Abstract
Objective
To review the current state of cerebral stimulation for neuropathic pain and to propose that cerebral stimulation should aim at the affective sphere of chronic pain rather than solely focusing on the primary sensory-discriminative sphere.
Methods
The past and current goals of cerebral stimulation are reviewed as well as its limitations. A novel deep brain stimulation approach is proposed to evaluate this conceptual shift fromsomatosensory to affective sphere of pain targeting
Approach
Thalamic and other central pain syndromes aretypically intractable to current treatment methods, including cerebral neuromodulation of somatosensory pathways, leading to long-term distress and disability. Our modern understanding of chronic pain pathophysiology is based largely on the neuromatrix theory, where cognitive, affective and sensory-discriminative spheres contribute equally to the overall pain experience. During the last decade, the safety and feasibility of chronic stimulation of neural pathways related to mood and affect has been explored with promising results. Here, we propose a novel approach to modulate the affective sphere of chronic pain by targeting similar networks in patients with treatment-refractory central pain. Our primary goal is not to produce (or measure) analgesia, but rather to modulate the affective burden of chronic.
Discussion
Cerebral neuromodulation for neuropathic pain has had limited efficacy thus far. Shifting our aim to neural networks related to the affective sphere of pain may allow us to reduce pain conditioning and pain-related disability. Our ultimate goal is to promote rehabilitation from chronic pain - social and occupational.
Introduction
Chronic pain is a leading cause of disability, resulting in enormous impact to individual health and the economy. Although back pain is perhaps the most common chronic pain disorder, as the second leading complaint in outpatient consults and the third in hospital admissions1, there are multiple pain syndromes that contribute to the overall burden of pain related disability. Neuropathic pain, defined as “pain initiated or caused by a primary lesion or dysfunction in the nervous system”2 has an estimated prevalence of 8% among adults enrolled in a family practice 3. Central pain, otherwise known as post-stroke pain, is a less common form of neuropathic pain4 that can be particularly devastating, due to its severity and refractoriness to management 5. Management of central pain has interested neurosurgeons for several decades, but remains a challenge6-7. This is likely due to the medical refractoriness of the disorder, lack of other therapeutic options as well as presence of an associated CNS lesion. Surgical management of these disorders is challenging and often frustrating, with several failed brain targets evaluated to date. However, the mechanistic hypothesis behind all of the targets evaluated thus far has emphasized modulation of the somatosensory pathways to produce analgesia, with outcomes generally based upon measurements made using a numerical pain scale. We think it is time to depart from this approach. Psychological factors are known to influence recovery, recurrence rates and the probability of returning to work for patients with chronic pain syndromes 8-11. Indeed, our understanding of chronic pain physiopathology is not limited to the sensory pathways but, rather, includes significant involvement of neural networks related to its cognitive and affective spheres. This is corroborated by the prior experience with ablative stereotactic procedures for chronic pain, in selected patients12. Although ablative procedures continue to be a relevant technical option in stereotactic and functional neurosurgery13, the reversibility of neuromodulatory techniques is an important safety feature. This can be particularly beneficial when exploring new targets and indications. As such, we propose a novel approach, utilizing neuromodulatory techniques such as deep brain stimulation (DBS) to target the networks related to the affective sphere – the suffering - of chronic pain. The goal also differs from simple analgesia. Instead of measuring outcome as a percentage of patients with greater than 50% pain relief, our intent is to reduce pain anticipation, suffering and, most importantly, pain related disability. If this approach proves to be successful in the most treatment-refractory conditions, it may also be a useful alternative or adjunct in the management of other disabling pain conditions.
