To the editor:
Nevus sebaceus is a common congenital skin hamartoma, classically appearing as a yellow-hued plaque on the scalp, face, or neck. It is the hallmark lesion of Schimmelpenning/nevus sebaceus syndrome (MIM: 163200), a multisystem disorder that includes a spectrum of central nervous system, ocular, skeletal, and cardiovascular defects. Secondary neoplasms arise within nevus sebaceus at a modest but elevated rate (Moody et al, 2012), prompting disagreement about whether they should be routinely excised (Shwayder, 2011). Determining the pathogenesis of nevus sebaceus would provide a framework to better understand this lesion and its associated syndrome.
The appearance of nevus sebaceus along Blaschko’s lines suggests that a mosaic genetic mutation causes the lesion, with more extensive multisystem involvement potentially underlying the syndromic form (Happle, 1993). Here, we report a case of an individual with nevus sebaceus syndrome. As individuals with this syndrome are uncommon, we sought to identify associated mutations by comparing the exome sequence of the nevus sebaceus from our patient with those of sporadic nevus sebaceus.
Our index patient is a 38-year old female who was born with Chiari malformation, myelomeningocele, and resultant paraplegia. Due to hydrocephalus and marked ventricomegaly, she required ventriculo-peritoneal shunt placement. Imaging studies demonstrated rotoscoliosis. She has had cognitive developmental delay and suffered from generalized seizures during childhood. In her early thirties, she experienced a middle right cerebral artery stroke. Despite her condition, she remains high functioning and lives in an assisted care facility. No other family members are affected by similar medical conditions, and no cause has been attributed to her findings.
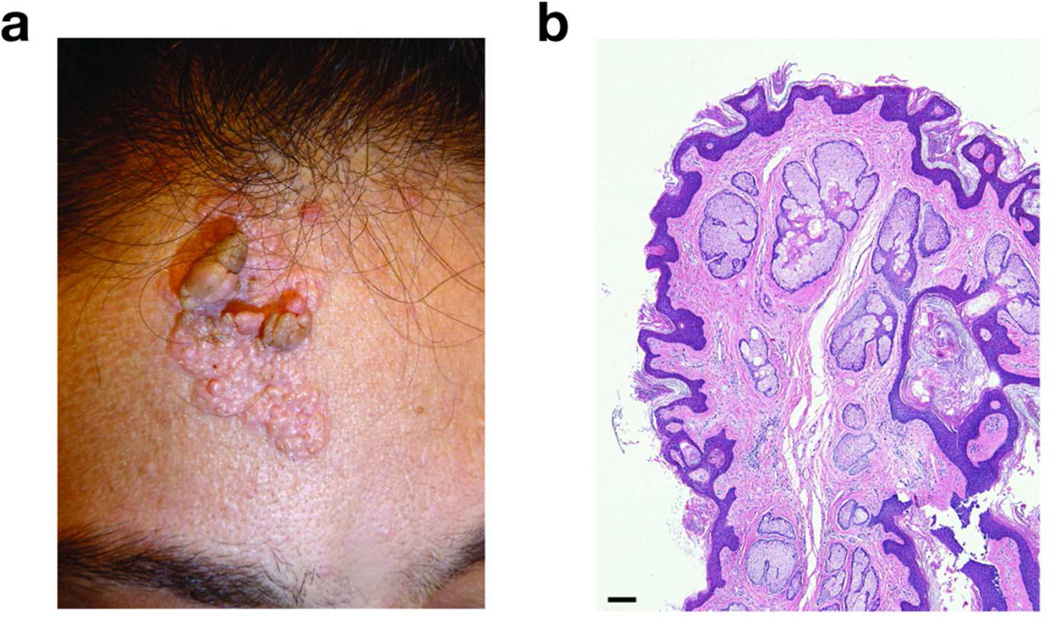
In the past year, the patient became bothered by growths on her forehead and presented to our clinic. On examination, she exhibited frontal bossing and a ~4 cm × ~3 cm yellow-tan, papillomatous plaque on the paramidline forehead that had been present since birth (Figure 1a). Several pedunculated papules were noted within the lesion. No other significant cutaneous findings were appreciated.
Figure 1. Clinical and histologic features of a patient with nevus sebaceus syndrome.
