Abstract
Background
The aim of this analysis is to describe the long-term changes in CD4 cell counts beyond 5 years of combination antiretroviral therapy (cART). If natural ageing leads to a long-term decline in the immune system via low-grade chronic immune activation/inflammation, then one might expect to see a greater or earlier decline in CD4 counts in older HIV-positive patients with increasing duration of cART.
Methods
Retrospective and prospective data were examined from long-term virologically stable HIV-positive adults from the Australian HIV Observational Database. We estimated mean CD4 cell counts changes following the completion of 5 years of cART using linear mixed models.
Results
A total of 37,916 CD4 measurements were observed for 892 patients over a combined total of 9,753 patient years. Older patients (>50 years) at cART initiation had estimated mean(95% confidence interval) change in CD4 counts by Year-5 CD4 count strata (<500, 501–750 and >750 cells/μL) of 14(7 to 21), 3(−5 to 11) and −6(−17 to 4) cells/μL/year. Of the CD4 cell count rates of change estimated, none were indicative of long-term declines in CD4 cell counts.
Conclusions
Our results suggest that duration of cART and increasing age does not result in decreasing mean changes in CD4 cell counts for long-term virologically suppressed patients. Indicating that level of immune recovery achieved during the first 5 years of treatment are sustained through long-term cART.
Keywords: ageing, CD4+ T cell, HIV infection, long-term, cART response
Introduction
Following the initiation of combination antiretroviral therapy (cART) and typically within 6 months of adherent therapy, around 80% of HIV-positive patients have undetectable plasma HIV RNA viral load (<50 copies/ml) (1). The suppression of HIV facilitates immune reconstitution and initial immunologic and virologic responses to cART are well documented from both randomised clinical trials and observational studies. The general immunologic response to therapy for the majority of patients is characterised by relatively rapid increases in mean CD4 cell counts during the first 2 years of therapy, followed by smaller but consistent increases through 3–6 years of treatment (2–6).
The long-term immunologic response to therapy with up to 6 years of cART has been studied in several cohorts (4–9). Investigation beyond these years has been evaluated in few cohorts to date, primarily due to lack data (8,10,11). Nevertheless several predictors of higher CD4 cell count trajectories have been identified consistently across these studies, namely younger age, higher CD4 cell count at cART initiation, and virological suppression while receiving cART.
If natural ageing in HIV-negative persons leads to a long-term decline in the immune system, including CD4 cell counts via low-grade chronic immune activation/inflammation (12–15), then one might expect to see a greater or earlier decline of CD4 cell counts in older HIV-positive patients with increasing duration of cART. Long-term HIV infection despite viral suppression from cART has been suggested to accelerate the body’s natural ageing trajectory primarily due to persistent immune activation and cART-induced pro-inflammatory effects leading to premature immunosenescence (ageing of the immune system) (16–21). It is not clear from these studies whether or not natural ageing (measured through the passage of time) coupled with long-term HIV viral suppression through cART impacts long-term CD4 cell count trends.
The aim of this analysis is to describe the long-term changes in CD4 cell counts beyond 5 years of cART. To our knowledge the specific relationship between long-term changes in CD4 cell counts by differing age strata and duration of cART (up to 13 years) has not been previously examined.
Methodology
Data Collection
The study population included in this analysis were patients from the Australian HIV Observational Database (AHOD) for which a comprehensive description of collection methodology has been described elsewhere (22). Briefly, AHOD data collection commenced in 1999 and currently 27 hospitals, sexual health clinics, and general medical practices throughout Australia contribute data every 6 months. All participating sites have ethics approval granted by relevant Human Research Ethics Committees (listed in acknowledgements). Written informed consent was obtained from all participants at enrolment and no further consent was required for the conduct of this analysis. Prospective data are collected on a core set of variables including sex, age, HIV exposure category, hepatitis B virus surface antigen (HBV), hepatitis C antibody status (HCV), CD4 and CD8 cell counts, viral load, cART history, AIDS illnesses, date and cause of death. Retrospective data are provided where available and data are transferred electronically to The Kirby Institute and are subjected to quality control and quality assurance procedures (22). This analysis is based on data collected up to 31st March 2011.