Invasive neurostimulation for central pain and its current limitations
Several clinical trials and retrospective case series have examined the potential role of neurostimulation, including both motor cortex stimulation (MCS) and DBS, in the management of central pain6, 14-20. Traditionally, DBS of the sensory thalamus has been reserved for neuropathic pain 6, 15 while periventricular gray (PVG) DBS, the therapeutic effects of which are mediated at least in part by endorphin release 16, 21, has been thought to be most effective for nociceptive or axial pain. This dichotomy has been challenged recently however, with at least one group showing that PVG stimulation may be effective in neuropathic deafferentation syndromes 14. Even though thalamic and central gray DBS has shown promising results for managing chronic treatment refractory pain in some patients with peripheral deafferentation 18, 22-23, it has not proven consistently effective for patients with central pain 19-20. Recent work by the Oxford group has focused on PVG stimulation for central pain. In a non-controlled study, Nandi et al reported that six of eight patients reported “satisfactory” relief of pain, representing approximately 30% improvement from the preoperative baseline24.
Motor cortex stimulation was proposed as a treatment for central pain, largely in response to growing frustration with inadequate DBS efficacy in this patient population25-26. However, the initial optimism derived from the preclinical model25 and from the first clinical trial26, was subsequently tempered when a trial comparing the effects of MCS on peripheral or central deafferentation in humans failed to observe efficacy in the latter group 27. Subsequent, larger series of MCS continued to report some efficacy with this technique, particularly for trigeminal neuropathic pain. Nguyen and colleagues followed 32 patients for an average of 27 months, reporting that five of 13 patients with central pain and nine of 12 patients with facial pain presented with “good” pain relief28. A gradual loss of efficacy was observed in six patients, however benefit was re-established through repositioning of the epidural leads assisted by neuronavigation. A similar decay of efficacy was also reported by Henderson and colleagues29 and pain relief was recovered with intense programming. Nuti and colleagues30 reported on a mean follow-up of four years in a series of 31 patients with central pain. Approximately 50% of patients reported greater than 40% pain relief in this non-controlled series. A two-center sham-controlled series of 10 patients was reported by Nguyen and colleagues, in which patients underwent two weeks of no stimulation or two weeks of active stimulation followed by double-blind cross-over. During the blinded phase, VAS pain scores reduced from a mean of 78 to 53 (32% improvement). Two of three patients with post-stroke pain did not respond. Although it was initially hypothesized that MCS would address the limitations of DBS in managing central pain, its effects largely replicated the results of prior DBS series, proving to be more effective for patients with peripheral deafferentation than central pain 31-32. The gap between the promising results of MCS in the animal model and the clinical trials outcomes may be partially explained by the location of the central lesion. While the feline model tested the effects of MCS following spinal cord injury33, the first human translation was attempted in patients with post-stroke pain26-27. Even though both represent CNS lesions per se, spinal cord injuries spare the corticothalamic pathways that are likely to contribute to the therapeutic mechanisms of MCS. Two decades following the first MCS studies, four decades after Akil’s central gray stimulation and five decades following the gate control theory we still do not have a reliable treatment for patients with chronic pain secondary to central lesions. The variety of brain regions that have been targeted and failed all have held the common goal of trying to produce analgesia through modulation of somatosensory pathways. In retrospect, it may be that the hypothesis itself was mis-postulated, with DBS or MCS doomed to fail in modulating a pathway that has been largely damaged in its central relay. This notion is underscored by the severity of pinprick anesthesia observed in many patients with thalamic pain syndrome.
A new approach for targeting the brain for chronic pain disorders
Knowledge of the different effects of DBS and MCS for peripheral and central pain syndromes has advanced along neurosurgical experience over the last decades. At the same pace, our understanding of physiopathology of chronic pain has also advanced dramatically. While modern investigation of neuromodulation for pain was instigated by the gate control theory in 196534, the current systems level understanding of chronic pain is largely based on Melzack’s theoretical re-postulation of pain related networks in the neuromatrix35. Under this ideology, sensory-discriminative pathways interact – with equal importance – with affective-motivational and cognitive-evaluative spheres to create the final pain experience (figure 1). While targeting neural pathways related to the sensory-discriminative sphere remains valid and clinically effective 36 for some patients, it may not be a viable option for patients presenting with little or no residual neurological substrate to mediate the therapeutic effect. In such cases, targeting an anatomically spared pathway may be a reasonable option.
Figure 1.