(a) A yellow-hued, papillomatous, oblong plaque on the paramidline forehead of the index patient. (b) Hematoxylin and eosin stained section (40× magnification) of a pedunculated papule from the patient’s lesion showing epidermal acanthosis, papillomatosis, absence of hair follicles, and ectopic sebaceous glands opening directly to the epidermal surface. Bar = 100 µm.
Per patient request, the lesion was excised and a portion of the excision specimen was collected with her written informed consent. Our study complied with the Declaration of Helsinki Principles and was approved by the Stanford IRB. Histologic evaluation confirmed features of nevus sebaceus with no secondary neoplasms (Figure 1b). Accordingly, in light of the extensive neurological and skeletal involvement, a diagnosis of Schimmelpenning/nevus sebaceus syndrome was made. In efforts to determine an underlying genetic mutation, four additional, independent nevus sebaceus were collected from elective excisions along with adjacent normal skin controls. The five samples were subjected to exome sequencing and analyzed for mutations using Seqgene (Deng, 2011) and DNAnexus (www.dnanexus.com) as described in the Supplemental Text.
Analysis of recurrent lesion-specific variants identified an HRAS point mutation (c.37G>C, p.Gly13Arg) in the index case and 2 of 4 isolated nevus sebaceus, with a variant allele frequency ranging from 17–43%. Sanger sequencing confirmed the HRAS mutation in all five lesional samples and its absence in all matched controls (Figure 2a). Examination of the two HRAS mutation-negative exomes showed low sequence coverage (<20 reads) at the mutation site, which may account for the false-negative calls.
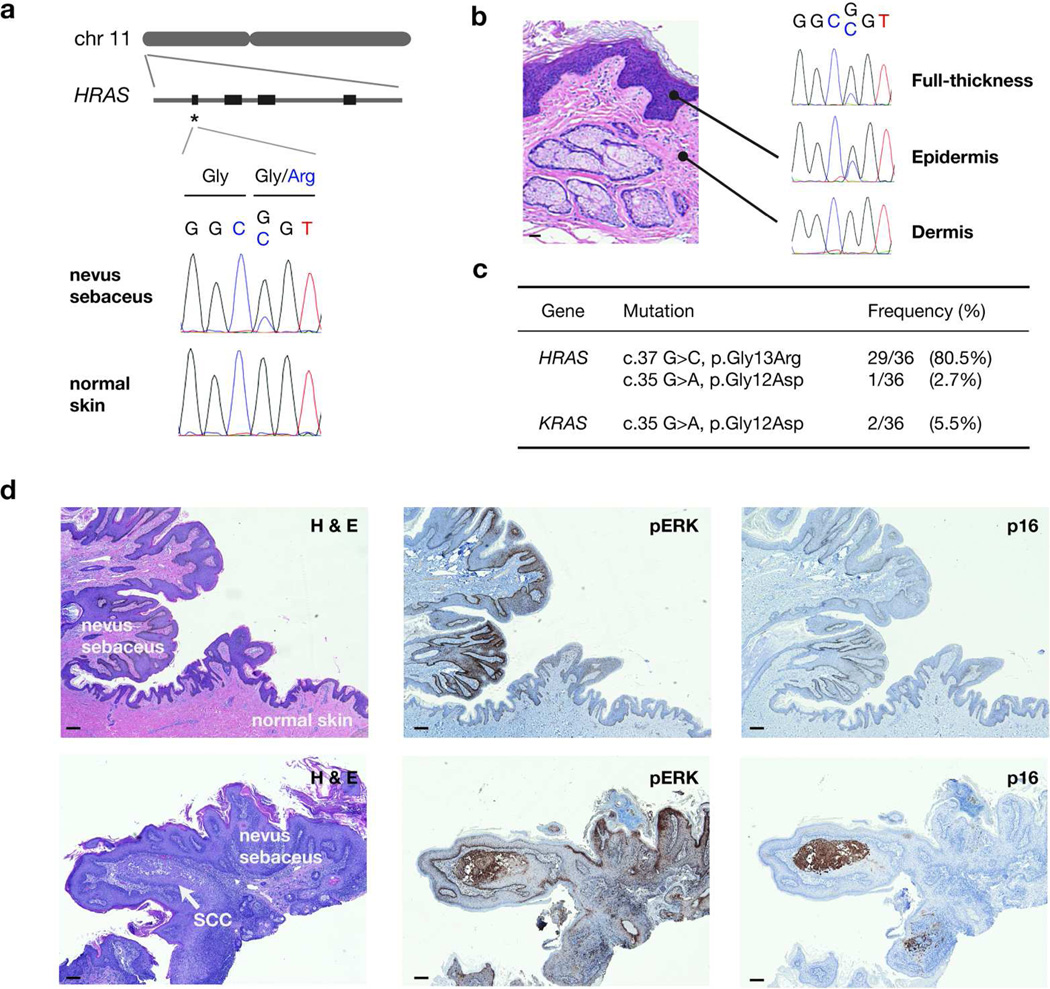
Figure 2. Activating mosaic RAS mutations in nevus sebaceus.
