: age (years), sex, education (≤ high school, technical school, ≥ bachelors), smoking history (never, former, current), and current khat consumption (yes, no) 22, 23,22, 23. Body Mass Index (BMI) was calculated as weight (kg)/height squared (m2). Different thresholds of BMI were set according to the World Health Organization (WHO) protocol (underweight: <18.5 kg/m2; normal: 18.5–24.9 kg/m2; overweight: 25.0–29.9 kg/ m2; and obese ≥30 kg/m2) 35. Participants were also asked the following question about their self-reported health status: “Would you say your health in general is excellent, very good, good, fair, or poor?” Self-reported health status was analyzed as a dichotomous variable (excellent, very good, or good versus fair or poor)
Data Analysis
We first examined frequency distributions of socio-demographic and behavioral characteristics of study participants. Prevalence estimates of migraine across socio-demographic groups, as well as age and sex-specific migraine prevalence estimates were reported. Using previously described methods, 95% confidence intervals (95% CI) for prevalence estimates were determined 36. We used multivariable logistic regression procedures to estimate odds ratios (OR) and 95% CI for the associations between migraine and socio-demographic factors. Forward logistic regression modeling procedures combined with the change-in-estimate approach were used to select the final models reported in this research 37. Variables of a priori interest (e.g., age and sex) were forced into final models. We also used unadjusted and multivariable-adjusted logistic regression models to ORs and 95% CIs of the association between migraine and psychiatric symptoms. Separate models were fitted for depression, anxiety and stress. In multivariable models, we adjusted for age, sex, occupation, and body mass index (BMI). These confounding variables were considered a priori on the basis of their relationship with both migraine and psychiatric disorders38. Additional adjustment for the other covariates listed in Table 1 did not substantially change the effect estimates. In multivariate analysis, to test for a linear trend across categories of psychiatric symptoms, we modeled the 4-level psychiatric symptom variables as continuous. All analyses were performed using STATA 11.0 statistical software for Windows (Statacorp, College Station, TX, USA). All reported p-values are two-sided and deemed statistically significant at α=0.05.
Table 1.
Characteristics of the study population according to migraine classification
| All N=2,151 | Migraine N=212 | No Migraine N=1,939 | ||
|---|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) | P-value* |
| Sex | ||||
| Women | 854 (39.7) | 122 (57.5) | 732 (37.7) | <0.01 |
| Men | 1,297 (60.3) | 90 (42.5) | 1,207 (62.3) | |
| Age (years) | ||||
| 18-29 | 926 (43.1) | 116 (54.7) | 810 (41.8) | 0.003 |
| 30-39 | 481 (22.3) | 40 (18.9) | 441 (22.7) | |
| 40-49 | 338 (15.7) | 32 (15.1) | 306 (15.8) | |
| 50-59 | 385 (17.9) | 23 (10.8) | 362 (18.7) | |
| ≥60 | 21 (1.0) | 1 (0.5) | 20 (1.0) | |
| Education | ||||
| ≤ High school | 628 (29.2) | 76 (36.4) | 552 (28.5) | 0.025 |