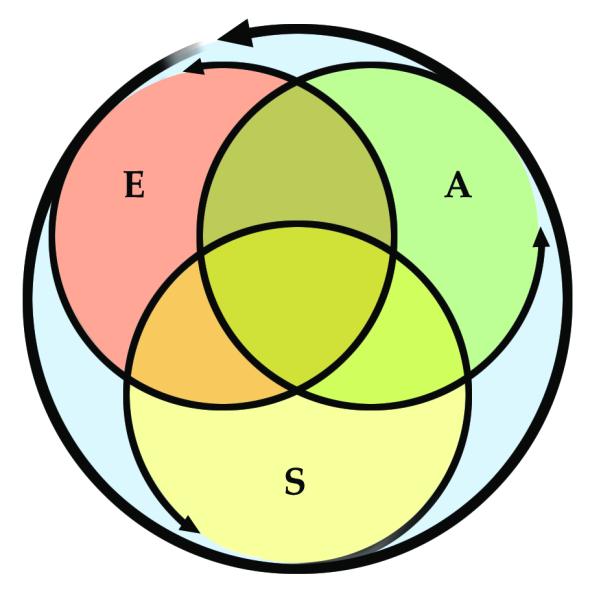
Modified from Melzack et al 1999. Pain experience is not determined by the intensity of activation of somatosensory pathways only. Pain processing involves also affective and cognitive spheres of interpretation. For example, the context in which the painful stimulus occurs can dramatically change the final perception of pain. The affective sphere can gain greater importance in chronic pain, when the experience is no longer dependent on external stimuli. This is particularly relevant in chronic deafferentation pain, when patients experience unrelenting continuous pain as a result of the central deafferentation. A: Affective, E: Evaluative, S: Sensory
It is also time to reconsider analgesia as the treatment goal. The current yardstick for outcomes in pain neuromodulation studies is the visual analog (or equivalent) scale. While this tool remains useful for bedside monitoring of acute interventions for acute pain, it can be misleading when managing chronic pain and fails to correlate with patient satisfaction or disability37. Instead of attempting to index analgesia by the percentage of reduction in pain intensity following intervention, a more reliable metric of therapeutic efficacy following neurostimulation would be improvement in pain-related disability38-39. Interventions that successfully facilitate rehabilitation and reduce dependence for self care should be regarded as valuable to patients and to society even if patients continue to report some persistence of measurable pain.
We postulate that chronic pain-related disability is not solely the result of the intensity of pain at a given point in time, but rather is profoundly influenced by the expectation of unrelenting pain. This is further aggravated by pain reinforcement caused by daily experiencing of allodynia when the affected side is used or touched. As such, reducing pain anticipation related to the use of the affected limb (or hemibody) and reducing pain fear – or dread – may also reduce disability. We propose that we shift our targeting choice away from neural pathways related to the sensory discriminative sphere of pain and focus on the neural networks related to the affective-motivational sphere. This is timely. We are now experiencing a renaissance of careful investigation of surgery for psychiatric disorders. Most relevant to this approach is the recent experience in DBS for obsessive compulsive disorder and depression.
Neural networks related to mood and affect: Recent DBS experience
Cortical control of emotion is manifested through processing within the circuit of Papez and the orbitofrontal cortico-striato-pallido-thalamo-cortical (CSPTC) system. The orbitofrontal cortex shares massive reciprocal excitatory projections with the thalamic mediodorsal nucleus, through the ventral anterior limb of the internal capsule (ALIC). This excitatory intercommunication is modulated by a longer loop, initiated by fibers that project from the orbitofrontal cortex to the ventral striatum, also passing through the ALIC. These fibers, in turn, connect with the ventral pallidum, which maintains inhibitory projections that further modulate thalamocortical activity40. This circuitry is highly implicated in the control of emotion and behavior as well as in the pathogenesis of psychiatric disorders41. Anterior capsulotomy, a stereotactic procedure in which the reciprocal projections between orbitofrontal cortex and thalamus are lesioned, has been shown to alleviate obsessive symptoms and anxiety. This corroborates the importance of the fibers projecting through the ALIC in the control of emotion and behavior. The densely arranged fibers bundles through the ventral ALIC connecting the orbitofrontal cortex to the mediodorsal thalamic nucleus and to the ventral striatum, makes it an ideal stereotactic target. Other surgical targets need to also be taken in consideration, including the anterior cingulate areas. There is robust literature supporting the efficacy of radiofrequency cingulotomy for obsessive compulsive disorder13, 42, chronic cancer pain12, 43 and chronic non-cancer pain44. . The procedure was also shown to be safe. In the Massachusetts General Hospital’s series of more than 800 cingulotomies, there were no reported deaths and two intracranial hemorrhages. As for other ablative procedures aimed at non-motor networks, there is a concern for cognitive changes that may be observed on follow-up45-46. Nevertheless, the efficacy of cingulotomy in alleviating pain conditions supports the concept of targeting subcortical networks related to affect and behavior with DBS for treatment-refractory neuropathic pain 47.
