Abstract
Background
Extensive data, primarily from animal studies, suggest that several classes of drugs may have anti-neuroplastic effects that could impede recovery from brain injury or reduce the efficacy of rehabilitation.
Aims
The Locomotor Experience Applied Post-Stroke (LEAPS) trial, a randomized controlled study of 408 subjects that tested the relative efficacy of two rehabilitation techniques on functional walking level at one year post-stroke, provided us the opportunity to prospectively assess the potential anti-neuroplastic effects of several classes of drug.
Methods
Subjects were randomized to receive one of the two rehabilitation therapies at 2-months post stroke. Drugs taken were recorded at time of randomization. Outcome was assessed at one year post-stroke. Regression models were used to determine the amount of variance in success in improving functional walking level, gains in walking speed, and declines in lower extremity, upper extremity, and cognitive impairment accounted for by α1 noradrenergic blockers + α2 noradrenergic agonists; benzodiazepines; voltage-sensitive sodium channel anticonvulsants; and α2δ voltage-sensitive calcium channel blockers.
Results
The maximum variance accounted for by any drug class was 1.66%. Drug effects were not statistically significant when using even our most lenient standard for correction for multiple comparisons.
Conclusions
Drugs in the classes we were able to assess do not appear to exert a clinically important effect on outcome over the period between 2- and 12-months post-stroke. However, the potential antineuroplastic effects of certain drugs remains an incompletely settled scientific question.
Keywords: neurorehabilitation, anticonvulsants, alpha-1 noradrenergic blockers, alpha-2 noradrenergic agonists, voltage-sensitive calcium channel blockers
Introduction
Neuroplasticity can be logically divided into reactive neuroplasticity and experience-dependent neuroplasticity. Reactive neuroplasticity consists of reduction in necrotic and apoptotic cell death and enhancement of angiogenesis, neurogenesis, neural migration, axonal growth, expansion of dendritic spines, and synaptogenesis, and it is maximal in the days and weeks following a neural injury (1, 2). Experience dependent neuroplasticity involves normal learning mechanisms, including non-declarative memory acquisition (e.g., procedural memory), which takes place directly in the neural structures supporting the functions involved, and declarative memory acquisition, which depends upon the hippocampus and associated mesial temporal structures. It predominantly involves genesis of dendritic spines, synaptogenesis, and modification of existing synapses (3). Neurorehabilitation most explicitly targets experience-dependent neuroplasticity and its impact on reactive neuroplasticity is largely unknown.
A number of drug classes have been shown, predominantly in animal studies but also in one study of human subjects (4), to inhibit neuroplasticity, as reflected in their effects on rate and magnitude of recovery. These include anti-cholinergic agents (see review (5)), voltage-sensitive sodium channel active anticonvulsants (e.g., phenytoin (6)), GABAergic anticonvulsants (7),α1 noradrenergic blockers (8),α2 noradrenergic agonists (9), GABAergic agents (e.g., benzodiazepines (10, 11) but not zopiclone (12)), and neuroleptics (e.g., haloperidol (8, 13, 14) but not clozapine (14) or risperidone (15)). Administration of anti-neuroplastic drugs to patients could both impede recovery from brain injury and reduce the efficacy of rehabilitation.
Only the adverse effects on learning produced by anticholinergic agents have been directly demonstrated in human subjects (all normal volunteers) (16, 17), and ethical concerns preclude the application of standard clinical trial methodology to the testing of potential anti-neuroplastic drug effects in subjects undergoing neurorehabilitation after brain injury. One prior prospective study of human subjects with stroke suggested that potentially anti-neuroplastic drugs prescribed by treating physicians had a small but statistically significant effect on motor outcome during the first three months after stroke (4).
The Locomotor Experience Applied Post-Stroke (LEAPS) trial is a large, multi-center, randomized controlled trial (RCT) of rehabilitation interventions for gait impairment after stroke (18, 19). It provides us the opportunity to pursue this secondary analysis in which we prospectively assess the impact of potentially anti-neuroplastic drugs on functional outcome between 2 and 12 months post-stroke in a considerably larger number of participants who, unlike the subjects in the study of Goldstein et al., participated in rehabilitation therapy of proven efficacy. LEAPS did not involve a drug intervention, but many of the 408 recruited subjects were taking potentially anti-neuroplastic drugs prescribed by their physicians.
The LEAPS trial compared two types of rehabilitation interventions provided by physical therapists to improve walking after disabling first stroke. LEAPS targeted adults who had hemiparesis due to a stroke severe enough to require inpatient rehabilitation, followed by discharge home. At entry 2-months after onset, participants were still limited to walking with assistance in the home or to walking short distances in the community. Interventions were: (1) a progressive, task-specific locomotor training program (LTP) that included walking on a treadmill with partial body weight-support and over-ground practice and (2) a progressive strength and balance exercise program delivered in the home (Home Exercise Program, HEP). LTP and HEP were delivered at 2-months (early) post-stroke in addition to usual care. A delayed LTP group received the intervention at 6-months post-stroke. Contrary to our original hypothesis, the task-specific LTP program provided early or late was not superior in improving 1-year walking ability compared to the impairment-targeted exercise program, HEP, and early LTP was not superior to late LTP. With both interventions, over 50% of the study population improved walking ability at 1-year, as defined by a transition to a higher functional walking level, regardless of severity of impairment. These results enable us to use all 408 LEAPS subjects in the present study.