Study Design
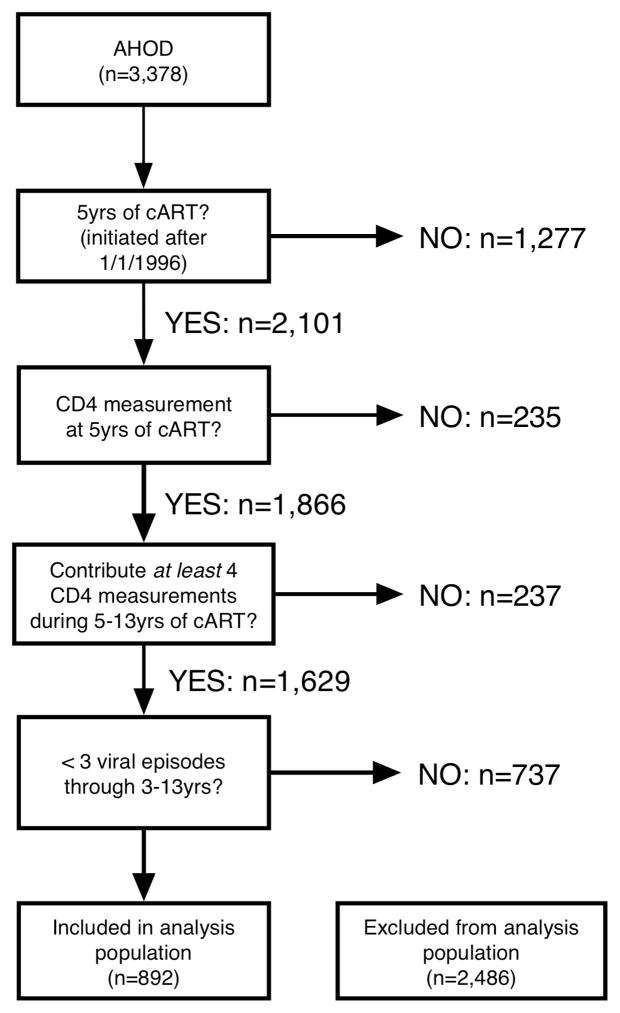
The analysis population inclusion criteria are outlined in Figure 1. The analysis population was selected to be reflective of patients who are under routine clinical care and are long-term virologically stable (VS; defined by follow-up on cART >3 years with at most 2 periods during 3–13 years on cART where median 6 monthly viral load was >1000 copies/ml). A patient was deemed lost to follow-up if they have not been seen at clinic for more than one year. We constructed our definition of long-term VS because it reflects clinical practice. In practice it is not uncommon for a patient to present with a detectable viral load, evaluate treatment options, receive adherence counseling, and subsequently visit with re-suppressed viral load. We chose to evaluate long-term CD4 cell counts trends from the completion of 5 years of cART time-point, as smaller annual rates change (or stabilisation) of CD4 cell counts during 3–5 years of cART have been previously reported in AHOD (9,23) and other studies (2–6).
Figure 1.
Flow diagram and number of patients eligible at each inclusion criteria step.
The longitudinal dataset of individual CD4 cell counts was summarised into mean 6 monthly CD4 cell counts from cART initiation. For patients with multiple observations during each time-point window (±3 months), we calculated a mean CD4 cell count. CD4 cell count values greater than 2,000 cells/μL were excluded from the analysis. Eligible patients needed to have a CD4 cell count measurement at 5 years of cART (±3 months), and contribute at least four CD4 cell count measurements after 5 years of cART.
Statistical Analysis
We modelled mean CD4 cell count changes from the end of the 5th year of cART using restricted maximum likelihood (REML) linear mixed models. To estimate fixed and random effects of covariates we assumed an unstructured covariance relationship for between patient variability and an exchangeable correlation structure for within patient variability. All statistical calculations performed with SAS/STAT software, Version 9.2 of the SAS System for Windows.
Building on prior CD4 cell count modelling work by our group (9,23,24) we selected the fixed effect covariates to be included in the model: sex, hepatitis C antigen serostatus at completion of 5 years of cART, reported mode of HIV transmission, age at cART initiation, CD4 cell count at 5 years of cART, year of cART initiation, prior mono/duo antiretroviral therapy and duration of cART. We allowed the model intercept and duration of cART coefficient to vary randomly with each patient. Covariates were included in the model a priori and no form of model selection was done. Covariate category levels are outlined Table 1. We investigated linear and quadratic rates of change in CD4 cell counts with increasing age and time receiving cART, by a three-way interaction between duration of cART (continuous: per year), age at cART initiation (categorical: <35, 35–50, >50 years old) and CD4 cell count at 5 years of cART (categorical: <500, 500–750, >750 cells/μL). We used a global test of effect size equality for significance and all p-values reported were tested at α=0.05 level unless stated otherwise. We estimated an intercept and slope (duration of cART coefficient) for each patient using the ‘best linear unbiased prediction’ routine as implemented in PROC MIXED, SAS Software v9.2.
Table 1.
Analysis population patient characteristics by age strata at cART initiation.