| ≥ College education | 1,523 (70.8) | 136 (63.6) | 1,387 (71.5) | |
| Smoking status | ||||
| Non smoker | 1,860 (86.5) | 196 (92.5) | 1,664 (85.8) | 0.004 |
| Current smoker | 192 (8.9) | 6 (2.8) | 186 (9.6) | |
| Former smoker | 99 (4.6) | 10 (4.7) | 89 (4.6) | |
| Alcohol consumption | ||||
| None | 486 (22.6) | 47 (22.2) | 439 (22.6) | 0.897 |
| Moderate | 1,508 (70.1) | 151 (71.2) | 1,357 (70.0) | |
| Heavy | 157 (7.3) | 14 (6.6) | 143 (7.4) | |
| Khat consumption | ||||
| No | 1,962 (91.2) | 198 (93.4) | 1,763 (91.0) | 0.236 |
| Yes | 189 (8.8) | 14 (6.6) | 175 (9.0) | |
| Self-reported health status | ||||
| Excellent/Very good/Good | 1,291 (60.0) | 104 (49.1) | 1,187 (61.2) | <0.001 |
| Poor/Fair | 860 (40.0) | 108 (50.9) | 752 (38.8) | |
| Body mass index (kg/m2) | ||||
| Underweight (<18.5) | 288 (13.5) | 29 (13.7) | 259 (13.4) | 0.05 |
| Normal (18.5-24.9) | 1,218 (56.9) | 133 (62.7) | 1,085 (56.3) | |
| Overweight (25.0-29.9) | 523 (24.5) | 35 (16.9) | 488 (25.3) | |
| Obese (≥30.0) | 109 (5.1) | 13 (6.6) | 96 (5.0) | |
P-value from Chi-Square test
Results
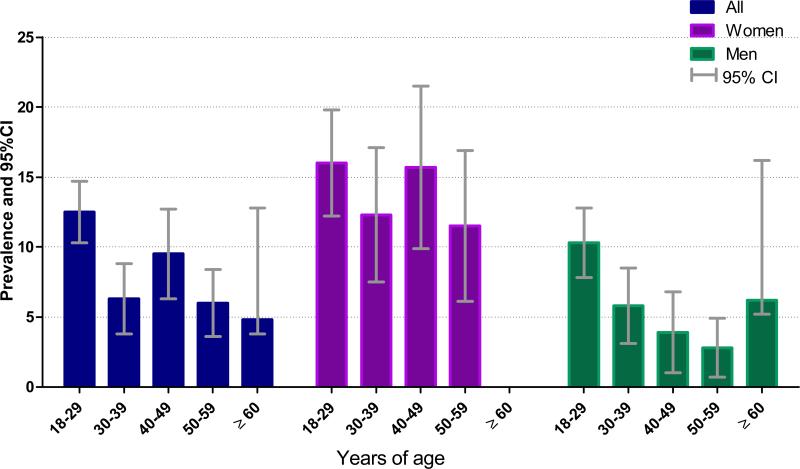
A summary of selected socio-demographic and lifestyle characteristics of study participants is presented in Table 1. A total of 2,151 participants between the ages of 18 and 67 years (mean age=35 years, standard deviation=11 years) participated in the study. The majority of participants were men (60%), unmarried (51%) and more likely to have a college diploma, bachelor's degree, or higher education (71%). Approximately 7% of participants reported that they were heavy drinkers and 9% reported that they were current smokers. Khat chewing was reported by 8.8% of participants. Approximately 40% of participants reported having a fair or poor health status. Distributions of the socio-demographic and lifestyle characteristics according to participants’ migraine status are also presented in Table 1. A total of 212 participants fulfilled ICHD-2 criteria for migraine (9.8%; 95%CI 8.6, 11.1) with more women fulfilling migraine criteria (14.3%; 95%CI 11.9, 16.6) than men (6.9%; 95%CI 5.5, 8.3). Migraineurs were more likely to be younger, have a lower educational level, and to report a poor health status, but to be less likely to report smoking. The prevalence of migraine according to sex and age groups is presented in Figure 1. Migraine prevalence varied with age among women and men. Overall, migraine prevalence was highest through early adult life (18-29 years), then declining after the fifth decade of life in men and women. The prevalence of migraine peaked in early adult life and during the fifth decade among women. Among men, migraine prevalence peaked during early adulthood (18-29 years).
Figure 1.
Prevalence of migraine according to sex and age groups
As shown in Table 2, migraine decreased with age (p-trend <0.001). The odds of migraine were more than 2-fold higher among women as compared with men (OR=2.16; 95% CI 1.62, 2.89). In the multivariate adjusted model participants reporting poor health status had a 60% increased odds of migraine (OR=1.62; 95%CI 1.21, 2.16).
Table 2.