Deep brain stimulation has revolutionized the field of stereotactic and functional neurosurgery in large part because of its superior safety profile compared to ablative procedures such as thalamotomy or capsulotomy. Although effective, recent investigation has shown significant risk for deterioration of executive function following capsulotomy 48. DBS is reversible and adjustable, allowing for adverse effects to be managed and stimulation to be activated or deactivated as needed. Nutin et al pioneered DBS of the ventral region of the ALIC in a group of patients with OCD49. The positive results led to subsequent exploration by other groups, demonstrating that DBS of the ventral ALIC region is safe and effective for OCD. Executive function has been prospectively assessed in patients undergoing long-term DBS of this area and permanent adverse effects were not seen 50.
In 2009 our group reported on the long-term effects of DBS of the ventral capsule and ventral striatal area (VC/VS) in patients with severe and treatment refractory depression51. This open label study showed a significant reduction in the two main outcome measures, the Montgomery-Asbrg Depression Rating Scale (MADRS) and Hamilton Depression Rating Scale (HDRS). The approximately 50% reduction occurred over three months and was sustained at 12-months of follow-up. Although confirmation from a controlled trial is still needed, the results are encouraging and demonstrate that it is feasible and safe to chronically modulate these networks in the human brain. Subsequent work in a subset of patients was conducted to evaluate the acute effects of stimulation in the VC/VS area and to study the topographic organization of the target region 52. It was observed that the most ventral contacts of the quadripolar DBS lead were consistently associated with both acute and chronic changes in mood and anxiety. These contacts correspond to the topography of the ventral striatal and the ventral-most part of the anterior limb of the internal capsule (ALIC). Contacts positioned more dorsally in the ALIC typically were not associated with changes in affect and behavior. Interestingly, the effects on the network can be seen acutely during surgery and subsequently reproduced with stimulation of the same sub-regions during outpatient programming. Electrode contacts and corresponding settings that produce positive changes in mood and anxiety were successfully selected for long-term stimulation. A follow-up study utilizing DBS modeling techniques showed that, in the topography of the ventral anterior capsule, pathways that coursed laterally were more commonly associated with positive responses53. We propose that the same pathways that have been safely targeted for OCD and depression may also be viable targets for modulating the affective sphere of chronic pain.
Deep brain stimulation of the ventral striatum and ventral capsular area for patients with chronic pain
As discussed above, we propose a conceptual shift in surgical target selection for central pain, one that moves away from emphasizing a compromised sensory-discriminative system and concentrates instead on networks related to the affective-motivational sphere of chronic pain. Such targets may be cortical (e.g., orbitofrontal, dorsolateral, insular, cingular) or subcortical, including the VC/VS area, mesial thalamus and Broadmann’s areas 24 and 25.
Based on our experience with DBS of the VC/VS area, its safety in patients with OCD and treatment refractory depression and our recent work on the functional topography of the area, we proposed the VC/VS target as a new conceptual approach to the treatment of central pain (figure 2). We are currently conducting the first federally funded prospective, double-blinded, controlled trial of VC/VS DBS aimed at modulating the affective sphere of thalamic pain syndrome54. We recognize the significant leap that this clinical trial proposes, regardless of the prior demonstrations of safety of VC/VS DBS in other patient populations. In order to ascertain well informed consent of participants and proper oversight, the clinical study is conducted not only under Institutional Review Board approval but also with federal oversight via a physician sponsored Food and Drug Administration Investigational Device Exemption. The primary outcome measures of the study is the pain disability index, which was selected to evaluate if modulation of the affective sphere of pain will alleviate disability and promote independence. Other outcome measures are incorporated to monitor the safety of DBS implantation and chronic stimulation, including depression and anxiety rating scales as well as detailed pre- and post-surgical cognitive assessments. Patients are prospectively evaluated with magnetoencephalography and functional magnetic resonance imaging in order to evaluate possible mechanisms underlying clinical effects.