Anti-neuroplastic effects of a drug can become evident only to the extent that there is a demonstrable neuroplastic response. In the LEAPS trial, mean baseline walking speed was 0.38 m/s (SD 0.23). Over the 10 months of the trial, walking speed increased by 0.24 m/s (SD 0.22), i.e., 63% (19). This increase, which we assume to predominantly reflect neuroplastic response, constituted the substrate for drug effect. The hypothesis motivating this study was that, because the drugs studied act to inhibit neurophysiologic processes involved in neuroplasticity, the use of these drugs during the 10 months of the LEAPS trial would significantly attenuate gains in function.
Methods
Study Design
The LEAPS trial was a multi-center, single-blind randomized controlled trial (RCT), stratified by walking impairment level at 2-months after onset of stroke (severe, < 0.40 m/s, and moderate, 0.40–<0.80 m/s), with randomization to three protocol intervention groups, early LTP, late LTP, and HEP (proportions 7:7:6).
The LEAPS protocol has been reported (18). Ethics review boards at all participating centers approved the protocol. All participants provided written informed consent. An independent medical monitor and a data safety monitoring board (DSMB) appointed by the National Institutes of Health (NIH) oversaw the conduct, safety, and efficacy of the trial and monitored adverse events. The 12-month primary outcomes have been reported (19).
Study Population and Screening
Participants were recruited from 6 inpatient rehabilitation sites in California and Florida. Inclusion criteria were age ≥ 18 years; stroke within 45 days and ability to be randomized at 2 months post-stroke; residual paresis in the lower extremity; ability to walk 10 feet with no more than 1-person assistance; ability to follow a three-step command; physician approval for participation; self-selected 10 meter walking speed less than 0.8 m/s; and living in the community by the time of randomization. Exclusion criteria included dependency in activities of daily living, exercise contraindications, pre-existing neurological disorders, and inability to travel to the treatment site.
Interventions
The LTP and HEP programs were controlled for exercise frequency (90 minute sessions, 3 times per week) and duration (12 to 16 weeks), for a total of 30–36 exercise sessions. LTP included stepping on a treadmill with partial body weight support for 20–30 minutes at 2.0 mph, with manual assistance as needed, followed by a progressive over ground walking program sustained for 15 minutes provided by a physical therapist and rehabilitation technician(s) (0–2) in the clinic. HEP included progressive flexibility, joint range of motion, upper- and lower-extremity strength, coordination, and static and dynamic balance exercises provided by a physical therapist in the home. In addition to the LTP and HEP interventions, all participants were allowed to receive any prescribed usual and customary care during the intervening periods.
Drugs
Drugs received by LEAPS participants were prescribed by their physicians and were not influenced either by participation in the Trial or by the Trial physicians. Drugs taken were assessed through examination of drug lists provided by patients, recording from pill bottle labels, and direct inquiry of participants at the time of randomization, 2 months post-stroke (Table 1). Patient self-report has been shown to have high agreement with pharmacy and medical records for the types of drug reported here (20–22). Neither drug dose nor duration of drug use post-randomization was documented.
Table 1.
Potentially anti-neuroplastic drugs taken by the study population
| Drug class | Drug | N |
|---|---|---|
| Agents with anticholinergic effects | ||
| Tricyclic antidepressants | 4 | |
| Other (diphenhydramine, benztropine) | 2 | |
| C2 | Benzodiazepines | 33 |
| C3 | α1-noradrenergic agents | |
| α1 noradrenergic blockers (prazosin, doxazosin, tamsulosin, terazosin) | 35 | |
| α2 noradrenergic agonists (clonidine) | 42 | |
| Neuroleptics | 7 | |
| C4 | Anticonvulsants | |
| C5 | Voltage-sensitive sodium channel anticonvulsants | |
| Phenytoin | 12 | |
| Carbamazepine | 1 | |
| Lamotrigine | 1 | |
| Topiramate | 1 | |
| Valproate | 2 | |
| GABAergic anticonvulsants (phenobarbital) | 1 | |
| C6 | α2δ voltage-sensitive calcium channel agents | |
| Gabapentin | 27 | |
| Pregabalin | 3 | |
| Levetiracetam | 13 |
Outcomes
Five outcomes served as the dependent measures in the present study: functional walking level, self-selected walking speed (the two LEAPs primary outcome measures), impairment based measures of lower and upper extremity function (Fugl-Meyer [FM] lower extremity [LE] and upper extremity [UE] motor scores, respectively) (23), and a cognitive measure. Improved functional walking level was defined as the ability to walk independently at a speed of 0.4 m/s or greater for persons with initially severe walking impairment (< 0.4 m/s), or at a speed of 0.8 m/s or greater for persons with initially moderate walking impairment (≥ 0.4 m/s – <0.8 m/s) (24, 25). In general, stroke patients who walk at less than 0.4 m/s are largely confined to home. Those who walk at between 0.4 and 0.8 m/s are capable of limited community ambulation. Those who walk at greater than 0.8 m/s are capable of full community ambulation. We employed the FM measures so that this study would be more nearly comparable to that of Goldstein et al. (4), who employed the Toronto Stroke Scale, which is also an impairment based measure. We included UE and cognitive function in our analysis to determine the possible impact of potentially anti-neuroplastic drugs on an untreated domain in which experience-dependent plasticity predominantly involves procedural memory recovery and re-acquisition, and an untreated domain in which experience-dependent plasticity substantially involves declarative memory recovery and re-acquisition (cognitive function). The cognitive measure was defined by the average of Z-scores for the Mini-Mental State Exam (MMSE) (26), the Wechsler Adult Intelligence Scale (WAIS) digit symbol subtest (27), and Trailmaking B-A (Trails B minus Trails A) (28). The change Z-score for each subject was computed by subtracting the baseline mean Z score from the 12-month mean, and then dividing by the population standard deviation at baseline.