Odds ratio (OR) and 95% confidence intervals (CI) for migraine
| Characteristic | Unadjusted OR (95%CI) | Age and sex adjusted OR (95%CI) | Multivariate *adjusted OR (95%CI) |
|---|---|---|---|
| Age (years) | |||
| 18-29 | Reference | Reference | |
| 30-39 | 0.63 (0.43,0.92) | 0.74 (0.51,1.08) | |
| 40-49 | 0.73 (0.48,1.10) | 0.65 (0.41,1.03) | |
| 50-59 | 0.44 (0.29,0.71) | 0.63 (0.41,0.98) | |
| ≥60 | 0.35 (0.05,2.62) | 0.27 (0.12,0.62) | |
| Sex | |||
| Men | Reference | Reference | |
| Women | 2.22 (1.67,2.97) | 2.16 (1.62,2.89) | |
| Education | |||
| ≤ High school | Reference | Reference | |
| ≥ College education | 0.69 (0.52,0.93) | 0.88 (0.69,1.20) | |
| Smoking status | |||
| Non smoker | Reference | Reference | |
| Current smoker | 0.27 (0.12,0.62) | 0.48 (0.20,1.11) | |
| Previous smoker | 0.94 (0.48,1.84) | 1.54 (0.77,3.01) | |
| Khat consumption | |||
| No | Reference | Reference | |
| Yes | 0.70 (0.40,1.24) | 1.02 (0.57,1.84) | |
| Alcohol consumption | |||
| None | Reference | Reference | |
| Moderate | 1.02 (0.73,1.44) | 1.22 (0.86,1.74) | |
| Heavy | 0.89 (0.48,1.67) | 1.62 (0.84,3.14) | |
| Body mass index (kg/m2) | |||
| Normal (18.5-24.9) | Reference | Reference | |
| Underweight (<18.5) | 0.91 (0.59,1.39) | 081 (0.52,1.25) | |
| Overweight (25.0-29.9) | 0.60 (0.41,0.88) | 0.66 (0.44,1.01) | |
| Obese (≥30.0) | 1.19 (0.66,2.14) | 1.05 (0.55,2.00) | |
| Self-reported health status | |||
| Excellent/Very Good/Good | Reference | Reference | Reference |
| Poor/Fair | 1.67(1.26,2.22) | 1.61 (1.20,2.14) | 1.62 (1.21,2.16) |
Each odds ratio is adjusted for age, occupation and all other covariates listed in the table
The odds of migraine increased with increasing severity of depressive symptoms as measured by the PHQ-9 questionnaire (p-trend <0.001) (Table 3). After adjusting for confounding variables moderately severe depressive symptoms were statistically significantly associated with over a 3-fold increased odds of migraine (OR=3.36; 95% CI 1.30, 8.70), compared with minimal/no depressive symptoms.
Table 3.
-Odds ratio (OR) and 95% confidence interval (CI) for migraine and severity of 4 depressive symptoms using the Patient Health Questionnaire-9 (PHQ-9)
| Migraine |
||||
|---|---|---|---|---|
| Depressive Symptoms(Score) | No (N=1,939) n (%) | Yes (N=212) n (%) | Unadjusted OR (95% CI) | *Adjusted OR (95% CI) |
| Minimal (<4) | 1,459 (75.2) | 133 (62.2) | 1.0 Reference | 1.00 Reference |
| Mild (5–9) | 377 (19.4) | 62 (28.9) | 1.80 (1.28,2.45) | 1.67 (1.20,2.32) |
| Moderate (10–14) | 83 (4.3) | 12 (5.6) | 1.59 (0.76,2.79) | 1.41 (0.73,2.74) |
| Moderately Severe (≥15) | 20 (1.0) | 7 (2.8) | 3.84 (1.59,9.24) | 3.36 (1.30,8.70) |
| Missing | 0 (0.0) | 1(0.4) | ||
| P-value for trend | <0.001 | 0.001 | ||
Adjusted for age, sex, occupation, and body mass index
As shown in Table 4, the odds of migraine were also positively and statistically significantly associated with depressive, anxiety and stress symptoms as measured using the DASS-21 questionnaire. Compared with the reference group, the OR and 95% CI for mild, moderate, and severe depressive symptoms were 1.31 (95% CI 0.59, 2.74), 2.29 (95% CI 1.03, 5.11) and 2.41 (95% CI 0.65, 8.30), respectively (p-trend=0.012). Anxiety symptoms were more strongly associated with migraine (p-trend<0.001). Compared with the reference group (DASS anxiety score ≤7) those with mild (DASS score 8-9), moderate (DASS score 10-14) and severe (DASS score ≥15) anxiety symptoms were associated with increased odds of migraine (OR=2.28; 95%CI 1.24, 4.21; OR=1.77; 95%CI 0.93, 3.35, and OR=5.39; 95%CI 2.19, 13.24, respectively). Lastly, that the odds of migraine increased with increasing severity of stress symptoms (p-value for trend=0.062), though the test of linear trend did not reach statistical significance. Compared with the reference group (DASS stress score ≤14), those with severe stress (DASS score ≥26) had a 3.57-fold increased odds of migraine (OR=3.57; 95%CI 1.35, 9.46).
Table 4.