Figure 2.
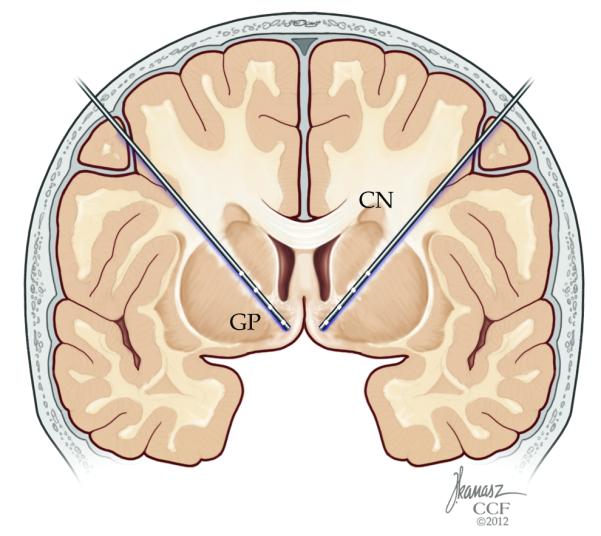
Targeting of the ventral striatal and ventral capsular area has been has been shown to be safe in patients with obsessive compulsive disorder as well as treatment refractory depression. In open label studies, it was shown that it is possible to modulate networks related to mood and affect. This figure illustrates deep brain stimulation targeting along the anterior limb of the internal capsule into the ventral striatal area, as intended in the ongoing clinical trial.
We anticipate that the results of this clinical trial will shed light on the role of the networks associated with mood and affect on the affective sphere of intractable chronic pain disorders. We expect that this structured evaluation of our conceptual approach will motivate our group and others to systematically evaluate targets related to the affective-motivational sphere in patients with central pain syndrome or other chronic pain disorders. We hope that future studies will be carried out in such fashion as to evaluate specific targets for specific pain disorders in controlled studies, thus avoiding the interpretational confounds imposed by studies that test a number of targets on heterogeneous populations. Further, we call upon our peers to favor quality of life and disability indices to measure the outcome of neuromodulatory interventions for chronic pain, thus retiring the visual analog pain scale as the yardstick of our field.
Acknowledgments
Funding: This work is funded by NIH New Innovator Award OD00646
Footnotes
Authorship: Drs. Machado, Baker and Malone contributed with the initial idea and concept of targeting neuromodulation in the networks related to the affective sphere of chronic pain. Dr. Machado wrote the initial draft with Dr. Ela Plow and this was extensively edited by Dr. Baker and Dr. Malone until a final version was achieved. Drs. Plow and Malone are working with Dr. Machado (the study PI) in advancing these concepts with invasive and non-invasive stimulation. Their contributions were key to the final manuscript. . All authors approved the final manuscript.
Conflicts of Interest Authors Andre G. Machado and Kenneth B. Baker have conflict of interest related to intellectual property assigned to Cleveland Clinic companies IntElect Medical, Cardionomics and ATI. The Conflict of Interest committee has managed these conflicts and a research plan has been approved.
Andre G. Machado was a consultant, member of the Member of Scientific Advisory Board and owner of stock or stock options for Intelect Medical / Boston Scientific (Intelect was recently purchased by Boston). This conflict ended in the beginning of 2011. Dr. Machado also owns stock or stock options for Cardionomics and ATI, and serves as a consultant for Monteris.
Kenneth B. Baker was a consultant and owner of stock or stock options for Intelect Medical / Boston Scientific (Intelect was recently purchased by Boston). This conflict ended in the beginning of 2011. Dr. Baker also owns stock or stock options for Cardionomics and ATI.