| Age strata | |||
|---|---|---|---|
| Age at cART initiation | <35 | 35–50 | >50 |
| Years, Mean (IQR) | 30(28–33) | 42(38–46) | 56(52–58) |
| N=241 | N=483 | N=168 | |
| Sex | N (%) | N (%) | N (%) |
| Female | 23 (10) | 23 (5) | 4 (2) |
| Male | 218 (90) | 460 (95) | 164 (98) |
| HIV Exposure Transmission | |||
| Heterosexual | 36 (15) | 45 (9) | 17 (10) |
| Intravenous drug use | 18 (7) | 19 (4) | 2 (1) |
| Other/Unknown | 23 (10) | 41 (8) | 23 (14) |
| Men who have sex with men | 164 (68) | 378 (78) | 126 (75) |
| HBV surface antigen at 5 years of cART | |||
| Yes | 12 (5) | 17 (4) | 2 (1) |
| No/Not tested | 229 (95) | 466 (96) | 166 (99) |
| HCV antibody at 5 years of cART | |||
| Yes | 18 (7) | 33 (7) | 4 (2) |
| No/not tested | 223 (93) | 450 (93) | 164 (98) |
| Prior cART HIV RNA plasma viral load (copies/ml) | |||
| 0–400 | 14 (6) | 34 (7) | 17 (10) |
| 400–104 | 22 (9) | 58 (12) | 15 (9) |
| 104–105 | 73 (30) | 124 (26) | 42 (25) |
| >105 | 70 (29) | 161 (33) | 48 (29) |
| Missing | 62 (26) | 106 (22) | 46 (27) |
| CD4 cell count (cells/μL) at cART initiation | |||
| 0–249 | 70 (29) | 176 (36) | 60 (36) |
| 250–499 | 93 (39) | 155 (32) | 64 (38) |
| 500–749 | 33 (14) | 66 (14) | 14 (8) |
| >750 | 11 (5) | 29 (6) | 8 (5) |
| Missing | 34 (14) | 57 (12) | 22 (13) |
| CD4 cell count (cells/μL) at 5 years of cART | |||
| 0–249 | 19 (8) | 30 (6) | 23 (14) |
| 250–499 | 61 (25) | 156 (32) | 49 (29) |
| 500–749 | 84 (35) | 172 (36) | 61 (36) |
| >750 | 77 (32) | 125 (26) | 35 (21) |
| AIDS prior to cART | |||
| Yes | 42 (17) | 96 (20) | 37 (22) |
| No | 199 (83) | 387 (80) | 131 (78) |
| Mono/Duo ARV usage prior to cART | |||
| Yes | 87 (36) | 206 (43) | 70 (42) |
| No | 154 (64) | 277 (57) | 98 (58) |
| Year of cART initiation | |||
| 1996–1999 | 187 (78) | 360 (75) | 121 (72) |
| 2000–2004 | 54 (22) | 123 (25) | 47 (28) |
| Follow-up time after cART initiation | |||
| median (IQR) years | 11.5 (9–13) | 11.5 (9–13) | 11.5 (9–13) |
| Number of CD4 measurements contributed after 5 years of cART | |||
| median (IQR) | 20 (12–27) | 20 (13–28) | 22 (14.5–31.5) |
We performed several sensitivity analyses to ascertain the robustness of our results. These included: (1) restricting analysis population to patients with strict virological control (i.e. patients who have maintained viral load <1000 copies/ml while on therapy); (2) excluded patients from the analysis population who received mono/duo antiretroviral therapy prior to commencing cART; (3) included CD4 cell count measurements >2000 cells/μL.
Results
Of the 3,378 AHOD patients recruited up to 31st March 2011, 892 (26.4%) were eligible for analysis. The numbers of patients excluded at each inclusion criteria step are outlined in Figure 1. A total of 37,916 CD4 measurements were observed over a combined total of 9,753 patient years (approximately 4 measurements per person per year). The average number of CD4 observations contributed from a patient after the completion of 5 years of cART was 20 (interquartile range [IQR]:13–28) and the median amount of follow-up per patient since initiating cART was 11.5 (IQR:9–13) years. The number of patients lost to follow-up was 91 (10%) and an additional 44 (5%) deaths were recorded. The overall number of patients with an observed virologically uncontrolled period at some stage during follow-up was N=168 (19%). The proportion of patients with observed uncontrolled viraemia during follow-up varied slightly across age at cART initiation strata: patients >50 years had a lower proportion (13%) than the 20% for both the <35 and 35–50 year age groups.
Table 1 describes the patient characteristics of the analysis population by age strata at cART initiation. Patient characteristics were similar across the age strata with the exception for a lower proportion of patients >50 years with positive hepatitis B surface antigen (>50=1% vs <35=5%, 35–50=4%) and hepatitis C antibody (>50=2% vs <35=7%, 35–50=7%). Patients included in the analysis were predominately male and MSM (men who have sex with men) HIV exposure risk. Prior to initiating cART, approximately 20% of patient had an AIDS diagnosis and 40% recorded mono/duo antiretroviral prior to cART. Three quarters of patients initiated cART during the period 1996–1999. Of the patients with a known CD4 cell count at cART initiation (N=779), approximately 40% initiated cART in the range 0–249 cells/μL and 40% in the range 350–500 cells/μL. At the completion of 5 years of cART 62% of patients recorded a CD4 count >500 cells/μL.