Statistical Analysis
In this paper, we studied the effects of the following classes of drugs: 1) benzodiazepines; 2) α1 noradrenergic blockers + α2 noradrenergic agonists; and 3) voltage-sensitive sodium channel anticonvulsants. α2 noradrenergic agonists were combined with α1 noradrenergic blockers for the purposes of this analysis because central nervous system α2 noradrenergic receptors sensitive to the action of clonidine (the only such drug in this study) are presynaptic and stimulation of them inhibits norepinephrine release (29). We also tested the hypothesis that agents active at the α2δ site of voltage-sensitive calcium channels (gabapentin, pregabalin) might inhibit neuroplasticity by reducing the quantity of neurotransmitter released by each action potential. Finally, we tested the effects of two classes of drugs that logically served as controls: β-noradrenergic blockers, for which there is no evidence of an anti-neuroplastic effect, and serotonin selective reuptake inhibitors (SSRIs), for which there is phase 2 RCT evidence of a possible adjuvant effect on neuroplasticity in the rehabilitation setting (30).
In our first analysis, the dependent variable was achievement of improvement in functional walking ability at 12 months (yes/no). We assessed the proportion of subjects who improved functional walking ability (defined in the previous section) among those prescribed one of the six different drug categories listed above; any anticonvulsant (voltage-sensitive sodium channel anticonvulsants, levetiracetam, or an α2δ active agent); and any of the potentially anti-neuroplastic agents listed in Table 1. A logistic regression model was developed including only baseline covariates: baseline severity of walking impairment (severe, < 0.40 m/s, and moderate, 0.40–<0.80 m/s), clinical site, age, stroke type (large vessel distribution, lacunar, or hemorrhage), side of hemiparesis, and depression as the independent variables. Eight additional logistic regression models were then created by adding one drug use indicator as an independent variable to the baseline logistic regression model in order to test the amount of additional variance in outcome accounted for by each drug class. The McKelvey-Zavoina method (31) was used to measure the increment in proportion of explained variance in the dichotomized response accounted for by each drug.
In our second analysis, self-selected walking speed at 12 months post-stroke was the dependent variable. This analysis, rather than testing the impact of drug class on the ability of a subject to achieve a functionally meaningful increment in walking level, tested the potential incremental impact of a drug class on walking speed. A linear regression model was developed that included the six baseline covariates employed in the first analysis as the independent variables. Eight additional linear regression models were then created by adding one drug use indicator as an independent variable to the baseline model in order to test the amount of additional variance in outcome accounted for by each drug class.
Further analyses using linear regression models were performed for each of the three additional dependent variables, FM-LE, FM-UE and the cognitive measure, assessed at 12-months post-stroke. In each case, we used the six baseline covariates employed in the first analysis plus the baseline value of the dependent measure as independent variables and proceeded to eight additional models testing the additional variance accounted for by each drug class.
In the first two analyses, the last-observation-carried-forward method was used to handle missing data. Complete case analyses were performed for FM-LE, FM-UE and the cognitive measure. None of the statistical results was corrected for multiple comparisons. We performed a total of 40 different regression analyses. Full Bonferroni correction would yield a threshold p value of 0.0013. Alternatively, each drug analysis could be viewed as testing an individual hypothesis, in which case a more lenient standard employing correction for the five outcome variables tested might be considered sufficient, yielding a threshold p value of 0.01. All statistical analyses were performed using SAS software, version 9.1.
Results
The LEAPS trial involved 408 participants. The mean age was 62.0±12.7 years; 54.9% of the participants were men and 22.1% were Black or African-American. At randomization participants were 63.8±8.5 days post-stroke; 40% had ischemic large vessel distribution strokes, 31% lacunar infarctions, 17% hemorrhages, and in 12% stroke type was undefined. Modified Rankin score was 1 in 0.5%, 2 in 13%, and 3–4 in 86%. Fifty-three percent walked < 0.4 m/s and 46.6% walked 0.4–<0.8 m/s. Twenty-seven percent had cardiovascular disease, 81% hypertension, 9% peripheral vascular disease, 6% chronic obstructive pulmonary disease, 36% musculoskeletal disease, 33% diabetes, and 10% depression.