Ela Plow has no conflicts of interest to disclose.
Donald A. Malone has received research funding from Medtronic.
Cerebral stimulation for the affective component of neuropathic pain Andre G. Machado, M.D., Ph.D., Kenneth B. Baker, Ph.D., Ela Plow, Ph.D., Donald A. Malone, M.D.
References
- 1.Deyo RA, Weinstein JN. Low back pain. The New England journal of medicine. 2001 Feb 1;344(5):363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 2.Turk D, Okifuji A. Pain terms and taxonomies of pain. In: Loeser J, Butler S, Chapman C, Turk D, editors. Bonica’s management of pain. Lippincott Williams & Wilkins; 2001. pp. 17–19. [Google Scholar]
- 3.Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. Apr. 2006;7(4):281–289. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain. 1995 May;61(2):187–193. doi: 10.1016/0304-3959(94)00144-4. [DOI] [PubMed] [Google Scholar]
- 5.Machado A, Azmi H, Rezai AR. Motor cortex stimulation for refractory benign pain. Clin Neurosurg. 2007;54:70–77. [PubMed] [Google Scholar]
- 6.Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic and internal capsule stimulation for the control of central pain. Surg Neurol. 1975 Jul;4(1):91–92. [PubMed] [Google Scholar]
- 7.Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans. Report of 122 cases (1970-1984) J Neurosurg. 1986 Apr;64(4):543–553. doi: 10.3171/jns.1986.64.4.0543. [DOI] [PubMed] [Google Scholar]
- 8.Kall LB. Psychological determinants of quality of life in patients with whiplash associated disorders-a prospective study. Disability and rehabilitation. 2009;31(3):227–236. doi: 10.1080/09638280801912030. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. The Journal of the American Academy of Orthopaedic Surgeons. 2006 Jul;14(7):397–405. doi: 10.5435/00124635-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberger PH, Kerns R, Jokl P, Ickovics JR. Mood and attitude predict pain outcomes following arthroscopic knee surgery. Ann Behav Med. 2009 Feb;37(1):70–76. doi: 10.1007/s12160-008-9078-z. [DOI] [PubMed] [Google Scholar]
- 11.Wall CL, Ogloff JR, Morrissey SA. The psychology of injured workers: health and cost of vocational rehabilitation. Journal of occupational rehabilitation. 2006 Dec;16(4):513–528. doi: 10.1007/s10926-006-9051-2. [DOI] [PubMed] [Google Scholar]
- 12.Ballantine HT, Jr., Cassidy WL, Flanagan NB, Marino R., Jr. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967 May;26(5):488–495. doi: 10.3171/jns.1967.26.5.0488. [DOI] [PubMed] [Google Scholar]
- 13.Cosgrove GR, Rauch SL. Stereotactic cingulotomy. Neurosurg Clin N Am. 2003 Apr;14(2):225–235. doi: 10.1016/s1042-3680(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 14.Owen SL, Green AL, Nandi DD, Bittar RG, Wang S, Aziz TZ. Deep brain stimulation for neuropathic pain. Acta Neurochir Suppl. 2007;97(Pt 2):111–116. doi: 10.1007/978-3-211-33081-4_13. [DOI] [PubMed] [Google Scholar]
- 15.Hosobuchi Y, Adams JE, Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol. 1973 Sep;29(3):158–161. doi: 10.1001/archneur.1973.00490270040005. [DOI] [PubMed] [Google Scholar]
- 16.Akil H, Mayer DJ, Liebeskind JC. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976 Mar 5;191(4230):961–962. doi: 10.1126/science.1251210. [DOI] [PubMed] [Google Scholar]
- 17.Richardson DE. Brain stimulation for pain control. IEEE Trans Biomed Eng. 1976 Jul;23(4):304–306. doi: 10.1109/tbme.1976.324589. [DOI] [PubMed] [Google Scholar]
- 18.Kumar K, Toth C, Nath RK. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 1997 Apr;40(4):736–746. doi: 10.1097/00006123-199704000-00015. discussion 746-737. [DOI] [PubMed] [Google Scholar]