(a) Genomic localization of HRAS to the short arm of chromosome 11 and schematic of its gene structure. A prominent mutational hotspot in exon 1 (codons 12–13) is marked with an asterisk. Sanger sequencing confirms a c.37G>C, p.Gly13Arg mutation specific to lesional tissue. (b) Laser capture microdissection of the epidermis and dermis of nevus sebaceus demonstrates presence of the HRAS mutation exclusively in the epidermis. (c) Summary of RAS mutations identified in nevus sebaceus. (d) Activated RAS/MAPK pathway in nevus sebaceus. Upper row: Nevus sebaceus transitioning into normal skin. Immunohistochemical staining for phosphorylated ERK (pERK), a downstream effector of the RAS pathway, is increased in nevus sebaceus compared to adjacent normal skin. Staining for p16 is homogenously negative. Lower row: An early focus of SCC arising within a nevus sebaceus. This area is characterized by stronger pERK signal and distinct p16 enrichment. All magnifications are at 40×. Bar = 100 µm.
Lesions arising along Blaschko’s lines are hypothesized to stem from a mosaic mutation affecting a specific cell lineage during development. To evaluate whether the candidate mutation fit this criterion, we used laser capture microdissection to isolate DNA from the lesional epidermis and dermis from the index case and one of the sporadic nevus sebaceus. In both cases, the mutation was limited to the epidermis, supporting the hypothesis of an acquired mutation affecting ectodermal precursors (Figure 2b). Both alleles were represented in approximately equal intensities, indicating that the mutation is likely heterozygous.
We next performed targeted Sanger sequencing on a validation set of 31 independent nevus sebaceus from archived tissues, and identified the HRAS p.Gly13Arg mutation in 24/31 samples and p.Gly12Asp in one sample. Remaining mutation-negative cases were evaluated for KRAS and NRAS hotspot mutations, identifying two samples carrying KRAS p.Gly12Asp mutations. Six validation samples had patient-matched normal skin tissue available, and the corresponding RAS mutations were absent in all six control samples. In total, 32 of 36 samples (89%) demonstrated HRAS or KRAS mutations, confirming a strong correlation between activating RAS mutations and nevus sebaceus (Figure 2c). We suspect that the remaining negative cases may be due to genetic heterogeneity, or to a low mutant allele frequency secondary to admixture with normal tissue.
RAS promotes cell growth through activation of multiple pathways, chief among them the mitogen-activated protein kinase (MAPK) signal transduction pathway. Activating mutations in this gene family have well-established links to cancer (Schubbert et al, 2007). Germline activating HRAS mutations cause Costello syndrome, which features predisposition to neoplasia and development of cutaneous papillomas (Gripp and Lin, 2012). Taken together, the known biologic features of activated RAS genes are consistent with the hamartomatous overgrowth and elevated neoplasia risk observed in nevus sebaceus.
To evaluate RAS-MAPK signaling, we performed phosphorylated ERK (pERK) staining on a set of nevi with confirmed HRAS mutations. Immunohistochemistry revealed increased pERK staining in lesional vs. normal epidermis, consistent with RAS-MAPK hyperactivation (Figure 2d). In one sample, a squamous cell carcinoma was identified arising from nevus sebaceus, highlighted by elevated p16 staining (Hodges and Smoller, 2002). The pattern of neoplasia arising from a background of upregulated pERK supports the hypothesis that RAS-MAPK hyperactivation may predispose towards development of secondary neoplasms in nevus sebaceus.