Odds ratio (OR) and 95% confidence intervals (CI) for migraine in relation to symptoms of depression, anxiety, and stress assessed using the Depression Anxiety Stress Scales (DASS-21)
| Migraine |
||||
|---|---|---|---|---|
| Psychiatric Symptoms | No (N=1,939) n (%) | Yes(N=212) n (%) | Unadjusted OR (95%CI) | *Adjusted OR (95% CI) |
| Depression | ||||
| Normal (0-9) | 1,823 (94.0) | 191 (89.6) | 1.00 (Reference) | 1.00 (Reference) |
| Mild (10-13) | 55 (2.8) | 8 (3.8) | 1.38 (0.65,2.95) | 1.31 (0.59,2.74) |
| Moderate (14-20) | 35 (1.8) | 10 (4.7) | 2.45 (1.16,5.19) | 2.29 (1.03,5.11) |
| Severe (≥21) | 12 (0.6) | 3 (1.4) | 2.38 (0.67,8.53) | 2.41 (0.65,8.90) |
| Missing | 14 (0.7) | 1 (0.4) | ||
| P-value for trend | 0.007 | 0.012 | ||
| Anxiety | ||||
| Normal (0-7) | 1,782 (91.9) | 179 (83.4) | 1.00 (Reference) | 1.00 (Reference) |
| Mild (8-9) | 62 (3.2) | 14 (6.6) | 2.24 (1.23,4.09) | 2.28 (1.24,4.21) |
| Moderate (10-14) | 67 (3.4) | 12 (5.6) | 1.78 (0.96,3.35) | 1.77 (0.93,3.35) |
| Severe (≥15) | 15 (0.8) | 8 (3.8) | 5.31 (2.22,12.69) | 5.39 (2.19,13.24) |
| Missing | 13 (0.6) | 1 (0.4) | ||
| P-value for trend | <0.001 | <0.001 | ||
| Stress | ||||
| Normal (0-14)) | 1,797 (94.4) | 196 (92.5) | 1.00 (Reference) | 1.00 (Reference) |
| Mild (15-18) | 61 (3.2) | 4 (1.9) | 0.60 (0.22,1.67) | 0.39 (0.12,1.29) |
| Moderate (19-25) | 31 (1.6) | 6 (2.8) | 1.77 (0.73,4.31) | 1.58 (0.64,3.90) |
| Severe (≥26) | 15 (0.8) | 6 (2.8) | 3.67 (1.41,9.56) | 3.57 (1.35,9.46) |
| Missing | 35 (1.8) | 0 (0.0) | ||
| P-value for trend | 0.002 | 0.062 | ||
Adjusted for age, sex, occupation, and body mass index
Discussion
In 2004, the WHO in partnership with the World Headache Alliance, the International Headache Society and the European Headache Federation launched the ‘Lifting The Burden: the Global Campaign to Reduce the Burden of Headache Worldwide’39. Since its inception, a number of studies have been conducted in South East Asia and Eastern Europe that increased our understanding of global burden of migraine and other headache disorders. Unfortunately, the burden of migraine morbidity has not been well studied or quantified among Africans 40. Our findings underscore the importance of understanding the epidemiology of migraine in understudied populations such as those in Ethiopia.
Historically it has been reported that migraine prevalence is greater among Caucasians than African Americans. However, in our present study we found a migraine prevalence among sub-Saharan African adults (9.9%) which is quite comparable to what is typically reported in predominantly Caucasian migraine cohorts41. As expected in our study, women were more than twice as likely to have migraine compared with men (OR=2.16; 95%CI: 1.62, 2.89). Further, and for the first time in a sub-Saharan African population, we also demonstrated that African migraineurs had increased odds of depressive (OR=2.37, 95%CI: 1.22, 4.61), anxiety (OR=2.32; 95%CI: 1.32, 3.38) and stress symptoms (OR= 2.26: 95%CI: 1.16, 4.38), when compared with non-migraineurs. Finally, self-reported health status has been shown to be a robust predictor of multiple adverse health outcomes42, 43. While the results of our study showing an association between migraine and self-reported health status (OR=1.62; 95%CI: 1.21, 2.16) was not surprising, the proportion of poor self-reported health status (40%) among our study participants was quite high which merits further investigation.
The prevalence of migraine among working adults in our study (9.9%) is higher than reported from some prior African studies7, 9, 44, 45 but lower than what is reported in Benin12 and Nigeria13. Collectively, the results of our study and those of others12, provide evidence to the hypothesis put forth by Stewart et al suggesting that migraine prevalence among Caucasians and African Americans are quite comparable. This suggests that greater attention and further research on the impact of migraine on health outcomes and work productivity specifically in African populations is warranted and remains in great need.
Another important issue that merits consideration is the emerging body of evidence on the growing burden of chronic disease including mental health46. According to the WHO, by 2030, depression alone is likely to be the single highest contributor to burden of disease in the world more so than heart disease, stroke, road traffic accidents, and HIV/AIDS 46. Importantly, studies conducted in sub-Saharan Africa among primary health clinic attendees show that 20-30% of patients present with depressive symptoms and other psychological disorders as the primary or secondary reason for seeking medical care [11-13]. Thus, increased recognition of migraine-psychiatric comorbidity will have important clinical and public health implications.
