Abstract
Purpose
Attempts to understand the causes of cognitive impairment in Obstructive Sleep Apnea (OSA) are complicated by the overlap among clinical and demographic factors that may impact cognition. The goal of the current study was to isolate the contribution of hypoxemia to cognitive impairment in OSA.
Methods
Two groups of 20 patients with newly diagnosed OSA were compared. The groups differed on severity of hypoxemia but not other demographic (e.g., age, gender, education, estimated premorbid IQ) or clinical (e.g., sleep related respiratory disturbances, daytime sleepiness, depressive symptoms) variables. Participants completed polysonmography and cognitive assessment.
Results
We compared patients with high and low hypoxemia on measures of memory, attention, executive functioning, and motor coordination using independent samples t-tests. The high hypoxemia group performed significantly better on immediate recall (HVLT-R; t=−2.50, p<.02) than the low hypoxemia group. No group differences were observed on other neuropsychological measures.
Conclusions
This study is one of the first to compare the cognitive performance of patients with high and low hypoxemia after controlling for demographic factors and aspects of OSA severity that could confound the relationship. In our carefully matched sample we observed an unexpected advantage of higher hypoxemia on memory. These preliminary findings are discussed in the context of basic science literature on the protective effects of adaptation to intermittent hypoxemia. Our data suggest that the association between hypoxemia and cognition may not straightforward. Future research targeting the effects of hypoxemia on cognition controlling for other clinical factors in large groups of patients with OSA will be important.
Keywords: OSA, Hypoxemia, Memory, Cognition
Introduction
Obstructive Sleep Apnea (OSA) is a disorder characterized by complete (apnea) or partial (hypopnea) cessations of breathing during sleep. This breathing problem results in fragmented sleep and intermittent drops in arterial blood oxygen saturation (hypoxemia). OSA affects 2% of middle-aged women, 4% of middle-aged men, and up to 42% of people over 65 years old [1] . The consequences of OSA can be significant, including daytime sleepiness, reduced quality of life[2], comorbid medical conditions such as vascular disease[3], and cognitive impairment.[4]
Cognitive deficits in OSA have been documented in several domains with most studies finding impaired memory, executive functioning, attention, and fine motor coordination.[4–7] Cognitive impairment has the potential to impact patients' daily functioning negatively. Many researchers over the past two decades have attempted to characterize the cause of cognitive dysfunction in OSA with mixed results. One important limitation of most studies examining cognitive impairment in OSA is that they have not controlled for other variables that might affect cognition. Severity of OSA is most commonly described using measures of hypoxemia/oxygen desaturation during the night (e.g., percent of sleep time below 90% saturation) and sleep related respiratory disturbances (e.g., apnea-hypopnea index [AHI]). A major challenge is to separate the cognitive sequelae of hypoxemia from sleep disturbance because the two occur simultaneously. We believe that this remains an important task in understanding completely the causes of cognitive dysfunction in OSA and potential methods to remedy them.
The goal of the current study was to isolate the potential contribution of hypoxemia to cognitive impairment in OSA. We compared the neuropsychological test performance of two groups of patients with newly diagnosed OSA who differed on their level of hypoxemia, but were matched on age, gender, and AHI on polysomnography (PSG; an assumed surrogate for sleep fragmentation). None of the participants had previously undergone treatment with positive airway pressure (PAP), eliminating the possibility of differential treatment effects across the two groups. Based on past clinical research, we hypothesized that individuals with OSA who had higher levels of hypoxemia would perform more poorly on cognitive testing than those with lower levels of hypoxemia. We focused on memory, executive functioning, attention/ vigilance, and fine motor coordination given findings from previous studies.
Methods and Materials
This study was approved by the institutional review boards at Rhode Island Hospital and Brown University (0122-01). Participants provided written informed consent prior to enrollment. After completing a full night of in-laboratory PSG, participants completed neuropsychological testing before treatment for OSA was initiated.