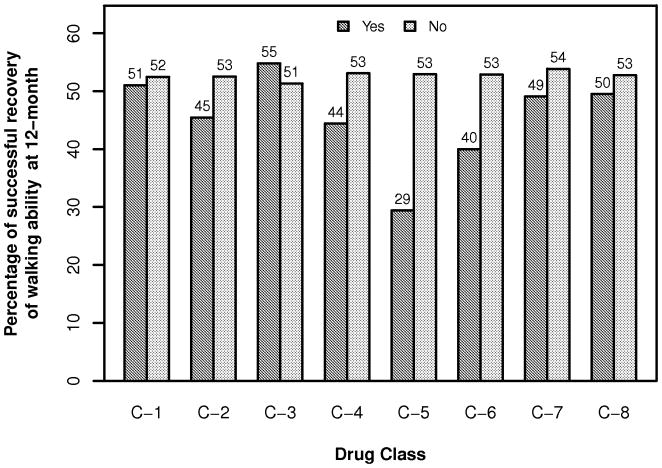
The number of subjects taking each class of drug is depicted in Table 1. In our first analysis, which involved achievement of improvement in functional walking level, potentially anti-neuroplastic drugs were associated with relatively slower walking speeds at 1-year (Figure 1, Table 2). Only 11.6% of the variability observed in outcome was accounted for by the baseline covariates, and only age (p<0.0001) was a significant predictor. However, the various classes of potentially anti-neuroplastic drugs accounted for only minimal additional variance (Table 3). Statistical significance (without correction for multiple comparisons) was achieved only for voltage-sensitive sodium channel anticonvulsants. No drug class met even our most lenient standard for correction for multiple comparisons, p<0.01.
Figure 1.
Proportion of subjects achieving an increase in functional walking level at 12 months post-stroke for each drug class. See Table 2 for key to drug classes.
Table 2.
Prevalence of drug use and summary of walking speed, successful recovery of walking ability, FM-LE, FM-UE and cognitive measure at 12 months by medication status *
| Drug Class | Prevalence of drug use | Improved Functional Walking Level | Mean ± SD | |||||
|---|---|---|---|---|---|---|---|---|
| Walking Speed | FM-LE | FM-UE | Cognitive Measure | |||||
| C1 | Any Potentially Anti- Neuroplastic Agent | Yes | 35.5% (145) | 51.0% (N=145) | 0.58±0.32 (N=145) | 25.29±6.20 (N=122) | 39.13±20.28 (N=122) | 0.01±0.72 (N=123) |
| No | 64.5% (263) | 52.5% (N=263) | 0.64±0.32 (N=263) | 26.90±5.54 (N=234) | 42.48±20.02 (N=233) | 0.11±0.72 (N=233) | ||
| C2 | Benzodiazepines | Yes | 8.1% (33) | 45.5% (N=33) | 0.58±0.30 (N=33) | 24.97±6.28 (N=28) | 43.50±19.11 (N=28) | 0.07±0.69 (N=29) |
| No | 91.9% (375) | 52.5% (N=375) | 0.62±0.33 (N=375) | 26.47±5.77 (N=328) | 41.14±20.25 (N=327) | 0.07±0.72 (N=327) | ||
| C3 | α1 Blocker or α2 Agonist | Yes | 17.9% (73) | 54.8% (N=73) | 0.57±0.30 (N=73) | 26.01±5.47 (N=61) | 42.93±18.08 (N=61) | 0.02±0.70 (N=61) |
| No | 82.1% (335) | 51.3% (N=335) | 0.63±0.33 (N=335) | 26.42±5.89 (N=295) | 41.00±20.56 (N=294) | 0.08±0.72 (N=295) | ||
| C4 | Any Anticonvulsant | Yes | 13.2% (54) | 44.4% (N=54) | 0.54±0.35 (N=54) | 23.80±6.59 (N=46) | 31.12±20.55 (N=46) | −0.04±0.83 (N=46) |
| No | 86.8% (354) | 53.1% (N=354) | 0.63±0.32 (N=354) | 26.73±5.61 (N=310) | 42.85±19.67 (N=309) | 0.09±0.70 (N=310) | ||
| C5 | Voltage-Sensitive Sodium Channel Anticonvulsant | Yes | 4.2% (17) | 29.4% (N=17) | 0.49±0.34 (N=17) | 22.22±6.75 (N=14) | 35.03±20.89 (N=14) | −0.13±1.06 (N=14) |
| No | 95.8% (391) | 52.9% (N=391) | 0.62±0.32 (N=391) | 26.52±5.73 (N=342) | 41.59±20.11 (N=341) | 0.08±0.70 (N=342) | ||
| C6 | α2δ Voltage-Sensitive | Yes | 7.4% (30) | 40.0% (N=30) | 0.46±0.34 (N=30) | 22.93±7.35 (N=24) | 28.00±20.88 (N=24) | −0.20±1.08 (N=24) |
| Calcium Channel Agent | No | 92.6% (378) | 52.9% (N=378) | 0.63±0.32 (N=378) | 26.59±5.62 (N=332) | 42.30±19.78 (N=331) | 0.09±0.68 (N=332) | |
| C7 | β Blocker | Yes | 40.0% (163) | 49.1% (N=163) | 0.58±0.33 (N=163) | 26.16±5.79 (N=140) | 42.00±19.51 (N=139) | 0.04±0.69 (N=141) |
| No | 60.0% (245) | 53.9% (N=245) | 0.64±0.32 (N=245) | 26.47±5.85 (N=216) | 40.90±20.58 (N=216) | 0.10±0.74 (N=215) | ||
| C8 | SSRI | Yes | 25.2% (103) | 49.5% (N=103) | 0.57±0.33 (N=103) | 26.28±6.17 (N=84) | 35.77±19.67 (N=84) | 0.05±0.86 (N=85) |
| No | 74.8% (305) | 52.8% (N=305) | 0.63±0.32 (N=305) | 26.37±5.72 (N=272) | 43.05±20.02 (N=271) | 0.08±0.67 (N=271) | ||
Many of the totals in this table do not reflect a simple addition of figures in Table 1 because some subjects were taking drugs from more than one class.