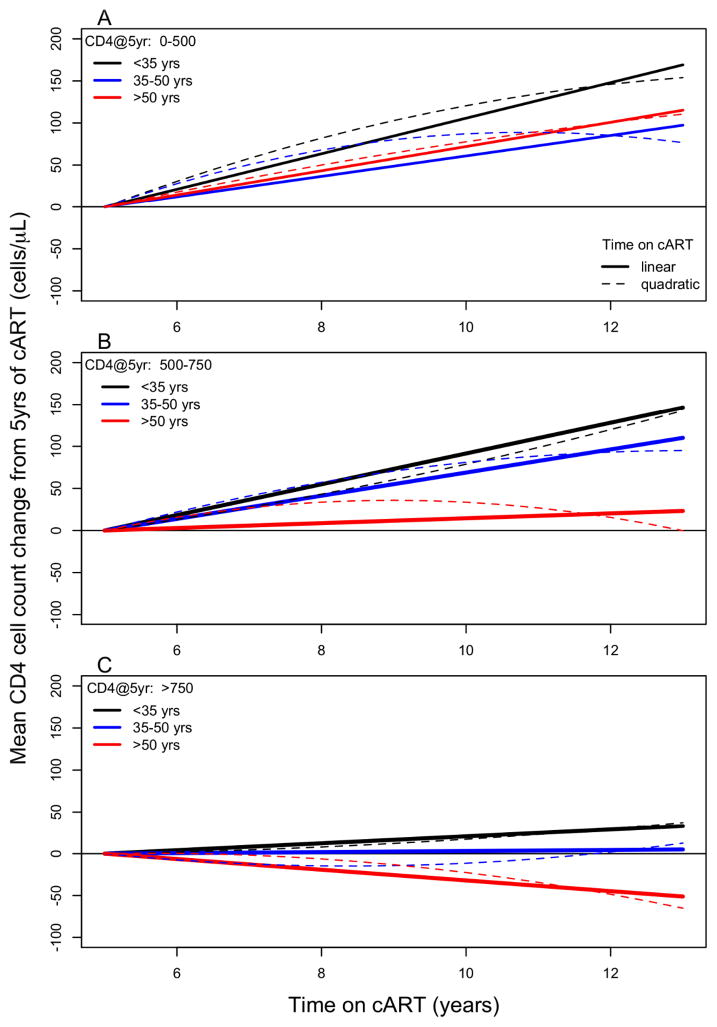
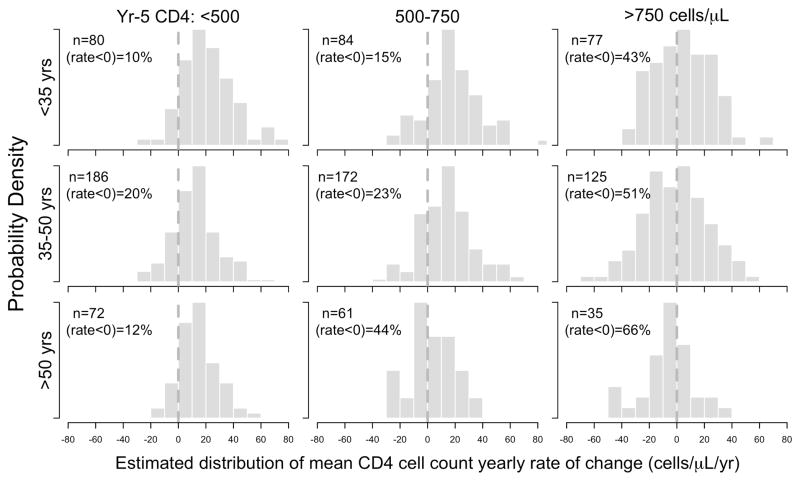
Mean CD4 cell count changes after 5 years of cART were predominately predicted by CD4 cell count strata at completion of 5 years of cART, age strata at cART initiation and their separate interactions with duration of cART (Year-5 CD4 count x time interaction p=0.0001, age x time interaction p=0.0061). The combined three-way interaction between age, CD4 count and time did not reach statistical significance (p=0.15). This signifies that the estimated interaction between age group and time is not significantly different across each level of the Year-5 CD4 count group. Table 2 and Figure 2 summarise the estimated long-term mean change in CD4 cell counts as specified under a combined three-way interaction model between age, CD4 count and duration of cART. Figure 3 plots the estimated distribution of yearly CD4 rates of change (cells/μL/year) for each age and year-5 CD4 cell count strata.
Table 2.
Model parameter estimates& for mean (95% confidence interval) CD4 cell count changes following completion of 5 years of cART.