Participants
Research records from 188 participants with OSA recruited from a sleep disorders clinic for a previous study (National Institutes of Health HL067209) were used to create the two groups of patients included in the current analysis. The goal was to create two groups who differed on severity of hypoxemia but were pair-matched on age, gender and AHI. To be included participants had to be 25–85 years of age, have a new diagnosis of OSA, and be fluent in English. Exclusion criteria were: (1) sleep disorder other than OSA including central sleep apnea, (2) previous treatment with PAP, and (3) medical disorder that might impact cognition (e.g., cardiovascular disease, chronic obstructive pulmonary disease (COPD), kidney failure, neurological illness, major psychiatric condition). Participants who reported treatment for depression were included if their treatment was stable without changes in the past three months.
OSA was diagnosed by a full night of in-laboratory, clinical PSG. The apnea-hypopnea index (AHI), an index of the number of apneas plus hypopneas per hour of sleep[8], was used as a measure of OSA severity and sleep related respiratory disturbance. An AHI of 15 events per hour was the lower limit for enrollment in the study. The amount of time spent below 90% blood oxygen saturation was also recorded during the overnight PSG.
The results of the overnight PSG, specifically the variable “time below 90% blood oxygenation” (Sa90), was used to create groups of participants with relatively high and low hypoxemia. Participants from the upper tertile of the sample distribution on the Sa90 variable were identified as having “high” levels of hypoxemia and spent at least 20% of their total sleep time below 90% blood oxygenation. Participants from the lower tertile on the Sa90 variable were considered to have “low” levels of hypoxemia and spent no more than 6% total sleep time below 90% blood oxygenation. This dichotomization was chosen to facilitate the comparison of those individuals with the highest and lowest levels of hypoxemia in our sample of patients with OSA. Of the participants identified as having high and low hypoxemia, a subset of 40 participants (22 male, 18 female) could be pair-matched on age, gender, and AHI. The final sample for the study included two groups of 20 matched participants between the ages of 32 and 77 differing only on their levels of hypoxemia.
Trained research assistants administered all neuropsychological measures based on standardized procedures. The examiners were blind to OSA severity and were not involved in participants' clinical care.
Measures
Epworth Sleepiness Scale,[9] a widely used self-report scale regarding how likely participants would be to fall asleep in eight situations. Scores range from 0–24 with higher scores indicating greater daytime sleepiness.
Beck Depression Inventory-II (BDI-II[10]), a 21-item self-report scale that evaluates symptoms of depression over the past two weeks. Scores range from 0–63 with higher scores reflecting greater depression.
Neuropsychological Test Battery
The neuropsychological test battery included the following measures: American National Adult Reading Test (AMNART[11]), Paced Auditory Serial Addition Test (PASAT[12]), Trail Making Test Part A[13], Controlled Oral Word Association Test (COWAT[14]), The Letter-Number Sequencing Test[15], Trail Making Test Part B[13], Hopkins Verbal Learning Test - Revised (HVLT-R[16]), and Grooved Pegboard[17]. Please see Table 1 for additional information about test administration and outcome measures.
Table 1.
