Abstract
Background
Altered behavioral performance and brain activation during spatial working memory (SWM) tasks have been demonstrated in individuals with an alcohol use disorder (AUD). It is possible that alterations in processing during SWM may be present prior to initiation of heavy alcohol use in adolescents with a family history of AUDs (FHP) and therefore represent a premorbid neural phenotype that could increase risk for developing an AUD. The goal of our study was to investigate group differences in brain activation during a SWM task between FHP adolescents and adolescents with no family history of AUDs (FHN), as well as examine the relationship between brain activation and individual differences in family history density (FHD) of AUDs.
Methods
18 FHP and 16 gender and age-matched FHN participants completed a SWM and vigilance task while undergoing a functional magnetic resonance imaging (fMRI) scan.
Results
There were no group differences in task performance. The FHN group demonstrated expected greater activation during the SWM than vigilance condition in the right middle frontal gyrus and dorsolateral prefrontal cortex, whereas the FHP group demonstrated comparable brain activation for both the more demanding and simple task conditions. Additionally, FHD was associated with greater activation of the right superior parietal cortex and less activation of the right cerebellum during the SWM task, but not during the vigilance task.
Conclusions
Results suggest FHP adolescents demonstrate alterations in activation of prefrontal regions that are related more generally to the maintenance of top-down cognitive control and alterations in parietal and cerebellar regions that are specific to spatial working memory. Alterations in top-down cognitive control may be a general risk factor for FHP adolescents, whereas SWM-specific alterations are seen as a function of family history loading.
Keywords: Family History, Alcoholism, Spatial Working Memory, fMRI, Adolescence
INTRODUCTION
While there are numerous factors that confer risk, familial history of alcoholism has been consistently associated with increased likelihood for developing an alcohol use disorder (AUD), compared to the general population (e.g., Cloninger et al., 1986). It is estimated that about 25% of adolescents have first or second degree relatives who have had an AUD (Grant, 2000). Due to the large burden that alcoholism has for both the individual and society, it is essential to identify neurobiological and behavioral endophenotypes that may increase risk for developing an AUD prior to the onset of heavy alcohol use. Many studies have begun to examine whether adolescents with a familial history of alcoholism (FHP) have premorbid brain and/or behavioral phenotypes that could be associated with risk for future alcohol abuse. Previous research has found that compared to family history negative (FHN) youth, substance naïve FHP adolescents have performance deficits on neuropsychological measures of attention, visuospatial processing, and set-shifting (Corral et al., 1999; 2003; Giancola et al., 1993; 1996; Harden and Pihl, 1995; Ozkaragoz et al., 1997; Tarter et al., 1989). In addition, imaging studies have shown that FHP youth, in the absence of heavy alcohol use, have differences in brain volume (e.g., Hill et al., 2007), white matter microstructure (Herting et al., 2010), and brain activity (Cservenka et al., 2012; Herting et al., 2011; Schweinsburg et al., 2004; Silveri et al., 2011; Spadoni et al., 2008). Interestingly, many of the findings in FHP youth are similar to those found in studies examining neural and behavioral phenotypes in adult alcoholics. Thus, in order to develop effective prevention strategies for at-risk adolescents, it is essential to determine to what extent the neurobiological deficits seen in those with AUDs represent premorbid abnormalities that may have conferred risk for developing alcoholism.
One of the most striking similarities reported, to date, is that both FHP youth and alcoholics have deficits in spatial working memory (SWM; Corral et al., 1999; Scaife and Duka, 2009), suggesting atypical abilities in actively maintaining visuospatial information. Since adolescence is a crucial developmental period for SWM skills (Luciana et al., 2005), it is important to understand whether FHP youth have deficits in these abilities that could indicate developmental delays in higher-order executive control of cognitive processes, thereby increasing risk for alcohol abuse. Functional magnetic resonance imaging (fMRI) studies have found abnormal brain functioning in SWM in adolescent alcohol abusers (Caldwell et al., 2005; Squeglia et al., 2011; Tapert et al., 2004) and adult alcoholics (Chanraud et al., 2010; Pfefferbaum et al., 2001). One study examining the effect of family history density (FHD) on blood oxygen level-dependent (BOLD) response during a SWM task found relationships between FHD scores and brain activity during vigilance (control task), and not SWM (Spadoni et al., 2008). To our knowledge, no studies have specifically examined group differences in SWM brain response between FHP and FHN youth, in the absence of heavy alcohol use.