Our finding of strong association between migraine and major depression is largely consistent with previous studies. Swartz et al 5 using the Epidemiology Catchment Area Follow-up Study reported a similar strong association between migraine and major depression (OR=3.1; 95% CI 2.0,4.8). Similarly, Breslau et al 10 in demonstrated that the prevalence of major depression was 3-times higher in persons with migraine compared to controls. Similar findings were likewise reported by Merikangas et al 47 from their Zurich Cohort study where participants with migraine exhibited elevated rates of depression. Although the effect sizes are smaller than prior studies, a recent study from Canada48 found that those with migraine were 60% more likely to develop major depression (HR=1.6; 95%CI 1.3,1.9). The authors 48also noted participants with depression were 40% more likely to develop migraine (HR=1.4; 95%CI 1.0,1.9) showing the bidirectional relationship of migraine and depression.
Our current study showing strong association of migraine and anxiety are likewise in concordance with the existing body of evidence. Juang et al 49 in their cross-sectional study of headache clinic patients found the prevalence of anxiety disorders to be significantly higher in patients with transformed migraine after controlling for age and sex (P=.02). Similarly, Lanteri-Minet et al 50 in France reported that migraineurs were more likely to exhibit anxiety symptoms compared to participants without migraine (P<0.01). Similar findings were reported by Merikangas et al 47. In addition, as shown by Merikangas et al 47 most headache patients who present with depression tend to have co-occurring anxiety disorders. There is also another growing body of evidence showing the comorbidity of posttraumatic stress disorder and migraine51, 52 that merits future research consideration in understanding the anxiety-migraine causal pathway.
Previous data from Peterlin et al has demonstrated that migraineurs report more stressful life events (4.2±3.4 events) than those without headache (2.5±2.4 events), p<0.001 41. Similarly we found that severe stress was associated with more than a 3-fold (OR=3.57; 95%CI 1.35, 9.46) higher odds of having migraine attacks. While the nature of stress was not evaluated in our study there is some emerging literature showing work stress as a significant risk factor for incident migraine53, 54. Maki et al 53 in their study among female employees in Finland reported that 6.2% new migraine cases were attributed to work related stress. Lin et al 54 in their study among nursing staff in Taiwan found stress at work to be significantly associated with migraine (p<0.001). Clearly, studies that evaluate sources of stress and their contribution to migraine attacks among working professionals, are warranted to move this area of literature forward.
Etiologic theories regarding the pathophysiologic mechanisms of migraine and psychiatric disorders remain unclear. Most have speculated common underlying neuroendocrine mechanisms including dysregulation of the hypothalamic-pituitary adrenalin (HPA) axis and dysfunction in central serotonergic availability may explain the observed association 55, 56. In addition activation of the HPA axis results in increased secretion of corticotrophin-releasing factor and changes in cortisol secretion which might play an important role in the pathophysiology of migraine and psychiatric disorders 55. Although not fully developed, there is also an emerging literature suggesting shared genetic factors between migraine and depression. Recently conducted twin studies have found some evidence for a shared genetic vulnerability for depression and migraine57, 58.
Some caveats should be considered when interpreting the results of our study. First, the cross-sectional study design precludes delineation of the temporal relation between migraine and psychiatric disorders. Second, one of the ICHD criteria was not included (aggravation of pain with activity) thus may have excluded some participants who may have fulfilled migraine criteria. As a result, the magnitudes of associations and prevalence estimates reported here are likely to be conservative. Although we adjusted for several potential confounders, we cannot exclude the possibility of residual confounding due to misclassification of adjusted variables or confounding by other unmeasured variables. In addition, the classification of psychiatric disorders was done using screening instruments that do not give definitive diagnosis of depression and/or anxiety. However, use of validated instruments including PHQ-9 and DASS-21 remains the most feasible method of data collection for large-scale epidemiological studies. Finally, our study findings may not be generalized to the broader Ethiopian population since our study was limited to a largely well-educated, urban dwelling, occupational cohort comprised of white-collar professionals in banking and academic sectors. The concordance of our results with those from other studies that have included individuals from various socio-economic status and geographically diverse populations, however, serve to attenuate some concerns about the generalizability of our findings.
In conclusion, our study provided strong evidence that migraine (1) is highly prevalent and associated with psychiatric symptoms in an African population, and (2) is similar to historically reported rates in predominantly Caucasian populations. Migraine and comorbid psychiatric disorders have significant impact on the quality of life with substantial individual, organizational as well as societal costs59. They result in lost productivity especially among an urban workforce. Given personal and financial burden of migraine 59 the high prevalence of migraine and psychiatric comorbidities in the present African cohort has clinical and public health importance. Specifically, the under-recognition of migraine and migraine Comorbidities in African populations may translate into under-treatment as well as negative personal and financial implications for African migraine sufferers. Thus, our current study strongly supports greater attention and further migraine research in African populations.