Neuropsychological Test Measures
| Test | Description | Outcome Variable |
|---|---|---|
| American National Adult Reading Test (AMNART[11]) | This measure provides an estimated verbal IQ based on the number of words that a person can correctly read aloud from a list of 50 words of varying difficulty. | Estimated Verbal IQ |
| Paced Auditory Serial Addition Test (PASAT[12]) | A measure of attention, vigilance, and complex mental manipulation. Participants must listen to a string of numbers read aloud from an audio recording and state the sum of the last two numbers presented in the string. | Total # of correct responses |
| Trail Making Test Part A[13] | A measure of attention and visual search skills. Participants are asked to draw lines to connect numbers scattered on a page in the correct sequential order as quickly as possible. | Time to completion |
| Controlled Oral Word Association Test (COWAT[14]) | A measure of rapid word generation. Participants generate as many words as possible in a 60-second trial to each of three letter cues. | Total # of words generated |
| Letter-Number Sequencing Test[15] | A task designed to test working memory and executive functioning. Participants read a string of numbers and letters, and state the numbers in sequential order followed by the letters in alphabetic order | Total # correct was used to generate a scaled score based on the testing manual. |
| Trail Making Test Part B[13] | A test designed to measure visual search and set shifting abilities. Participants are required to quickly connect numbers and letters in alphanumeric order. | Time to completion |
| Hopkins Verbal Learning Test - Revised (HVLT-R[16]) | A test of verbal list learning, free recall, and recognition memory. The examiner presents a list of words to recall across three trials. After a 25-minute delay, participants must recall the list, and discriminate between target words and foils. | # words recalled for trials 1–3 # words recalled after the delay Percent retention on the delay trial |
| Grooved Pegboard[17] | Used as a measure of fine motor coordination, participants must place pegs into a pegboard as quickly as possible using only one hand. The task is completed with the dominant and non-dominant hand separately. | Time to completion |
Analyses
Data were analyzed using SPSS 11.5 (SPSS Inc., Chicago, IL) with the significance level set at 0.05. Independent t-tests were utilized to examine group differences in demographic and clinical variables. For the primary analysis comparing neuropsychological test scores, independent t-tests were employed. We planned to follow-up any significant group differences in neuropsychological test scores by examining the performance of each group compared to published normative data. These normative t-scores represent each group's performance relative to healthy individuals of the same age. The mean value of a t-score is 50 with a standard deviation of 10. Lower scores represent poorer performance.
Results
Table 2 depicts participant demographic information by hypoxemia severity group. The two groups were pair-matched on age, gender, and AHI, and did not significantly differ on education, body mass index (BMI), estimated premorbid verbal intelligence quotient, symptoms of daytime sleepiness, or depression. Participants were generally middle-aged Caucasian males with 1–2 years of post-secondary education. All participants were either overweight (BMI≥25) or obese (BMI≥30), and reported clinically significant subjective sleepiness but minimal depressive symptoms. By design the two groups differed on severity of hypoxemia (i.e., Sa90).
Table 2.
Demographics for Study Sample by Group
| Variable | Low Hypoxemia (n=20) | High Hypoxemia (n=20) | p-value |
|---|---|---|---|
| Gender (# of males) | 11/20 | 11/20 | |
| Mean (SD) | Mean (SD) | ||
| Age (years) | 53.5 (9.5) | 55.6 (9.4) | 0.50 |
| BMI (kg/m2) | 35.3 (7.3) | 35.9 (9.4) | 0.82 |
| Epworth Sleepiness Scale | 11.3 (4.0) | 12.6 (5.4) | 0.41 |
| BDI-II | 9.9 (6.5) | 10.7 (8.2) | 0.78 |
| Education (years) | 13.8 (1.8) | 14.5 (3.0) | 0.38 |
| AMNART (Estimated Verbal IQ score) | 112.2 (8.2) | 112.5 (9.0) | 0.91 |
| AHI (events/hour) | 37.5 (19.8) | 38.1 (20.3) | 0.93 |
| Sa90 (% sleep time <Sa90) | 3 (2) | 43 (25) | 0.00* |
(SD= Standard Deviation; BMI= Body Mass Index, BDI-II= Beck Depression Inventory-II, AMNART= American National Adult Reading Test, AHI=Apnea-Hypopnea Index, Sa= oxygen saturation)
Independent t-tests conducted for all continuous variables, p < 0.05.
Table 3 shows the neuropsychological test performance for the low hypoxemia group and the high hypoxemia group. The groups differed significantly on verbal memory, specifically verbal immediate recall (HVLT-R; t=−2.50, p<0.02). The effect size for this difference was large (Cohen's d=−.78). There was a trend towards significance for delayed recall (t=−1.77, p<0.09), though not statistically significant. No other group differences were observed on any neuropsychological measure.