| Intercept# (cells/μL) | Slope# (cells/μL/year) | ||
|---|---|---|---|
| CD4 count @ 5 Years of cART: 0 – 500 cells/μL | |||
| Age (years) | <35 | 396 (360 to 432)* | 21 (14 to 28)* |
| 35–50 | 412 (386 to 437)* | 12 (8 to 17)* | |
| >50 | 336 (298 to 373)* | 14 (7 to 21)* | |
| CD4 count @ 5 Years of cART: 500 – 750 cells/μL | |||
| Age (years) | <35 | 649 (614 to 683)* | 18 (11 to 25)* |
| 35–50 | 650 (624 to 676)* | 14 (9 to 18)* | |
| >50 | 647 (607 to 687)* | 3 (−5 to 11) | |
| CD4 count @ 5 Years of cART: >750 cells/μL | |||
| Age (years) | <35 | 937 (901 to 972)* | 4 (−3 to 11) |
| 35–50 | 956 (927 to 985)* | 1 (−5 to 6) | |
| >50 | 936 (885 to 987)* | −6 (−17 to 4) | |
| Interaction P-values^ | |||
| Age x Duration of cART | 0.0061 | ||
| CD4@5 years x Duration of cART | 0.0001 | ||
| Age x CD4@5 years of cART x Duration of cART | 0.1489 | ||
Wald significance test: Ho: parameteri=0,
significant at α=0.05 level
Type III F-test (global significance)
Model adjusted for sex (ref=male), mode of HIV exposure (ref=MSM), HCV antibody (ref=no/not tested), year of cART initiation (ref=1996–1999) and prior usage of antiretroviral therapy (ref=no).
Figure 2(A–C).
Estimated mean long-term CD4 cell count changes following the completion of 5 years of cART for equivalent patients differing only by age at cART initiation. Presented by age strata and Year-5 CD4 cell count strata.
Figure 3.
Estimated distribution of mean CD4 cell count yearly rate of change (cells/μL/year) by age and Year-5 CD4 cell count strata.
Younger patients (<35 years) at cART initiation had estimated mean (95% confidence interval) yearly change in CD4 counts (cells/μL/year) from 5 years of cART of 21(14 to 28), 18(11 to 25) and 4(−3 to 11) cells/μL/year by Year-5 CD4 count strata 0–500, 501–750, and >750 cells/μL respectively. Patients who initiated cART aged 35–50 years had estimated mean yearly CD4 counts changes of 12(8 to 17), 14(9 to 18) and 1(−5 to 6) cells/μL/year by Year-5 CD4 count strata respectively. Patients >50 years of age at cART initiation had estimated mean yearly CD4 count changes of 14(7 to 21), 3(−5 to 11) and −6(−17 to 4) cells/μL/year by Year-5 CD4 count strata respectively. Of the yearly rates of changes estimated for each level of the Year-5 CD4 count and age interaction, none were found to be indicative of decreasing mean CD4 cell count change, even for the older patients who are aged up to 60–70 years old.
The quadratic trend for duration of cART used to assess accelerated declining rates of change in CD4 with increasing age did not reach statistical significance (p=0.15). Table S1 describe the model parameter estimates and associated standard errors. The quadratic trend model did not indicate superior model fit as determined by common model selection criteria (results not shown) and by inspection of Figure 2. Qualitatively similar results were obtained for all modeling sensitivity analyses [1]–[3] as defined in the Methodology section, Table S2.
Discussion
We found that long-term CD4 cell count changes following 5 years of cART in virologically controlled patients were predicted predominantly by age strata at cART initiation, Year-5 CD4 cell count strata, and their interaction with duration of cART. Our results suggest that independent of Year-5 CD4 cell count, older persons (>50 years old) tended to have smaller annual changes in CD4 cell count year-to-year compared to those <35 years old. Inspecting Figure 2 we conclude that older patients who achieve a CD4 cell count >500 cell/μL after 5 years of cART will typically further experience only minimal increases of CD4 cell counts year-to-year. Our analysis did not suggest that on average CD4 counts would decrease in older patients, at least in those aged up to around 65 years and with 10+ years experience of cART.
Under the mixed modeling framework, we are able to calculate individual patient intercepts and slopes. Figure 3 shows the estimated distribution of yearly rates of change by age at cART initiation and Year-5 CD4 cell count strata. This plot shows that although we are reporting “on average” no evidence of declining CD4 cell counts with increasing older age, there are clearly some patients within our analysis population that are experiencing declines in mean CD4 cell count over time. This is evident in patients across all Year-5 CD4 strata and especially those who with Year-5 CD4 count >750 cells/μL. Within each age grouping for Year-5 CD4 count stratum >750 cells/μL, the proportion of patients whose yearly rate of change less than 0 cells/μL/year is between 43–66%. We are therefore cautious in interpreting any differences in estimated mean CD4 rates of change between older (>50 years) and younger patients(<35 years) within this stratum, 4(−3 to 11) vs. −6(−17 to 4) cells/μL/year respectively.
Differing model and analysis specifications make it difficult to compare results exactly across studies, though qualitatively our results are consistent with other studies. A recent analysis from the Multicenter AIDS Cohort Study (MACS) (11) investigating CD4 cell counts and plasma HIV RNA levels beyond 5 years of cART reported minimal changes in CD4 cell counts 5–12 years after cART initiation by differing CD4 counts strata at cART initiation; 11 (7 to 15) cells/μL/year for pre-cART CD4 count ≤350 cells/μL and 2 (−4 to 8) cells/μL/year for pre-cART CD4 count >350 cells/μL. The authors also reported that equivalent patients differing only by age (younger and older) have significantly different long-term immunological outcomes. Specifically, older patients would need to initiate cART with a CD4 counts higher than younger patients to achieve the same levels of immunological success at 5–12 years of cART, suggesting smaller rates of change in CD4 counts per year for older patients.