Acknowledgment
This research was supported by an award from the National Institutes of Health, National Institute of Minority Health and Health Disparities (T37-MD001449). The authors wish to thank the staff of Addis Continental Institute of Public Health for their expert technical assistance. The authors would also like to thank the participants in the study, the Commercial Bank of Ethiopia and the Addis Ababa Education Office for granting access to conduct the study.
Footnotes
Conflict of Interest:
None to declare
References
- 1.Burton WN, Landy SH, Downs KE, Runken MC. The impact of migraine and the effect of migraine treatment on workplace productivity in the United States and suggestions for future research. Mayo Clinic proceedings Mayo Clinic. 2009;84:436–445. doi: 10.1016/S0025-6196(11)60562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen BK. Epidemiology of headache. Cephalalgia. 1995;15:45–68. doi: 10.1046/j.1468-2982.1995.1501045.x. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Word Health Organization [January 16, 2012];Headache disorders.Fact sheet N°277. 2004 Available at: http://www.who.int/mediacentre/factsheets/fs277/en/
- 4.Mateen FJ, Dua T, Steiner T, Saxena S. Headache disorders in developing countries: research over the past decade. Cephalalgia. 2008;28:1107–1114. doi: 10.1111/j.1468-2982.2008.01681.x. [DOI] [PubMed] [Google Scholar]
- 5.Dent W, Stelzhammer B, Meindl M, Matuja WB, Schmutzhard E, Winkler AS. Migraine attack frequency, duration, and pain intensity: disease burden derived from a community-based survey in northern Tanzania. Headache. 2011;51:1483–1492. doi: 10.1111/j.1526-4610.2011.02009.x. [DOI] [PubMed] [Google Scholar]
- 6.Amayo EO, Jowi JO, Njeru EK. Migraine headaches in a group of medical students at the Kenyatta National Hospital, Nairobi. East African medical journal. 1996;73:594–597. [PubMed] [Google Scholar]
- 7.Dent W, Spiss H, Helbok R, Matuja W, Scheunemann S, Schmutzhard E. Prevalence of migraine in a rural area in South Tanzania: a door-to-door survey. Cephalalgia. 2004;24:960–966. doi: 10.1111/j.1468-2982.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 8.Omoti AE, Waziri-Erameh MJ. Pattern of neuro-ophthalmic disorders in a tertiary eye centre in Nigeria. Nigerian journal of clinical practice. 2007;10:147–151. [PubMed] [Google Scholar]
- 9.Tekle Haimanot R, Seraw B, Forsgren L, Ekbom K, Ekstedt J. Migraine, chronic tension-type headache, and cluster headache in an Ethiopian rural community. Cephalalgia. 1995;15:482–488. doi: 10.1046/j.1468-2982.1995.1506482.x. [DOI] [PubMed] [Google Scholar]
- 10.Takele GM, Tekle Haimanot R, Martelletti P. Prevalence and burden of primary headache in Akaki textile mill workers, Ethiopia. J Headache Pain. 2008;9:119–128. doi: 10.1007/s10194-008-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IHS The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 12.Adoukonou T, Houinato D, Kankouan J, et al. Migraine among university students in Cotonou (Benin). Headache. 2009;49:887–893. doi: 10.1111/j.1526-4610.2009.01408.x. discussion 894. [DOI] [PubMed] [Google Scholar]
- 13.Ofovwe GE, Ofili AN. Prevalence and impact of headache and migraine among secondary school students in Nigeria. Headache. 2010;50:1570–1575. doi: 10.1111/j.1526-4610.2010.01776.x. [DOI] [PubMed] [Google Scholar]
- 14.Moschiano F, D'Amico D, Canavero I, Pan I, Micieli G, Bussone G. Migraine and depression: common pathogenetic and therapeutic ground? Neurol Sci. 2011;32(Suppl 1):S85–88. doi: 10.1007/s10072-011-0545-0. [DOI] [PubMed] [Google Scholar]
- 15.Radat F, Swendsen J. Psychiatric comorbidity in migraine: a review. Cephalalgia. 2005;25:165–178. doi: 10.1111/j.1468-2982.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 16.Baskin SM, Lipchik GL, Smitherman TA. Mood and anxiety disorders in chronic headache. Headache. 2006;46(Suppl 3):S76–87. doi: 10.1111/j.1526-4610.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 17.Burton WN, Conti DJ, Chen CY, Schultz AB, Edington DW. The economic burden of lost productivity due to migraine headache: a specific worksite analysis. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2002;44:523–529. doi: 10.1097/00043764-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Wai WS, Dhami RS, Gelaye B, et al. Comparison of Measures of Adiposity in Identifying Cardiovascular Disease Risk Among Ethiopian Adults. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran A, Gelaye B, Girma B, et al. Prevalence of Metabolic Syndrome among Working Adults in Ethiopia. Int J Hypertens. 2011;2011:193719. doi: 10.4061/2011/193719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . Primary Health Care Management of Trachoma. World Health Organization; Geneva: 1993. [Google Scholar]