Table 3.
Neuropsychological Test Scores for Study Sample by Group in Mean (SD)
| Variable | Low Hypoxemia (n=20) | High Hypoxemia (n=20) | p-value |
|---|---|---|---|
| Attention | |||
| Trails A (sec.) | 36.2 (17.4) | 33.0 (11.8) | 0.50 |
| PASAT total correct | 98.9 (43.4) | 105.2 (42.0) | 0.49 |
| Executive Functioning | |||
| Trails B (sec.) | 84.2 (44.5) | 82.6 (36.3) | 0.91 |
| LNS total | 9.9 (2.1) | 10.4 (2.7) | 0.56 |
| COWAT total | 37.2 (8.0) | 35.2 (9.6) | 0.49 |
| Memory | |||
| HVLT trial 1 | 5.6 (1.6) | 7.0 (1.7) | 0.01* |
| HVLT trial 2 | 8.4 (2.0) | 9.4 (1.6) | 0.09 |
| HVLT trial 3 | 9.0 (1.8) | 10.2 (1.7) | 0.04* |
| HVLT-R Total Immediate Recall | 23.0 (4.8) | 26.5 (4.2) | 0.02* |
| HVLT-R Delayed Recall | 8.1 (2.4) | 9.4 (2.1) | 0.09 |
| HVLT-R Percent Retention | 85.5 (17.4) | 89.0 (12.8) | 0.48 |
| Motor Coordination | |||
| Pegs dominant hand (sec.) | 82.3 (18.0) | 80.9 (14.1) | 0.78 |
(SD= Standard Deviation; PASAT = Paced Auditory Serial Addition Test; LNS = Letter Number Sequencing Test; COWAT = Controlled Oral Word Fluency Test; HVLT-R = Hopkins Verbal learning Test – Revised, Trails= Trail Making Test)
Independent t-tests performed on all variables, p <0.05.
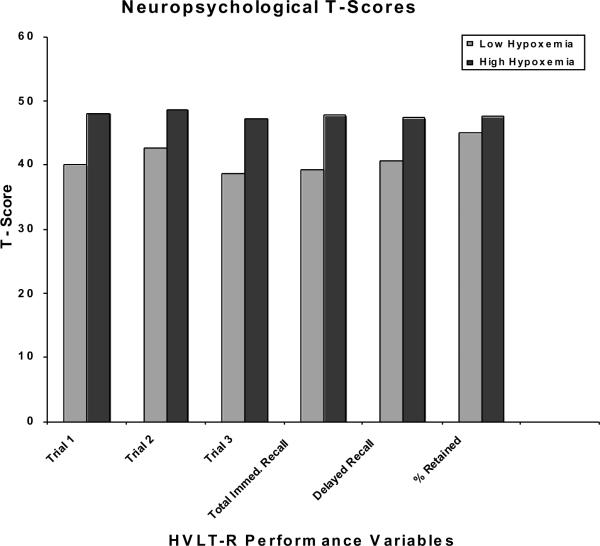
Figure 1 illustrates the t-scores calculated using published normative data for each of the memory scores. The high hypoxemia group performed largely in the average range (i.e., t-scores 40–60). Performance on the HVLT-R was more variable for the low hypoxemia group, with t-scores falling in the borderline/low average ranges (i.e., t-scores 38–40).
Figure 1.
Learning and Memory Test t-scores for Study Sample by Group
Discussion
The goal of the current study was to examine the association between hypoxemia and cognitive performance among patients with OSA. Two groups of patients were carefully matched to isolate the impact of hypoxemia on cognition from other demographic factors and aspects of OSA severity (i.e. age, sleep related respiratory disturbance) that could confound the relationship. The study compared patients with high and low hypoxemia that were pair-matched on age, gender, and severity of sleep related respiratory disturbance (AHI), and did not differ on education, estimated premorbid intelligence, BMI, self-reported daytime sleepiness, or depressive symptoms. We found that individuals with greater hypoxemia performed better on learning and memory than those with lower levels of hypoxemia.