Table 3.
Variability in walking speed, successful recovery of walking ability, FM-LE, FM-UE and cognitive measure at 12 months explained by independent variables
| Drug Class | Improved Functional Walking Level | Walking Speed | FM-LE | FM-UE | Cognitive Measure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAV | Sig (p) | Pwr | IAV | Sig (p) | Pwr | IAV | Sig (p) | Pwr | IAV | Sig (p) | Pwr | IAV | Sig (p) | Pwr | ||
| Baseline covariates | 11.64% | - | - | 44.61% | - | 64.02% | - | 78.82% | - | 50.38% | - | |||||
| C1 | Any potentially anti-neuroplastic agent | 0.22% | 0.408 | 0.13 | 0.26% | 0.174 | 0.28 | 0.07% | 0.400 | 0.13 | 0.00% | 0.974 | 0.05 | 0.56% | 0.048 | 0.51 |
| C2 | Benzodiazepine | 0.23% | 0.396 | 0.14 | 0.09% | 0.430 | 0.12 | 0.01% | 0.734 | 0.06 | 0.02% | 0.611 | 0.08 | 0.00% | 0.906 | 0.05 |
| C3 | α-1 blocker or α-2 agonist | 0.00% | 0.846 | 0.06 | 0.10% | 0.401 | 0.13 | 0.00% | 0.978 | 0.05 | 0.12% | 0.165 | 0.28 | 0.02% | 0.719 | 0.07 |
| C4 | Any anticonvulsant | 0.81% | 0.113 | 0.35 | 0.29% | 0.148 | 0.30 | 0.01% | 0.829 | 0.06 | 0.02% | 0.592 | 0.08 | 0.72% | 0.026 | 0.61 |
| C5 | Voltage-sensitive Na-channel anticonvulsant | 1.66% | 0.033 | 0.57 | 0.47% | 0.068 | 0.45 | 0.08% | 0.390 | 0.14 | 0.06% | 0.339 | 0.16 | 0.54% | 0.053 | 0.49 |
| C6 | α2δ voltage-sensitive Ca-channel agent | 0.71% | 0.144 | 0.31 | 0.69% | 0.026 | 0.61 | 0.04% | 0.515 | 0.10 | 0.12% | 0.156 | 0.29 | 0.77% | 0.021 | 0.64 |
| C7 | β-blocker | 0.04% | 0.626 | 0.08 | 0.18% | 0.261 | 0.20 | 0.02% | 0.703 | 0.07 | 0.14% | 0.130 | 0.33 | 0.07% | 0.478 | 0.11 |
| C8 | SSRI | 0.46% | 0.272 | 0.20 | 0.32% | 0.129 | 0.33 | 0.20% | 0.166 | 0.28 | 0.03% | 0.489 | 0.11 | 0.08% | 0.452 | 0.12 |
IAV – Incremental addition to variance explained
Sig (p) – Significance of adding each drug to the model after controlling for the baseline covariates, uncorrected for multiple comparisons. P < 0.05 in bold. Pwr – Post-hoc power at 0.05 significance level to detect a potential neuroplastic effect, positive or negative. For drugs with a relatively large effect, as measured by the IAV (e.g., C5 and Improved Functional Walking Level), there is considerable power despite a modest number of subjects. For drugs with a small effect (e.g., C7 and Improved Functional Walking Level), the power is low and a much larger number of subjects would be needed to confidently establish the presence or absence of a neuroplastic effect.