- 19.Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2001 Sep;2(3):183–192. doi: 10.1046/j.1526-4637.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- 20.Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 1987 Dec;21(6):885–893. doi: 10.1227/00006123-198712000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Richardson DE, Akil H. Long term results of periventricular gray self-stimulation. Neurosurgery. 1977 Sep-Oct;1(2):199–202. doi: 10.1097/00006123-197709000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Hamani C, Schwalb JM, Rezai AR, Dostrovsky JO, Davis KD, Lozano AM. Deep brain stimulation for chronic neuropathic pain: Long-term outcome and the incidence of insertional effect. Pain. 2006 Jun 21; doi: 10.1016/j.pain.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Gorecki J, Hirayama T, Dostrovsky JO, Tasker RR, Lenz FA. Thalamic stimulation and recording in patients with deafferentation and central pain. Stereotact Funct Neurosurg. 1989;52(2-4):219–226. doi: 10.1159/000099504. [DOI] [PubMed] [Google Scholar]
- 24.Nandi D, Aziz T, Carter H, Stein J. Thalamic field potentials in chronic central pain treated by periventricular gray stimulation -- a series of eight cases. Pain. 2003 Jan;101(1-2):97–107. doi: 10.1016/s0304-3959(02)00277-4. [DOI] [PubMed] [Google Scholar]
- 25.Hirayama T, Tsubokawa T, Yamamoto T. Chronic changes in activity of thalamic relay neurons following spino thalamic tractotomy in cat. Pain. 1990;5(Suppl):273. [Google Scholar]
- 26.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:12, 137–139. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- 27.Meyerson BA, Lindblom U, Linderoth B, Lind G, Herregodts P. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl (Wien) 1993;58:150–153. doi: 10.1007/978-3-7091-9297-9_34. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen JP, Lefaucher JP, Le Guerinel C, et al. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch Med Res. 2000 May-Jun;31(3):263–265. doi: 10.1016/s0188-4409(00)00078-3. [DOI] [PubMed] [Google Scholar]
- 29.Henderson JM, Boongird A, Rosenow JM, LaPresto E, Rezai AR. Recovery of pain control by intensive reprogramming after loss of benefit from motor cortex stimulation for neuropathic pain. Stereotact Funct Neurosurg. 2004;82(5-6):207–213. doi: 10.1159/000082447. [DOI] [PubMed] [Google Scholar]
- 30.Nuti C, Peyron R, Garcia-Larrea L, et al. Motor cortex stimulation for refractory neuropathic pain: four year outcome and predictors of efficacy. Pain. 2005 Nov;118(1-2):43–52. doi: 10.1016/j.pain.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Lefaucheur JP, Drouot X, Cunin P, et al. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain. 2009 Jun;132(Pt 6):1463–1471. doi: 10.1093/brain/awp035. [DOI] [PubMed] [Google Scholar]
- 32.Rasche D, Ruppolt M, Stippich C, Unterberg A, Tronnier VM. Motor cortex stimulation for long-term relief of chronic neuropathic pain: a 10 year experience. Pain. 2006 Mar;121(1-2):43–52. doi: 10.1016/j.pain.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Hirayama T, Tsubokawa T, Katayama Y, Yamamoto Y, Koyama S. Chronic changes in activity of the thalamic relay neurons following spinothalamic tractotomy in cats. Effects of motor cortex stimulation. Pain. 1990;5(suppl):273. [Google Scholar]
- 34.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965 Nov 19;150(699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 35.Melzack R. From the gate to the neuromatrix. Pain. 1999 Aug;(Suppl 6):S121–126. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 36.Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008 Oct;63(4):762–770. doi: 10.1227/01.NEU.0000325731.46702.D9. discussion 770. [DOI] [PubMed] [Google Scholar]
- 37.Sears NC, Machado AG, Nagel SJ, et al. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation. 2011 Jul-Aug;14(4):312–318. doi: 10.1111/j.1525-1403.2011.00372.x. discussion 318. [DOI] [PubMed] [Google Scholar]