To our knowledge, the current data are the first published findings in a clinical sample of patients with OSA suggesting a protective advantage of higher levels of hypoxemia on memory. Our results were unexpected and may seem counterintuitive. Past studies of patients with OSA typically have suggested that greater hypoxemia is associated with poorer memory performance[5,4,18]. There are some potential explanations for our different findings. There is evidence in the clinical and animal literature that exposure to intermittent hypoxemia has protective effects on the cardiovascular system, brain, and memory specifically[19]. Adaptation to intermittent hypoxemia has been used therapeutically for many years in the nations of the former Soviet Union to reduce future cardiovascular risk [20,21]/.Effects of intermittent hypoxemia and cerebral response to apnea have gained more widespread attention in English medical literature over the past several years[22,23]. Adaptation to intermittent hypoxemia has been shown to confer cardiovascular protection against more severe and sustained hypoxia in the future, and to transfer protection to some other stressors such as ischemia[19][24,25][26].
Most research examining adaptation to hypoxemia has not, however, been conducted among clinical disorders like sleep apnea. Almendros and colleagues [27,28] have recently examined changes in oxygen partial pressure of brain tissue (PtO2) in an animal model of obstructive apnea. The model is designed to apply recurrent obstructive apneas in a stable and controlled way without other comorbidities that can occur in OSA. The authors recently posited that a physiological response in cerebral hemodynamics such as hypercapnia could compensate, in part, for reduction in arterial oxygen saturation (SpO2), the measure of oxygen on pulse oximetry that is most commonly used clinically to capture oxygen saturation during apneas[28]…
In the clinical sleep literature, one relatively large study identified an unexpected survival advantage associated with moderate sleep apnea in older adults[29]. The study examined all-cause-mortality over eight years comparing 611 elderly (aged ≥ 65) individuals with a diagnosis of OSA to age-, gender- and ethnicity- matched national mortality data. Elderly patients with moderate OSA had significantly lower mortality rates than the matched population cohort, while those with severe apnea had the same mortality as a comparison cohort. Together with the basic literature on protective effects of chronic intermittent hypoxia, the finding of a survival advantage of moderate OSA suggests that a protective effect of intermittent hypoxemia is plausible in OSA although the mechanisms and specific levels of hypoxemia that are beneficial have not been determined.
We observed differences on memory testing, but no significant group differences on attention, executive functioning, or motor coordination measures. The reason for the specificity of in the memory domain is unknown. The hippocampus has historically been implicated as vulnerable in different clinical populations of patients who experience hypoxia.[4,30,31] Experimental paradigms in animals have also demonstrated that acute severe hypobaric hypoxia causes extensive neuronal damage in hippocampus and neocortex, and that preconditioning with three-time exposure to mild hypobaric hypoxia prevents severe hypoxia-induced neuronal death in these regions.[32,33] Past animal work following exposure to intermittent hypoxia has focused on memory tasks (e.g., retention of conditioned passive avoidance[34]) as outcome measures providing consistency with our memory finding. Our data, however, do not offer information about the reason for group differences in memory specifically.
The current study is the first to focus specifically on the relationship between hypoxemia and cognition, controlling for other clinical variables in a sample of patients with OSA; however, strengths and weaknesses of the study design need to be considered. First, we examined only a small sample of carefully matched patients. Second, the study used retrospective data from a larger study. Prospective data collection might have enabled matching on a wider range of variables; however, using retrospective data enable us to match groups on key variables (i.e., age, gender, and AHI) and examine other variables (i.e., education, BMI, estimated premorbid intelligence) between groups. Still, we cannot rule out that other factors associated with OSA could account for our findings. Another limitation is that both of our groups suffered from intermittent hypoxemia. The low hypoxemia group simply had less total hypoxemia time than did the high hypoxemia group. Finally, limited information from the PSG was available for analysis (i.e., AHI and % sleep time <Sa90). It is possible that the groups different on aspects of sleep, which were not included in our analysis. Given these limitations it is possible that the better performance of the high hypoxemia group was a chance finding. It will be necessary to replicate this type of analysis to be confident in the observed effects..