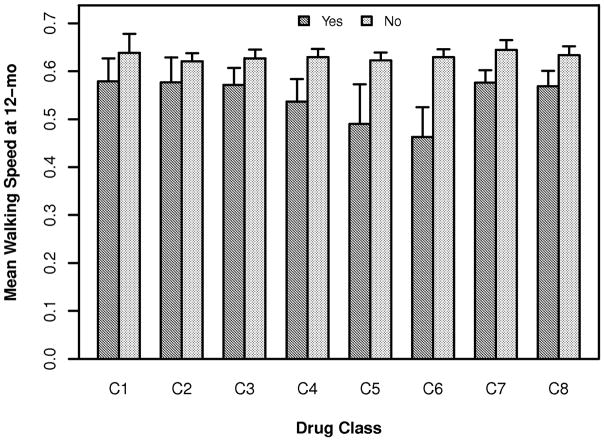
The results of our other four analyses, in which the walking speed (Figure 2), FM-LE, FM-UE, and cognitive measure at 12 months were the dependent variables, were quite similar (Table 2). The baseline covariates accounted for 44.61%, 64.0%, 78.8% and 50.4% of the variability in these outcomes, respectively. In contrast, the various drug classes accounted for very minimal additional variance (Table 3). For walking speed at 12 months, baseline severity (p < 0.0001), age (p < 0.0001) and our depression indicator (Personal Health Questionnaire 9-Depression Scale, PHQ9) (32) (p = 0.004) were the major predictors. Statistical significance was achieved only for α2δ active agents. For FM-LE score at 12 months, baseline FM-LE (p < 0.0001) was the only significant predictor. The baseline FM-UE (p < 0.0001) and baseline severity (p = 0.010) were the major predictors for FM-UE score at 12 months. None of the drug classes achieved statistical significance at the p=0.05 level for these two outcomes. For the cognitive measure, the baseline value (p < 0.0001) and the depression indicator (p = 0.011) were the major predictors and statistical significance (uncorrected for multiple comparisons) was achieved only for any potentially anti-neuroplastic agent andα2δ-active agents. No drug class met even our most lenient standard for correction for multiple comparisons, p<0.01.
Figure 2.
Mean and standard error (bars) of walking speed at 12 months post-stroke for each drug class. See Table 2 for key to drug classes.
Discussion
This prospective analysis of the effect of potentially anti-neuroplastic drugs determined that there was an association between several major classes of these drugs and lesser gains in functional walking level (Figure 1, Table 2). However, our regression models (Table 3) suggest that the increment in outcome variance accounted for by these drugs was very small and would fail to achieve statistical significance if corrected for multiple comparisons, even using our most lenient correction standard.
Even if the increments in outcome variance accounted for by the drugs assessed achieved statistical significance when corrected for multiple comparisons, by virtue of the small magnitude of these effects, they are unlikely to be of clinical significance. As shown in the first row of Table 3, the baseline covariates accounted for 44.6% of the variance in walking speed, 64% of the variance in FM-LE, 78.8% of the variance in FM-UE, and 50.4% of the variance in cognitive function at 12 months. The largest amount of variance accounted for by any drug tested, which was by voltage sensitive sodium channel anticonvulsants on improvement in functional walking level, was 1.66%, a small fraction of any of these figures. The baseline covariates accounted for only 11.64% of the variance in achieving improvement in functional walking level but this was because the strongest predictor of this outcome was closeness to one of the two points that defined improvement, 0.4 and 0.8 m/s (data not shown).
Our findings also suggest that the association between these drug classes and reduced success in achieving a gain in functional walking level (Figure 1) must be related to the fact that more impaired patients were more likely to be taking these drugs, rather than that the drugs inhibit neuroplasticity. The results were the same for gains in walking speed (Figure 2; Tables 2 and 3); an impairment based measure (FM); untreated functions (UE and cognitive measures); and an outcome substantially involving recovery and re-acquisition of declarative memory (cognitive).
In our analysis, β-blockers, which have not been implicated in inhibiting neuroplasticity, and SSRIs, which have been shown in a Phase II RCT to possibly act as an adjuvant to neuroplasticity, as determined by improvement in the FM motor score (30), served as a form of drug control. Neither was associated with a significant decrement in outcome, but neither was associated with an increment either.
Strengths and Limitations
The strengths of this investigation include the large number of subjects treated (and the substantial number of subjects receiving drugs of the classes investigated), the prospective nature of the study, the evidence that the treatment was effective (19), the high compliance with treatment protocols, the completeness of follow-up, the inclusion of both an impairment based measure (Fugl-Meyer) and functional measures (including a cognitive measure), and measures of both treated and untreated functions. However, there are several limitations. We have not ruled out the possibility of invidious effects of potentially anti-neuroplastic drugs before the time of randomization (2-months post stroke), i.e., the period addressed in published animal studies. Our results suggest that these drug classes do not have a clinically significant effect on the mix of normal learning mechanisms engaged by practice-based therapy and reactive neuroplasticity in the 2–12 month post-stroke epoch. Goldstein et al. (4) assessed potentially anti-neuroplastic effects of these same classes of drugs during the first three months after stroke, precisely the period during which reactive neuroplastic processes are likely to be most active. The presence of these drugs was associated with adverse effects and accounted for 2% of the variance in their primary outcome measure, the Toronto Stroke Scale Motor Subscore, and 4% of the variance in the Barthel Index. On the other hand, several of the classes of drugs examined in our study (benzodiazepines, voltage-sensitive sodium channel anticonvulsants, and α2δ voltage-sensitive calcium channel agents) act by mechanisms that theoretically could impact normal learning mechanisms, for example, by reducing neural firing rates or efficacy of neural transmission. Finally, to the extent that the participants were still taking the drugs of interest at the time of their 1-year evaluation, we cannot separate anti-neuroplastic effects from direct effects on neural function.