- 38.Tait RC, Chibnall JT, Krause S. The Pain Disability Index: psychometric properties. Pain. 1990 Feb;40(2):171–182. doi: 10.1016/0304-3959(90)90068-O. [DOI] [PubMed] [Google Scholar]
- 39.Vranken JH, Dijkgraaf MG, Kruis MR, van Dasselaar NT, van der Vegt MH. Iontophoretic administration of S(+)-ketamine in patients with intractable central pain: a placebo-controlled trial. Pain. 2005 Nov;118(1-2):224–231. doi: 10.1016/j.pain.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Haber SN, Gdowski MJ. The Basal Ganglia. In: Paxinos G, Mai J, editors. The Human Nervous System. Academic Press; 2003. [Google Scholar]
- 41.Modell JG, Mountz JM, Curtis GC, Greden JF. Neurophysiologic dysfunction in basal ganglia/limbic striatal and thalamocortical circuits as a pathogenetic mechanism of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 1989 Winter;1(1):27–36. doi: 10.1176/jnp.1.1.27. [DOI] [PubMed] [Google Scholar]
- 42.Dougherty DD, Baer L, Cosgrove GR, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry. 2002 Feb;159(2):269–275. doi: 10.1176/appi.ajp.159.2.269. [DOI] [PubMed] [Google Scholar]
- 43.Hassenbusch SJ, Pillay PK, Barnett GH. Radiofrequency cingulotomy for intractable cancer pain using stereotaxis guided by magnetic resonance imaging. Neurosurgery. 1990 Aug;27(2):220–223. doi: 10.1097/00006123-199008000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson HA, Davidson KM, Davidson RI. Bilateral anterior cingulotomy for chronic noncancer pain. Neurosurgery. 1999 Nov;45(5):1129–1134. doi: 10.1097/00006123-199911000-00023. discussion 1134-1126. [DOI] [PubMed] [Google Scholar]
- 45.Faillace LA, Allen RP, McQueen JD, Northrup B. Cognitive deficits from bilateral cingulotomy for intractable pain in man. Dis Nerv Syst. 1971 Mar;32(3):171–175. [PubMed] [Google Scholar]
- 46.Cohen RA, Paul R, Zawacki TM, Moser DJ, Sweet L, Wilkinson H. Emotional and personality changes following cingulotomy. Emotion. 2001 Mar;1(1):38–50. doi: 10.1037/1528-3542.1.1.38. [DOI] [PubMed] [Google Scholar]
- 47.Broggi G. Pain and psycho-affective disorders. Neurosurgery. 2008 Jun;Discussion 919-920;62(6 Suppl 3):901–919. doi: 10.1227/01.neu.0000333760.53748.9e. [DOI] [PubMed] [Google Scholar]
- 48.Ruck C, Andreewitch S, Flyckt K, et al. Capsulotomy for refractory anxiety disorders: long-term follow-up of 26 patients. Am J Psychiatry. 2003 Mar;160(3):513–521. doi: 10.1176/appi.ajp.160.3.513. [DOI] [PubMed] [Google Scholar]
- 49.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999 Oct 30;354(9189):1526. doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- 50.Greenberg BD, Gabriels LA, Malone DA, Jr., et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2008 May 20; doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malone DA, Jr., Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009 Feb 15;65(4):267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machado A, Haber S, Sears N, Greenberg B, Malone D, Rezai A. Functional topography of the ventral striatum and anterior limb of the internal capsule determined by electrical stimulation of awake patients. Clin Neurophysiol. 2009 Nov;120(11):1941–1948. doi: 10.1016/j.clinph.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 53.Lujan JL, Chaturvedi A, Malone DA, Rezai AR, Machado AG, McIntyre CC. Axonal pathways linked to therapeutic and nontherapeutic outcomes during psychiatric deep brain stimulation. Hum Brain Mapp. 2011 Apr 21; doi: 10.1002/hbm.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machado A. Safety Study of Deep Brain Stimulation to Manage Thalamic Pain Syndrome (DBS) 2011 http://clinicaltrials.gov/ct2/show/NCT01072656.