The findings from the current study suggest that the association between hypoxemia and cognition is not straightforward. Given the small sample size findings are not definitive; however, future research on cognition in OSA should aim to examine the effects of different aspects of OSA separately. Research targeting the effects of hypoxemia on cognition while also controlling for other clinical factors that might impact cognition (e.g., sleep disturbance, daytime sleepiness, obesity, and depression) will be important to advance our understanding of the relationship between hypoxemia and cognition.
Acknowledgements
This work was supported by the National Institutes of Health [Grant HL067209]. Dr. Aloia takes responsibility as the guarantor of the work presented in the manuscript. All authors assisted in reviewing and editing of the manuscript. Additionally, Dr. Hoth, Ms. Meschede, Dr. Zimmerman, and Dr. Aloia engaged in data analysis and interpretation, Dr. Aloia and Dr. Zimmerman contributed to the design of the study, and Dr. Arnedt assisted in data collection. The authors acknowledge Dr. Richard P. Millman, Leisha Smith, Jaime Skrekas, Sarah Harris, and Charleen Pysz for assistance with patient recruitment, neuropsychological assessment, and data entry.
Footnotes
Conflict of Interest Disclosures: Dr. Zimmerman has received investigator initiated research funding from Merck Sharp and Dohme Corp., and Bristol-Myers Squibb. Dr. Aloia is a paid employee and stockholder for Philips/Respironics, Inc.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24(2):249–259. doi: 10.1016/s0272-5231(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 3.Golbin JM, Somers VK, Caples SM. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc. 2008;5(2):200–206. doi: 10.1513/pats.200708-143MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10(5):772–785. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 5.Twigg GL, Papaioannou I, Jackson M, Ghiassi R, Shaikh Z, Jaye J, Graham KS, Simonds AK, Morrell MJ, Twigg GL, Papaioannou I, Jackson M, Ghiassi R, Shaikh Z, Jaye J, Graham KS, Simonds AK, Morrell MJ. Obstructive sleep apnea syndrome is associated with deficits in verbal but not visual memory. American Journal of Respiratory & Critical Care Medicine. 2009;182(1):98–103. doi: 10.1164/rccm.200901-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quan S, Chan C, Dement W, Gevins A, Goodwin J, Gottlieb D, Green S, Guilleminault C, Hirshkowitz M, Hyde P, Kay G, Leary E, Nichols D, Schweitzer P, Simon R, Walsh J, Kushida C. The association between Obstructive Sleep Apnea and neurocognitive performance- The Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2011;34(3):303–314. doi: 10.1093/sleep/34.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alchanatis M, Zias N, Deligiorgis N, Liappas I, Chroneou A, Soldatos C, Roussos C. Comparison of cognitive performance among different age groups in pateints with obstructive sleep apnea. Sleep Breath. 2008;12:17–24. doi: 10.1007/s11325-007-0133-y. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 9.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 10.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 11.Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. 2nd ed. Oxford University Press; US, New York, NY: 1998. [Google Scholar]
- 12.Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percet Mot Skills. 1977;44(2):367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 13.Army Individual Test Battery: Manual of Directions and Scoring. War Department, Adjuctant General's Office; Washington DC: 1944. [Google Scholar]
- 14.Spreen O. Controlled Oral Word Association Test. 1977 [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale. Third Edition Pearson Assessment; San Antonio: 1997. [Google Scholar]