Analyses from the EuroSIDA group have calculated rates of change for CD4 cell counts from two different methodologies, successive CD4 records (8) and CD4 time-series equated into concurrent viral load strata (25). Extracting comparable rates, the first analysis presented annual rates of change by CD4 count at cART initiation and time on cART. For patients who initiated treatment with a CD4 count >350 cells/μL and received treatment for >5 years, the rate of change was 21 cells/μL/year (confidence interval −12 to 54 cells/μL/year) and no significant interaction with age was found. From the second analysis, using the most comparable stratum (viral load<500 copies/ml), the reported rates of increase in CD4 cell counts was 30 (27 to 35) cells/μL/year. The higher annual rate of change (compared to our result) is likely explained by analysis design differences. In the EuroSIDA analysis, patients were eligible for analysis shortly after cART initiation whereas in our study patients were eligible after 5 years of cART, a period in which the largest increases in CD4 cell counts have largely occurred.
We hypothesised that additional immune activation experienced in all long-term virologically suppressed HIV-positive persons would eventually be reflected in a decreasing mean change in CD4 cell counts per year with increasing older age. Our data shows that older persons at cART initiation (>50 years old), tended to have either on average slightly increasing CD4 cell counts year-to-year (where Year-5 CD4 count <500 cells/μL) through 5–13 years of cART; or on average no change in CD4 cell counts year-to-year (where Year-5 CD4 count 500–750 or >750 cells/μL) through 5–13 years of cART. Our results further iterates the findings that long-term immune reconstitution as measured by CD4 count recovery, continually occurs in patients who have low CD4 counts despite long-term viral suppression (3,5,6,26,27). We found no evidence of accelerated or gradual long-term declines in CD4 cell counts across age strata and Year-5 CD4 count strata. Although we have reported different long-term responses to cART by age strata, we are uncertain as to how these differences would translate into long-term clinical outcomes.
A potential criticism to our analysis is the lack of a healthy HIV-negative control group to delineate the effects of HIV, cART and ageing on long-term CD4 cell count trends. To our knowledge there are no published population-comparable longitudinal data for long-term CD4 cell count trends in HIV-negative populations. Several cross-sectional studies have established relationships between CD4 cell counts and older age in HIV-negative populations (13,28). Both analyses evaluate CD4 cell counts across a broad range of age groupings and report a declining trend in absolute counts (13,28) and percentages (28) with increasing age. It should be noted that even though both studies report a declining trend, mean CD4 cell counts for older HIV-negative individuals (>60 years old) are well above 700 cells/μL. This is considerably higher than most mean CD4 cell counts observed across the different age strata in AHOD, even after 5–10 years of cART.
There are limitations to our analysis. First, AHOD is an observational cohort study of HIV-positive adults under routine clinical care from a non-random selection of sexual health clinics, hospitals and private general practitioners. AHOD is unlikely to be representative of all HIV-positive persons in Australia, particularly those not seeking treatment or those from marginalised populations. However given the strict eligibility inclusion criteria for our analysis, we argue that our results presented here would be “best-case” long-term trends for persons living with HIV, as patients included in the analysis are able to achieve long-term virus suppression and maintain routine clinical care.
Second, although the statistical methods used in this analysis account for lost-to-follow-up and missing data as best as possible to reduce bias in our results, we were not able to quantify and adjust for population selection bias. The analysis study population in our analysis does not contain patients who have had substantial uncontrolled viraemia for large periods of time. In this group we expect to see declines in CD4 cell counts due high levels of circulating viraemia and by selectively removing this group from our analysis we can eliminate some confounding of the effects of untreated HIV (which impact negatively on CD4 cell counts) from any CD4 cell count changes attributed to increasing age.
Third, our results are based on data from patients that have mostly been receiving cART for 9–13 years and our older persons are aged up to 70 years. Therefore extrapolating our results beyond these boundaries could be problematic. Further monitoring and evaluation of CD4 cell count trends or other markers in immunity in older long-term HIV-positive patients should continue.
In summary, our results suggest that duration of cART and increasing age does not result in decreasing mean changes in CD4 cell counts for long-term virologically suppressed patients, indicating that sustained levels of immune recovery achieved during the first 5 years of treatment are ongoing under long-term on-going cART, at least up to 15 years of treatment.
Supplementary Material
Acknowledgments
The Australian HIV Observational Database is funded as part of the Asia Pacific HIV Observational Database, a program of The Foundation for AIDS Research, amfAR, and is supported in part by a grant from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID) (Grant No. U01-AI069907) and by unconditional grants from Merck Sharp & Dohme; Gilead; Bristol-Myers Squibb; Boehringer Ingelheim; Roche; Pfizer; GlaxoSmithKline; Janssen-Cilag. The Kirby Institute (formally the National Centre in HIV Epidemiology and Clinical Research) is funded by The Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to acknowledge all of the contributors and collaborators of the Australian HIV Observational Database cohort study without whom, this work would not have been possible (Appendix 1).