- 21.World Health Organization . STEPs manual. World Health Organization; Geneva: 2008. [Google Scholar]
- 22.Belew M, Kebede D, Kassaye M, Enquoselassie F. The magnitude of khat use and its association with health, nutrition and socio-economic status. Ethiop Med J. 2000;38:11–26. [PubMed] [Google Scholar]
- 23.Kalix P. Khat: scientific knowledge and policy issues. Br J Addict. 1987;82:47–53. doi: 10.1111/j.1360-0443.1987.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 24.Henry P, Michel P, Brochet B, Dartigues JF, Tison S, Salamon R. A nationwide survey of migraine in France: prevalence and clinical features in adults. GRIM. Cephalalgia : an international journal of headache. 1992;12:229–237. doi: 10.1046/j.1468-2982.1992.1204229.x. discussion 186. [DOI] [PubMed] [Google Scholar]
- 25.ICHD The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):S24–101. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adewuya AO, Ola BA, Afolabi OO. Validity of the patient health questionnaire (PHQ-9) as a screening tool for depression amongst Nigerian university students. J Affect Disord. 2006;96:89–93. doi: 10.1016/j.jad.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Monahan PO, Shacham E, Reece M, et al. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. J Gen Intern Med. 2009;24:189–197. doi: 10.1007/s11606-008-0846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omoro SA, Fann JR, Weymuller EA, Macharia IM, Yueh B. Swahili translation and validation of the Patient Health Questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med. 2006;36:367–381. doi: 10.2190/8W7Y-0TPM-JVGV-QW6M. [DOI] [PubMed] [Google Scholar]
- 30.Lovibond PF. Long-term stability of depression, anxiety, and stress syndromes. Journal of abnormal psychology. 1998;107:520–526. doi: 10.1037//0021-843x.107.3.520. [DOI] [PubMed] [Google Scholar]
- 31.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour research and therapy. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 32.Cheung HN, Power MJ. The development of a new multidimensional depression assessment scale: preliminary results. Clinical psychology & psychotherapy. 2012;19:170–178. doi: 10.1002/cpp.1782. [DOI] [PubMed] [Google Scholar]
- 33.Reise SP, Widaman KF, Pugh RH. Confirmatory factor analysis and item response theory: two approaches for exploring measurement invariance. Psychological bulletin. 1993;114:552–566. doi: 10.1037/0033-2909.114.3.552. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization . Global status report on alcohol. WHO, Department of Mental Health and Substance Abuse; Geneva: 2004. [Google Scholar]
- 35.WHO . Report of a WHO Expert Committee. World Health Organization; Geneva: 1995. Physical status: the use and interpretation of anthropometry. [PubMed] [Google Scholar]
- 36.Brown LD, Cai TT, DasGupta A. Interval Estimation for a Binomial Proportion. Statistical Science. 2001;16:101–133. [Google Scholar]
- 37.Rothman KJ, Greenland S. Modern epidemiology. Lippincott-Raven; Philadelphia: 1998. [Google Scholar]
- 38.Evans RW, Williams MA, Rapoport AM, Peterlin BL. The association of obesity with episodic and chronic migraine. Headache. 2012;52:663–671. doi: 10.1111/j.1526-4610.2012.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steiner TJ. Lifting the burden: The global campaign against headache. Lancet neurology. 2004;3:204–205. doi: 10.1016/S1474-4422(04)00703-3. [DOI] [PubMed] [Google Scholar]
- 40.Steiner TJ, Birbeck GL, Jensen R, Katsarava Z, Martelletti P, Stovner LJ. Lifting the burden: the first 7 years. J Headache Pain. 2010;11:451–455. doi: 10.1007/s10194-010-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipton RB, Scher AI, Kolodner K, Liberman J, Steiner TJ, Stewart WF. Migraine in the United States: epidemiology and patterns of health care use. Neurology. 2002;58:885–894. doi: 10.1212/wnl.58.6.885. [DOI] [PubMed] [Google Scholar]