- 16.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test--Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 17.Reitan RM, Davison LA. Clinical neuropsychology: Current status and applications. V H Winston & Sons; England, Oxford, England: 1974. [Google Scholar]
- 18.Findley LJ, Barth JT, Powers DC, Wilhoit SC, Boyd DG, Suratt PM. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90(5):686–690. doi: 10.1378/chest.90.5.686. [DOI] [PubMed] [Google Scholar]
- 19.Manukhina EB, Downey HF, Mallet RT. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp Biol Med. 2006;231(4):343–365. doi: 10.1177/153537020623100401. [DOI] [PubMed] [Google Scholar]
- 20.Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT, Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med. 2008;233(6):627–650. doi: 10.3181/0710-MR-267. [DOI] [PubMed] [Google Scholar]
- 21.Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens. 2011;29:2265–2272. doi: 10.1097/HJH.0b013e32834b5846. [DOI] [PubMed] [Google Scholar]
- 22.Koo BB. Cerebral response to obstructive apnea: the times they are a-changin'. Sleep Breath. 2012;16:269–270. doi: 10.1007/s11325-011-0533-x. [DOI] [PubMed] [Google Scholar]
- 23.Goryacheva AV, Kruglov SV, Pshennikova MG, Smirin BV, Malyshev IY, Barskov IV, Viktorov IV, Downey HF, Manukhina EB, Goryacheva AV, Kruglov SV, Pshennikova MG, Smirin BV, Malyshev IY, Barskov IV, Viktorov IV, Downey HF, Manukhina EB. Adaptation to intermittent hypoxia restricts nitric oxide overproduction and prevents beta-amyloid toxicity in rat brain. Nitric Oxide. 2010;23(4):289–299. doi: 10.1016/j.niox.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Sharp F, Ran R, Lu A, Tang Y, Strauss K, Glass T, Ardozzone T, Bernaudin M. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx. 2004;1:24–35. doi: 10.1602/neurorx.1.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Ramos J, Yi P, Eleore L, Madronal N, Rueda A, Delgado-Garcia J. Classical eyeblink conditioning during acute hypobaric hypoxia is improved in acclimatized mice and involves FOS expression in selected brain areas. J Appl Physiol. 2007;103:1479–1478. doi: 10.1152/japplphysiol.00384.2007. [DOI] [PubMed] [Google Scholar]
- 27.Almendros I, Farré R, Planas A, Torres M, Bonsignore M, Navajas D, Montserrat J. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34(8):1127–1133. doi: 10.5665/SLEEP.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almendros I, Montserrat JM, Torres M, Gonzalez C, Navajas D, Farre R, Almendros I, Montserrat JM, Torres M, Gonzalez C, Navajas D, Farre R. Changes in oxygen partial pressure of brain tissue in an animal model of obstructive apnea. Respir Res. 2010;11(1) doi: 10.1186/1465-9921-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnea. Journal of Sleep Research. 2009;18:397–403. doi: 10.1111/j.1365-2869.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 30.Petito K, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurol. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 31.Rempel-Clower NL, Zola-Morgan SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rybnikova E, Sitnik N, Gluschenko T, Tjulkova E, Samoilov MO. The preconditioning modified neuronal expression of apoptosis-related proteins of Bcl-2 superfamily following severe hypobaric hypoxia in rats. Brain Res. 2006;1089:195–202. doi: 10.1016/j.brainres.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 33.Rybnikova E, Vataeva L, Tyulkova E, Gluschenko T, Otellin V, Telto-Huikko M, Samoilov MO. Mild hypoxia preconditioning prevents impairment of passive avoidance learning and suppression of brain NGFI-A expression induced by severe hypoxia. Behav Brain Res. 2005;160:107–114. doi: 10.1016/j.bbr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Manukhina EB, Pshennikova MG, Goryacheva AV, Khomenko IP, Mashina SY, Pokidyshev DA, Malyshev IY. Role of nitric oxide in prevention of cognitive disorders in neurodegenerative brain injuries in rats. Bull Exp Biol Med. 2008;146(4):391–395. doi: 10.1007/s10517-009-0315-7. [DOI] [PubMed] [Google Scholar]