Basal cell carcinomas were once thought to arise commonly from nevus sebaceus, but others have subsequently contended that the majority of these tumors are actually trichoblastomas (Cribier et al, 2000). Our data provide genetic support for the latter opinion, as most basal cell carcinomas arise from Hedgehog pathway dysregulation and lack RAS mutations (Reifenberger et al, 2005). Our findings also raise the possibility that tumors arising from nevus sebaceus, such as syringocystadenoma papilliferum and trichoblastomas, may be associated with RAS mutations as well.
Using targeted sequencing and SNaPshot assays, Hafner and colleagues have recently profiled oncogenic hotspot mutations in epidermal and sebaceous nevi (Groesser et al, 2012; Hafner et al, 2012). Together with the results presented here and by others in this issue (Levinsohn et al, 2012), the cumulative data demonstrate that keratinocytic epidermal nevi and sebaceous nevi are both associated with activating HRAS p.Gly13Arg and KRAS p.Gly12Asp mutations, supporting the belief held by some clinicians they represent a spectrum of the same entity (Sybert, 2010). We postulate that the phenotypic difference between these nevi may be related to the extent of the mutation, as well as body site-specific embryologic patterns and environment. The knowledge of the genetic basis of nevus sebaceus and its associated syndrome represents a further step towards understanding genotype-phenotype correlations arising from genetic mosaicism.
Supplementary Material
Acknowledgements
We thank the patients and their families for taking part in this project. We also thank S. Aasi, R. Khosla, and P. Lorenz for their valuable assistance.
Abbreviations
- MAPK
mitogen-activated protein kinase
- pERK
phosphorylated ERK
- SCC
squamous cell carcinoma
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: A study of 596 cases. J Am Acad Dermatol. 2000;42(2 Pt 1):263–268. doi: 10.1016/S0190-9622(00)90136-1. [DOI] [PubMed] [Google Scholar]
- Deng X. SeqGene: a comprehensive software solution for mining exome- and transcriptome- sequencing data. BMC Bioinformatics. 2011;12:267. doi: 10.1186/1471-2105-12-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp K, Lin A. Costello syndrome: a Ras/mitogen activated protein kinase pathway syndrome (rasopathy) resulting from HRAS germline mutations. Genet Med. 2012;14(3):285–292. doi: 10.1038/gim.0b013e31822dd91f. [DOI] [PubMed] [Google Scholar]
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44(7):783–787. doi: 10.1038/ng.2316. [DOI] [PubMed] [Google Scholar]
- Hafner C, Toll A, Gantner S, et al. Keratinocytic epidermal nevi are associated with mosaic RAS mutations. J Med Genet. 2012;49(4):249–253. doi: 10.1136/jmedgenet-2011-100637. [DOI] [PubMed] [Google Scholar]
- Happle R. Mosaicism in human skin. Understanding the patterns and mechanisms. Arch Dermatol. 1993;129(11):1460–1470. [PubMed] [Google Scholar]
- Hodges A, Smoller B. Immunohistochemical comparison of P16 expression in actinic keratoses and squamous cell carcinomas of the skin. Mod Pathol. 2002;15(11):1121–1125. doi: 10.1097/01.MP.0000032536.48264.D1. [DOI] [PubMed] [Google Scholar]
- Levinsohn J, Tian L, Boyden L, et al. Whole exome sequencing reveals somatic mutations in HRAS and KRAS which cause nevus sebaceus. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.379. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics (Oxford, England) 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M, Landau J, Goldberg L. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29(1):15–23. doi: 10.1111/j.1525-1470.2011.01562.x. [DOI] [PubMed] [Google Scholar]
- Reifenberger J, Wolter M, Knobbe C, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005;152(1):43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7(4):295–208. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Shwayder T. Re: Management of nevus sebaceous and the risk of basal cell carcinoma: an 18-year review. By Rosen et al: Pediatric Dermatology v26, n6, 676–681, Nov/Dec 2009. Pediatr Dermatol. 2011;28(1):82. doi: 10.1111/j.1525-1470.2011.01242.x. author reply 82. [DOI] [PubMed] [Google Scholar]
- Sybert V. Genetic Skin Disorders (Oxford Monographs on Medical Genetics) 2nd ed. USA: Oxford University Press; 2010. p. 784. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.