- 42.Idler EL, Angel RJ. Self-rated health and mortality in the NHANES-I Epidemiologic Follow-up Study. American journal of public health. 1990;80:446–452. doi: 10.2105/ajph.80.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Idler EL, Russell LB, Davis D. Survival, functional limitations, and self-rated health in the NHANES I Epidemiologic Follow-up Study, 1992. First National Health and Nutrition Examination Survey. American journal of epidemiology. 2000;152:874–883. doi: 10.1093/aje/152.9.874. [DOI] [PubMed] [Google Scholar]
- 44.Winkler AS, Dent W, Stelzhammer B, et al. Prevalence of migraine headache in a rural area of northern Tanzania: a community-based door-to-door survey. Cephalalgia. 2010;30:582–592. doi: 10.1111/j.1468-2982.2009.01994.x. [DOI] [PubMed] [Google Scholar]
- 45.Houinato D, Adoukonou T, Ntsiba F, Adjien C, Avode DG, Preux PM. Prevalence of migraine in a rural community in south Benin. Cephalalgia. 2010;30:62–67. doi: 10.1111/j.1468-2982.2009.01894.x. [DOI] [PubMed] [Google Scholar]
- 46.WHO . The Global Burden of Disease 2004 Update. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- 47.Merikangas KR, Merikangas JR, Angst J. Headache syndromes and psychiatric disorders: association and familial transmission. Journal of psychiatric research. 1993;27:197–210. doi: 10.1016/0022-3956(93)90008-p. [DOI] [PubMed] [Google Scholar]
- 48.Modgill G, Jette N, Wang JL, Becker WJ, Patten SB. A Population-Based Longitudinal Community Study of Major Depression and Migraine. Headache. 2011 doi: 10.1111/j.1526-4610.2011.02036.x. [DOI] [PubMed] [Google Scholar]
- 49.Juang KD, Wang SJ, Fuh JL, Lu SR, Su TP. Comorbidity of depressive and anxiety disorders in chronic daily headache and its subtypes. Headache. 2000;40:818–823. doi: 10.1046/j.1526-4610.2000.00148.x. [DOI] [PubMed] [Google Scholar]
- 50.Lanteri-Minet M, Radat F, Chautard MH, Lucas C. Anxiety and depression associated with migraine: influence on migraine subjects’ disability and quality of life, and acute migraine management. Pain. 2005;118:319–326. doi: 10.1016/j.pain.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Peterlin BL, Rosso AL, Sheftell FD, Libon DJ, Mossey JM, Merikangas KR. Post-traumatic stress disorder, drug abuse and migraine: new findings from the National Comorbidity Survey Replication (NCS-R). Cephalalgia. 2011;31:235–244. doi: 10.1177/0333102410378051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peterlin BL, Tietjen GE, Brandes JL, et al. Posttraumatic stress disorder in migraine. Headache. 2009;49:541–551. doi: 10.1111/j.1526-4610.2009.01368.x. [DOI] [PubMed] [Google Scholar]
- 53.Maki K, Vahtera J, Virtanen M, Elovainio M, Keltikangas-Jarvinen L, Kivimaki M. Work stress and new-onset migraine in a female employee population. Cephalalgia. 2008;28:18–25. doi: 10.1111/j.1468-2982.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 54.Lin KC, Huang CC, Wu CC. Association between stress at work and primary headache among nursing staff in Taiwan. Headache. 2007;47:576–584. doi: 10.1111/j.1526-4610.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 55.Overeem S, van Vliet JA, Lammers GJ, Zitman FG, Swaab DF, Ferrari MD. The hypothalamus in episodic brain disorders. Lancet neurology. 2002;1:437–444. doi: 10.1016/s1474-4422(02)00191-6. [DOI] [PubMed] [Google Scholar]
- 56.McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Schur EA, Noonan C, Buchwald D, Goldberg J, Afari N. A twin study of depression and migraine: evidence for a shared genetic vulnerability. Headache. 2009;49:1493–1502. doi: 10.1111/j.1526-4610.2009.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stam AH, de Vries B, Janssens AC, et al. Shared genetic factors in migraine and depression: evidence from a genetic isolate. Neurology. 2010;74:288–294. doi: 10.1212/WNL.0b013e3181cbcd19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jette N, Patten S, Williams J, Becker W, Wiebe S. Comorbidity of migraine and psychiatric disorders--a national population-based study. Headache. 2008;48:501–516. doi: 10.1111/j.1526-4610.2007.00993.x. [DOI] [PubMed] [Google Scholar]