Our results speak only to the drug classes used by a sufficient number of subjects to enable statistical analysis. Insufficient subjects were taking neuroleptics or drugs with anticholinergic effects for us to assess the impact of these important drug classes. We cannot assess potential dose effects, the extent of time over which drugs were maintained was not recorded, and no attempt was made to assess patient adherence. The drugs were being given to outpatients beyond the acute stage of medical care after stroke and they are by their nature drugs that tend to be maintained chronically. However, recent studies have demonstrated average adherence rates in the 35–65% range, even for drugs as important as post-myocardial infarction medications, antihypertensives, and anticonvulsants (33–36). The major contributors to non-adherence are self-initiated discontinuation of the drug and erratic dosing. To the extent that the drugs used by subjects in the LEAPS trial were not continuously maintained throughout the full 10 months of this study, their potential antineuroplastic effects might be underestimated. Furthermore, because neither this study nor that of Goldstein et al. (4) constituted randomized trials of drug effects, neither study can rule out the possibility that it was the conditions for which the drugs were prescribed, rather than the drugs themselves, that accounted for the minor negative, albeit probably insignificant effects observed.
Conclusion
Within the limits of our study but with the benefits of a prospective trial and planned analysis, the small amounts of variance accounted for, relative to our baseline covariates, suggests that there are no clinically significant effects on neuroplasticity relatable to several major drug classes in common use, including benzodiazepines; α1 noradrenergic blockers + α2 noradrenergic agonists (clonidine); and anticonvulsants, including voltage-sensitive sodium channel agents and α2δ voltage-sensitive calcium channel agents. Several classes of drug had effects that achieved statistical significance in our analyses but these effects would no longer be significant when using even our most lenient standard for correction for multiple comparisons. Future phase III trials can build on our results by providing converging evidence, refining drug analysis through assessment of impact of dose and duration of administration, and explicitly monitoring drug adherence.
Acknowledgments
LEAPS Investigators include: Duke University Administrative Coordinating Center: Pamela Duncan, PT, PhD, FAPTA, FAHA; Sarah Hayden; Mysha Sissine; Quishi Feng, PhD; Brooks Rehabilitation Hospital, Jacksonville, FL: Deborah Stewart, MD; Trevor Paris, MD; Joann Gllichio, PT, DSc; Florida Hospital, Orlando, FL: Mitchell Freed, MD; Michelle Dolske, PhD; Craig Moore, PT; Bettina Brutsch, PT; Long Beach Memorial Hospital, Long Beach, CA; H. Richard Adams, MD; Diehma Hoang, MD; Anita Correa, PT; Sharp Rehabilitation Center, San Diego, CA; Jerome Stenehjem, MD; Roxanne Hon, MD; Molly McLeod, PT; University of Southern California: David Alexander, MD, UCLA Medical Center; Julie Hershberg, DPT; Samneang Ith-Chang, DPT; Official Coordinating Center - University of Florida: Andrea L. Behrman, PT, PhD, FAPTA; Dorian K. Rose, PT, PhD; Clinical Coordinating Center – University of Southern California: Julie K. Tilson, DPT, MS; Data management and Analysis Center - University of Southern California: Steven Cen, PhD; Chris Han, MS; James Gardener; University of Florida, Gainesville, FL: Yunfeng Dai, MS; Xiaomin Lu, PhD; Consultants: Anatole D. Martin, PhD, University of Florida; Richard Schofield, MD, University of Florida; Steering Committee: Pamela Duncan, PT, PhD, FAPTA, FAHA; Andrea L. Behrman, PT, PhD, FAPTA; Stanley P. Azen, PhD, University of Southern California; Samuel S Wu, PhD, University of Southern California; Bruce H. Dobkin, MD, University of California, Los Angeles; Stephen Nadeau, MD, University of Florida; Sarah K. Hayden, Duke University.
Sources of Funding
This work was supported by funding from National Institute of Neurological Disorders and Stroke and the National Center for Medical Rehabilitation Research (RO1 NS050506). Trial registration: NCT0024391.
Footnotes
Disclosures
None.