Appendix 1 Australian HIV Observational Database collaborators
Asterisks indicate steering committee members in 2012.
New South Wales: D Ellis, General Medical Practice, Coffs Harbour; M Bloch, T Franic*, S Agrawal, L McCann, N Cunningham, T Vincent, Holdsworth House General Practice, Darlinghurst; D Allen, JL Little, Holden Street Clinic, Gosford; D Smith, C Gray, Lismore Sexual Health & AIDS Services, Lismore; D Baker*, R Vale, East Sydney Doctors, Surry Hills; DJ Templeton*, CC O’Connor, C Dijanosic, RPA Sexual Health Clinic, Camperdown; E Jackson, K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba; M Grotowski, S Taylor, Tamworth Sexual Health Service, Tamworth; D Cooper, A Carr, F Lee, K Hesse, K Sinn, R Norris, St Vincent’s Hospital, Darlinghurst; R Finlayson, I Prone, Taylor Square Private Clinic, Darlinghurst; E Jackson, J Shakeshaft, Nepean Sexual Health and HIV Clinic, Penrith; K Brown, C McGrath, V McGrath, S Halligan, Illawarra Sexual Health Service, Warrawong; L Wray, P Read, H Lu, Sydney Sexual Health Centre, Sydney; D Couldwell, Parramatta Sexual Health Clinic; D Smith, V Furner, Albion Street Centre; Dubbo Sexual Health Centre, Dubbo; J Watson*, National Association of People living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; B Mulhall*, Department of Public Health and Community Medicine, University of Sydney; M Law*, K Petoumenos*, S Wright*, H McManus*, C Bendall*, M Boyd*, The Kirby Institute, University of NSW.
Northern Territory: A Kulatunga, P Knibbs, Communicable Disease Centre, Royal Darwin Hospital, Darwin.
Queensland: J Chuah*, M Ngieng, B Dickson, Gold Coast Sexual Health Clinic, Miami; D Russell, S Downing, Cairns Sexual Health Service, Cairns; D Sowden, J Broom, K Taing, C Johnston, K McGill, Clinic 87, Sunshine Coast-Wide Bay Health Service District, Nambour; D Orth, D Youds, Gladstone Road Medical Centre, Highgate Hill; M Kelly, A Gibson, H Magon, Brisbane Sexual Health and HIV Service, Brisbane.
South Australia: W Donohue, O’Brien Street General Practice, Adelaide.
Victoria: R Moore, S Edwards, R Liddle, P Locke, Northside Clinic, North Fitzroy; NJ Roth*, J Nicolson*, H Lau, Prahran Market Clinic, South Yarra; T Read, J Silvers*, W Zeng, Melbourne Sexual Health Centre, Melbourne; J Hoy*, K Watson*, M Bryant, S Price, The Alfred Hospital, Melbourne; I Woolley, M Giles, T Korman, J Williams, Monash Medical Centre, Clayton.
Western Australia: D Nolan, J Skett, J Robinson, Department of Clinical Immunology, Royal Perth Hospital, Perth.
CoDe reviewers
AHOD reviewers: D Sowden, DJ Templeton, J Hoy, L Wray, J Chuah, K Morwood, T Read, N Roth, I Woolley, M Kelly, J Broom.
TAHOD reviewers: PCK Li, MP Lee, S Vanar, S Faridah, A Kamarulzaman, JY Choi, B Vannary, R Ditangco, K Tsukada, SH Han, S Pujari, A Makane, , OT Ng, AJ Sasisopin.
Independent reviewers: F Drummond, M Boyd.
Footnotes
Conflict of interest: nothing to declare.
