Abstract
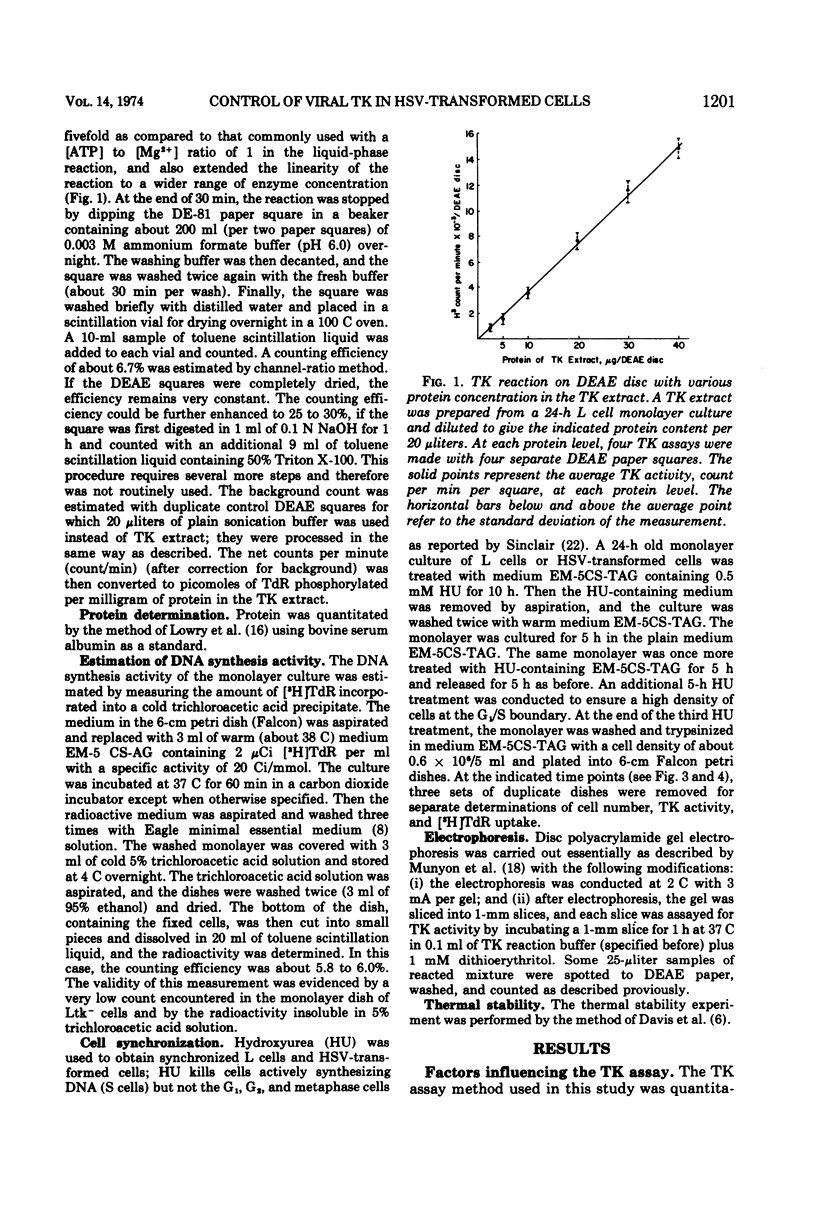
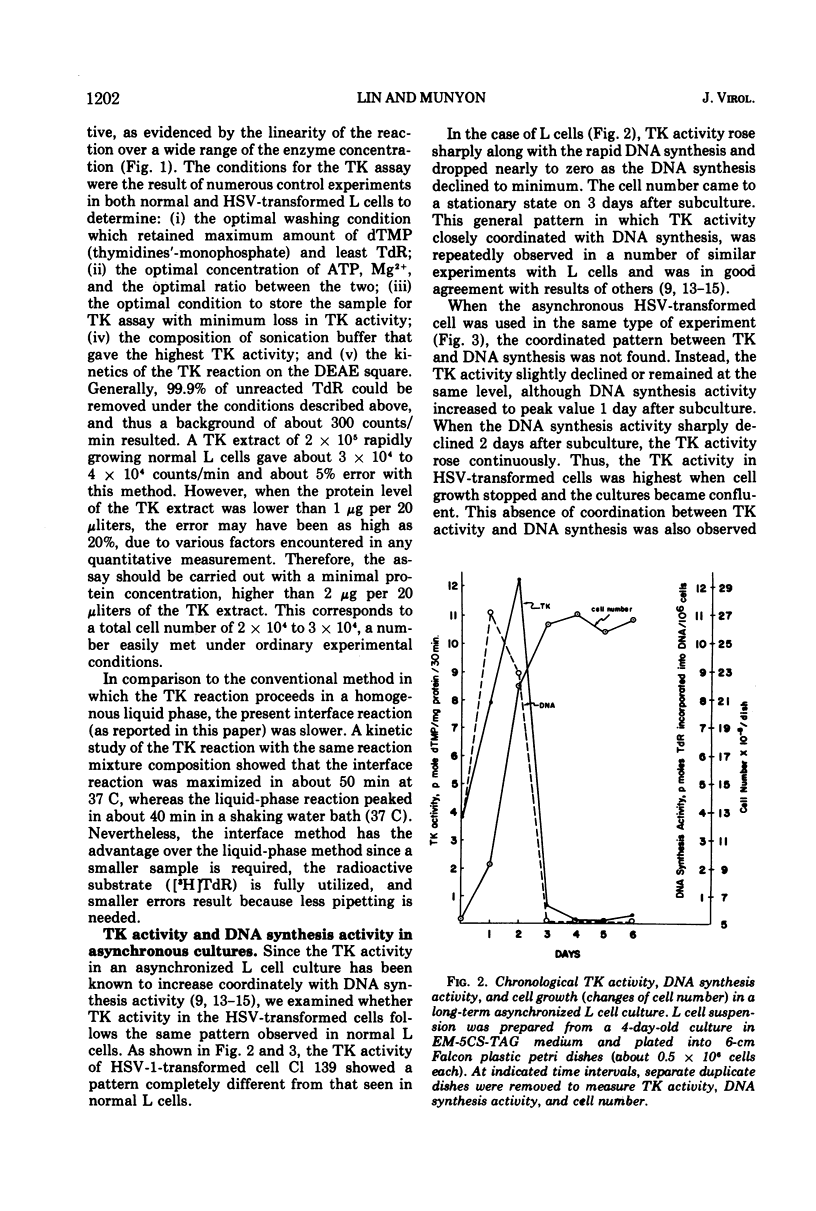
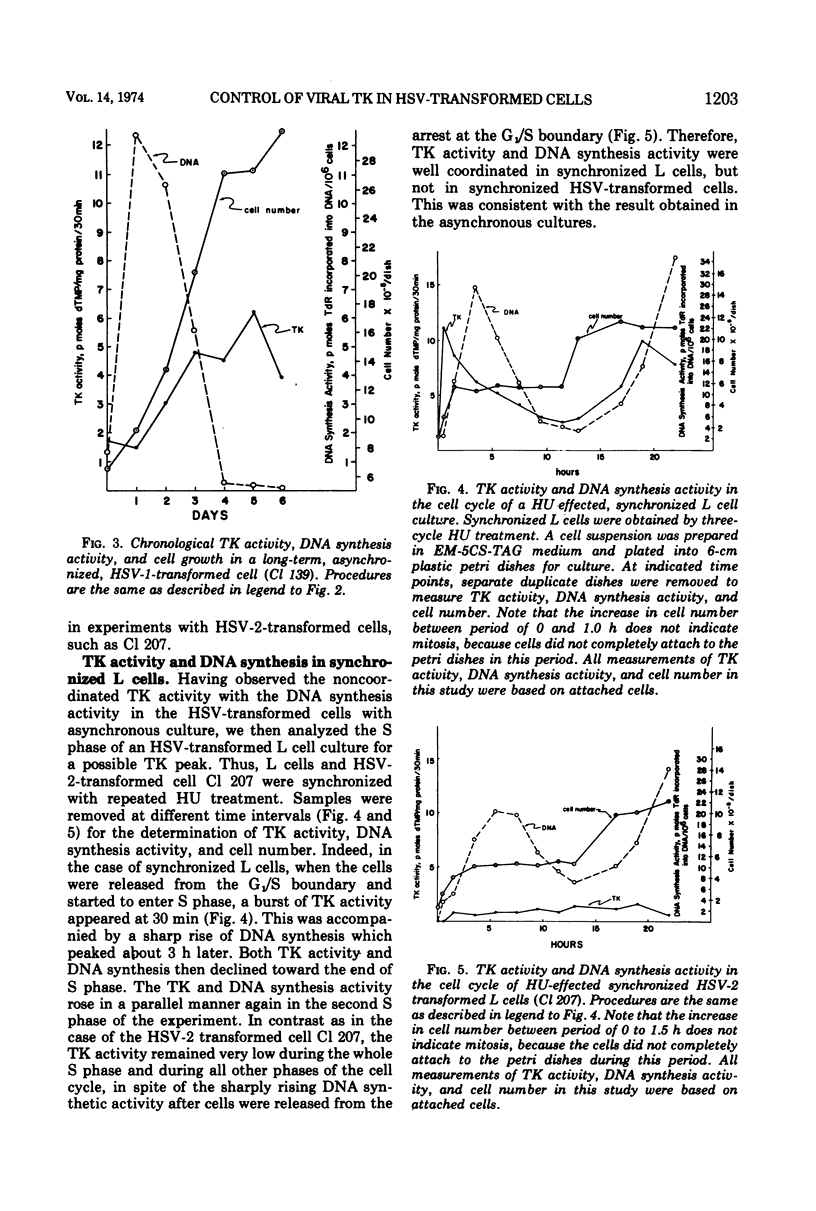
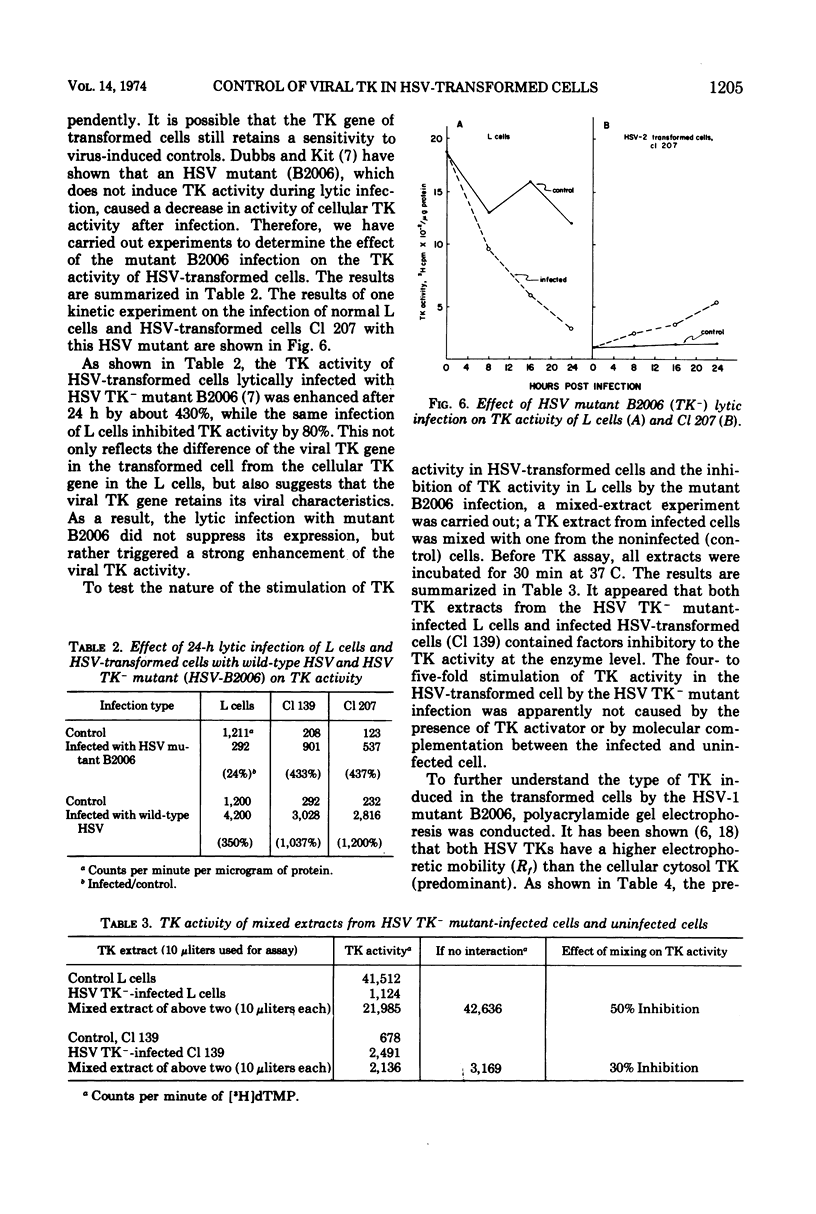
In these studies, the expression of thymidine kinase (TK) in normal and herpes simplex virus (HSV)-transformed L cells has been compared. In asynchronously dividing cultures of L cells, the TK activity rose and declined rapidly and coordinately with DNA synthesis. When net cell increase stopped, TK activity was at a minimum. In contrast, TK activity of HSV-transformed cells remained at a minimum during rapid DNA synthesis and gradually increased as the rate of DNA synthesis decreased. When net cell increase stopped, TK activity was at a maximum. In synchronous cultures of L cells, TK activity rose and fell coordinately with the rate of DNA synthesis. In synchronous cultures of HSV-transformed cells, no increase in TK activity was observed during the period of rapid DNA synthesis, i.e., the S phase. These findings indicated that the viral TK gene in HSV-transformed cells was not placed under the control of the cellular mechanisms which normally modulate the host cell TK gene. Lytic infection of HSV-transformed