References
- 1.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nature Neurosci. 2011;14:1363–8. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr Opin Neurol. 2006;16:258–64. doi: 10.1016/j.conb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 2011;192:273–95. doi: 10.1016/B978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein LB Sygen in Acute Stroke Study Investigators. Common drugs may influence motor recovery after stroke. Neurology. 1995;45:865–71. doi: 10.1212/wnl.45.5.865. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez Rothi LJ, Fuller R, Leon SA, et al. Errorless practice as a possible adjuvant to donepezil in Alzheimer’s disease. J Int Neuropsychol Soc. 2009;15:311–21. doi: 10.1017/s1355617709090201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brailowsky S, Knight RT, Efron R. Phenytoin increases the severity of cortical hemiplegia in rats. Brain Res. 1986;376:71–7. doi: 10.1016/0006-8993(86)90900-5. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez TD, Holling LC. Disruption of behavioral recovery by the anti-convulsant phenobarbital. Brain Res. 1994;635:300–6. doi: 10.1016/0006-8993(94)91451-6. [DOI] [PubMed] [Google Scholar]
- 8.Feeney DM, Westerberg VS. Norepinephrine and brain damage: alpha noradrenergic pharmacology alters functional recovery after cortical trauma. Can J of Psychol. 1990;44:233–52. doi: 10.1037/h0084243. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein LB, Davis JN. Clonidine impairs recovery of beam-walking in rats. Brain Res. 1990;362:305–9. doi: 10.1016/0006-8993(90)90413-6. [DOI] [PubMed] [Google Scholar]
- 10.Schallert T, Hernandez TD, Barth TM. Recovery of function after brain damage: severe and chronic disruption by diazepam. Brain Res. 1986;379:104–11. doi: 10.1016/0006-8993(86)90261-1. [DOI] [PubMed] [Google Scholar]
- 11.Moratalla R, Barth TM, Bowery NG. Benzodiazepine receptor autoradiography in corpus striatum of rat after large frontal cortex lesions and chronic treatment with diazepam. Neuropharmacology. 1989;28:893–900. doi: 10.1016/0028-3908(89)90187-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhao CS, Puurunen K, Schallert T, et al. Behavioral effects of photothrombotic ischemic cortical injury in aged rats treated with the sedative-hypnotic GABAergic drug zopliclone. Behav Brain Res. 2005;160:260–6. doi: 10.1016/j.bbr.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect the rate of recovery after motor cortex injury. Science. 1982;217:855–7. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein LB, Bullman S. Differential effects of haloperidol and clozapine on motor recovery after sensorimotor cortex injury in rats. Neurorehabil Neural Repair. 2002;16:1–5. doi: 10.1177/154596830201600402. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Puurunen K, Schallert T, et al. Behavioral and histological effects of chronic antipsychotic and antidepressant drug treatment in aged rats with focal ischemic brain injury. Behav Brain Res. 2005;158:211–20. doi: 10.1016/j.bbr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Drachman DA, Leavitt J. Human memory and the cholinergic system. Arch Neurology. 1974;30:113–21. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 17.Wezenberg E, Verkes RJ, Sabbe BGC, et al. Modulation of memory and visuospatial processes by biperiden and rivastigmine in elderly healthy subjects. Psychopharmacology. 2005;181:582–94. doi: 10.1007/s00213-005-0083-7. [DOI] [PubMed] [Google Scholar]
- 18.Duncan PW, Sullivan KJ, Behrman AL, et al. Protocol for the Locomotor Experience Applied Post-Stroke (LEAPS) trial: a randomized controlled trial. BMC Neurol. 2007;7:39:1–23. doi: 10.1186/1471-2377-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight—supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026–36. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamiae G-B, Michel R, Elodie A, et al. Agreement between patients’ self-report and physicians’ prescriptions on cardiovascular drug exposure: the PGRx database experience. Pharmacoepidemiol Drug Safety. 2010;19:591–5. doi: 10.1002/pds.1952. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen MW, Søndergaard B, Kjøller M, et al. Agreement between self-reported data on medicine use and prescriptions records vary according to method of analysis and therapeutic groups. J Clin Epidemiol. 2008;61:919–24. doi: 10.1016/j.jclinepi.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Solomon DH, Stedman M, Licari A, et al. Agreement between patient report and medical record review for medications used for rheumatoid arthritis: the accuracy of self-reported medication information in patient registries. Arthritis Rheum. 2007;57:234–9. doi: 10.1002/art.22549. [DOI] [PubMed] [Google Scholar]
- 23.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232–40. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 24.Perry J, Garrett M, Gronley JK, et al. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 25.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychol Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.The Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 28.Corrigan JD, Hinkeldey NS. Relationships between Parts A and B of the Trail Making Test. J Clin Psychol. 1987;43:402–8. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Gamo N, Arnsten AF, et al. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–3. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chollet F, Tardy J, Albucher J-F, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–30. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 31.DeMaris A. Explained variance in logistic regression: a Monte Carlo study of proposed measures. Sociol Methods Res. 2002;31:27–74. [Google Scholar]
- 32.Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke. 2005;36:635–8. doi: 10.1161/01.STR.0000155688.18207.33. [DOI] [PubMed] [Google Scholar]
- 33.Blaschke TF, Osterberg L, Vrijens B, et al. Adherence to medications: insights arising from studies on the unrelaible link between prescribd and actual drug dosing hiistories. Ann Rev Pharmacol and Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 34.Choudhry HK, Avorn J, Glynn RJ, et al. Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) Trial. N Engl J Med. 2011;365:2088–97. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 35.Fitz-Simon N, Bennett K, Feely J. A review of studies of adherence with antihypertensive drugs using prescription databases. Ther Clin Risk Manag. 2005;1:93–106. doi: 10.2147/tcrm.1.2.93.62915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeber JE, Copeland LA, Pugh MJV. Variation in antiepileptic drug adherence among older patients with new-onset epilepsy. Ann Pharmacother. 2010;44:1896–904. doi: 10.1345/aph.1P385. [DOI] [PubMed] [Google Scholar]