References
- 1.May MT, Sterne JAC, Costagliola D, Sabin CA, Phillips AN, Justice AC, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006 Aug 5;368(9534):451–8. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003 Oct 13;163(18):2187–95. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 3.Hunt PW, Deeks SG, Martin JN. 12. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003 Sep 4;17(13):1907–15. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 4.Viard J-PJ, Burgard MM, Rouzioux CC. 9. Impact of 5 years of maximally successful highly active antiretroviral therapy on CD4 cell count and HIV-1 DNA level. AIDS. 2004 Jan 1;18(1):45–9. doi: 10.1097/00002030-200401020-00005. [DOI] [PubMed] [Google Scholar]
- 5.Le Moing V, Thiébaut R Group ACAS. 11. Long-term evolution of CD4 count in patients with a plasma HIV RNA persistently. HIV Med. 2007 Mar 31;8(3):156–63. doi: 10.1111/j.1468-1293.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 6.Moore RD, Keruly JC. CD4(+) cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clinical Infectious Diseases. 2007;44(3):441–6. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 7.Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. The Lancet Infectious Diseases Elsevier. 2006;6(5):280–7. doi: 10.1016/S1473-3099(06)70463-7. [DOI] [PubMed] [Google Scholar]
- 8.Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007 Aug 4;370(9585):407–13. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 9.Egger S, Petoumenos K, Kamarulzaman A, Hoy J, Sungkanuparph S, Chuah J, et al. Long-term patterns in CD4 response is determined by an interaction between baseline CD4 cell count, viral load and time: the Asia Pacific HIV Observational Database (APHOD) J Acquir Immune Defic Syndr NIH Public Access. 2009;50(5):513. doi: 10.1097/qai.0b013e31819906d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes R, Sterne J, Sabin C. 19. Long-term trends in CD4 cell counts and impact of viral failure in individuals starting antiretroviral therapy: UK Collaborative HIV Cohort (CHIC) study. HIV Med. 2011 May 15;15(4):583–93. doi: 10.1111/j.1468-1293.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Margolick JB, Jamieson BD, Rinaldo CR, Phair JP, Jacobson LP. CD4+ T-cell counts and plasma HIV-1 RNA levels beyond 5 years of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2011 Aug 15;57(5):421–8. doi: 10.1097/QAI.0b013e31821e9f21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rea IM. BELFAST nonagenarians: nature or nurture? Immunological, cardiovascular and genetic factors. Immun Ageing. 2010;7(1):6. doi: 10.1186/1742-4933-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wikby AA, Månsson IAI, Johansson BB, Strindhall JJ, Nilsson SES. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology. 2008 Sep 30;9(5):299–308. doi: 10.1007/s10522-008-9138-6. [DOI] [PubMed] [Google Scholar]
- 14.Dorshkind K, Montecino-Rodriguez E, Signer RAJ. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009 Jan;9(1):57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 15.Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, et al. The immune system in extreme longevity. Exp Gerontol. 2008 Feb;43(2):61–5. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Appay V, Rowland-Jones SL. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 2002 Dec;23(12):580–5. doi: 10.1016/s1471-4906(02)02338-4. [DOI] [PubMed] [Google Scholar]
- 17.Capeau J. Premature Aging and Premature Age-Related Comorbidities in HIV-Infected Patients: Facts and Hypotheses. Clinical Infectious Diseases. 2011 Oct 12; doi: 10.1093/cid/cir628. [DOI] [PubMed] [Google Scholar]
- 18.De Biasi S, Pinti M, Nasi M, Gibellini L, Bertoncelli L, Manzini S, et al. HIV-1 Infection and the Aging of the Immune System: Facts, Similarities and Perspectives. J Exp Clin Med Elsevier. 2011;3(4):143–50. [Google Scholar]
- 19.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2008 Dec 31;338:a3172–2. doi: 10.1136/bmj.a3172. (jan26 2) [DOI] [PubMed] [Google Scholar]
- 20.Guaraldi G, Orlando G, Palella F. 10. Premature Age-Related Comorbidities Among HIV-Infected Persons Compared With the General Population. Clinical Infectious Diseases. 2011 Oct 12; doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 21.Hearps AC, Angelovich TA, Jaworowski A, Mills J, Landay AL, Crowe SM. HIV infection and aging of the innate immune system. Sex Health. 2011 Dec;8(4):453–64. doi: 10.1071/SH11028. [DOI] [PubMed] [Google Scholar]
- 22.Australian HIV Observational Database. Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med. 2002;3(1):28. doi: 10.1046/j.1464-2662.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 23.Wright ST, Carr A, Woolley I, Giles M, Hoy J, Cooper DA, et al. CD4 cell responses to combination antiretroviral therapy in patients starting therapy at high CD4 cell counts. J Acquir Immune Defic Syndr. 2011 Sep 1;58(1):72–9. doi: 10.1097/QAI.0b013e318225ba62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajasuriar R, Gouillou M, Spelman T, Read T, Hoy J, Law M, et al. Clinical Predictors of Immune Reconstitution following Combination Antiretroviral Therapy in Patients from the Australian HIV Observational Database. PloS one Public Library of Science. 2011;6(6):e20713. doi: 10.1371/journal.pone.0020713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mocroft A, Phillips AN, Ledergerber B, Smith C, Bogner JR, Lacombe K, et al. Estimated average annual rate of change of CD4(+) T-cell counts in patients on combination antiretroviral therapy. Antivir Ther (Lond) 2010;15(4):563–70. doi: 10.3851/IMP1559. [DOI] [PubMed] [Google Scholar]
- 26.Gras L, Kesselring AM, Griffin JT, van Sighem AI, Fraser C, Ghani AC, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr. 2007 Jun 1;45(2):183–92. doi: 10.1097/QAI.0b013e31804d685b. [DOI] [PubMed] [Google Scholar]
- 27.Smith CJ, Sabin CA, Youle MS, Kinloch-de Loes S, Lampe FC, Madge S, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. J Infect Dis. 2004 Nov 15;190(10):1860–8. doi: 10.1086/425075. [DOI] [PubMed] [Google Scholar]
- 28.Rea IM, Stewart M, Campbell P, Alexander HD, Crockard AD, Morris TC. Changes in lymphocyte subsets, interleukin 2, and soluble interleukin 2 receptor in old and very old age. Gerontology. 1996;42(2):69–78. doi: 10.1159/000213775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.