cells with a TK− mutant of HSV-1 induced a four-to fivefold increase in viral TK. The TK of HSV-1 was induced in the HSV-1-transformed cells and HSV-2 in the HSV-2-transformed cells by this TK− mutant. The same infection of normal L cells decreased the cellular TK activity by 80%. This stimulation, rather than inhibition, suggest that the viral gene in HSV-transformed cells retain some of its original viral characteristics.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brent T. P., Butler J. A., Crathorn A. R. Variations in phosphokinase activities during the cell cycle in synchronous populations of HeLa cells. Nature. 1965 Jul 10;207(993):176–177. doi: 10.1038/207176a0. [DOI] [PubMed] [Google Scholar]
- Bresnick E., Williams S. S., Mossé H. Rates of turnover of deoxythymidine kinase and of its template RNA in regenerating and control liver. Cancer Res. 1967 Mar;27(3):469–475. [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Adelstein S. J., Oxman M. N. Herpes simplex virus as a source of thymidine kinase for thymidine kinase-deficient mouse cells: suppression and reactivation of the viral enzyme. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1912–1916. doi: 10.1073/pnas.70.7.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Munyon W., Buchsbaum R., Chawda R. Virus type-specific thymidine kinase in cells biochemically transformed by herpes simplex virus types 1 and 2. J Virol. 1974 Jan;13(1):140–145. doi: 10.1128/jvi.13.1.140-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EKER P. ACTIVITIES OF THYMIDINE KINASE AND THYMINE DEOXYRIBONUCLEOTIDE PHOSPHATASE DURING GROWTH OF CELLS IN TISSUE CULTURE. J Biol Chem. 1965 Jun;240:2607–2611. [PubMed] [Google Scholar]
- HAMADA C., KAPLAN A. S. KINETICS OF SYNTHESIS OF VARIOUS TYPES OF ANTIGENIC PROTEINS IN CELLS INFECTED WITH PSEUDORABIES VIRUS. J Bacteriol. 1965 May;89:1328–1334. doi: 10.1128/jb.89.5.1328-1334.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., FREARSON P. M. DECLINE OF THYMIDINE KINASE ACTIVITY IN STATIONARY PHASE MOUSE FIBROBLAST CELLS. J Biol Chem. 1965 Jun;240:2565–2573. [PubMed] [Google Scholar]
- Kaplan A. S. A brief review of the biochemistry of herpesvirus-host cell interaction. Cancer Res. 1973 Jun;33(6):1393–1398. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. STUDIES ON THYMIDINE KINASE IN CULTURED MOUSE FIBROBLASTS. Biochim Biophys Acta. 1965 Jan 11;95:14–22. doi: 10.1016/0005-2787(65)90206-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littlefield J. W. The periodic synthesis of thymidine kinase in mouse fibroblasts. Biochim Biophys Acta. 1966 Feb 21;114(2):398–403. doi: 10.1016/0005-2787(66)90319-4. [DOI] [PubMed] [Google Scholar]
- Mittermayer C., Bosselmann R., Bremerskov V. Initiation of DNA synthesis in a system of synchronized L-cells: rhythmicity of thymidine kinase activity. Eur J Biochem. 1968 May;4(4):487–489. doi: 10.1111/j.1432-1033.1968.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A. B., Tarro G. Herpes simplex and herpes genitalis viruses in etiology of some human cancers. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3225–3229. doi: 10.1073/pnas.70.11.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. C., Kuff E. L. The association of deoxyribonucleic acid with liver microsomes. J Biol Chem. 1969 Sep 25;244(18):4843–4851. [PubMed] [Google Scholar]
- Sinclair W. K. Hydroxyurea: differential lethal effects on cultured mammalian cells during the cell cycle. Science. 1965 Dec 24;150(3704):1729–1731. doi: 10.1126/science.150.3704.1729. [DOI] [PubMed] [Google Scholar]