The goal of the present study was to examine brain activation and behavior related to SWM in FHP and FHN adolescents, in the absence of heavy alcohol use. The presence of altered brain activation or behavior could help elucidate biomarkers or endophenotypes for risk in FHP adolescents, prior to the initiation of heavy drinking. We investigated neural alterations at a group level, by comparing FHP and FHN adolescents, as well as individual differences in family history loading, by examining brain response relationships with FHD. Based on previous findings of decreased dorsolateral prefrontal cortex (DLPFC), inferior frontal cortex, and parietal cortex activity during SWM in adults with an AUD (Tapert et al., 2001), we expected FHP adolescents to demonstrate less activity in these regions than FHN adolescents. Additionally, given previous findings of FHD relationships with activation to vigilance and not SWM in FHP youth (Spadoni et al., 2008), we expected activation in frontal regions during vigilance to be positively associated with FHD.
Materials and Methods
Participants
Participants consisted of 35 adolescents between the ages of 13 and 15 (18 FHP, 17 FHN). FHP participants were selected from an on-going longitudinal study to be matched on age and gender with FHN participants. Given the block design of the fMRI task, which requires examining blocked signal comprised of correct and incorrect responding, an accuracy threshold (>80% accuracy on each task) was used in an attempt to reduce the impact of error signaling on the results (Dosenbach et al., 2006). One FHN participant did not meet the accuracy threshold; therefore the final sample consisted of 18 FHP and 16 FHN adolescents. See Table 1 for demographic information.
Table 1.
Characteristics and performance for each group.
| FHP1 | FHN2 | |
|---|---|---|
| N | 18 | 16 |
| Female/Male | 7/11 | 8/8 |
| Age | 14.52 (0.85) | 14.18 (0.70) |
| Puberty3 | 2.92 (0.55) | 2.76 (0.59) |
| IQ4 | 112.67 (11.28) | 112.69 (11.85) |
| GPA5 | 3.42 (0.57) | 3.62 (0.41) |
| SES6 | 33.89 (12.80) | 28.06 (14.28) |
| FHD7 | 0.57 (0.19) | 0 |
| Parental history of AUD (Paternal/Maternal) | 7/0/18 | 0/0/17 |
| Grandparent history of AUD | 7/18 | 0/17 |
| Aunt/Uncle history of AUD | 10/18 | 0/17 |
| Spatial Working Memory | ||
| Accuracy | 93.47 (5.44) | 92.66 (4.52) |
| Reaction Time (ms) | 549.03 (123.81) | 508.48 (82.80) |
| Vigilance Task | ||
| Accuracy | 98.78 (3.24) | 97.27 (3.59) |
| Reaction Time (ms) | 499.93 (60.73) | 494.73 (73.17) |
Family History Positive for alcoholism.
Family History Negative for alcoholism.
Pubertal Development Scale (Petersen et al., 1988); scores have been translated to Tanner stages, range 1–5, with higher scores reflecting greater maturity (Carskadon & Acebo, 1993)
Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)
Grade Point Average; values range from 0–4, with higher scores reflecting higher grades.
Hollingshead Index of Social Position; higher scores indicate lower socioeconomic status (Hollingshead, 1975)
Family History Density of alcohol use disorders (Zucker et al., 1994); total possible values range from 0 to 2, and in this study ranged from 0.25 to 1.0. Higher values represent greater familial loading.
Alcohol use disorder.
After obtaining written consent and assent from all youth and their parents in accordance with the Oregon Health & Science University (OHSU) Institutional Review Board, separate structured telephone interviews were conducted with both the youth and one of their parents. Exclusionary criteria for youth included the inability of a parent to provide family history information, reported family history of psychotic or bipolar disorder (Family History Assessment Module (FHAM) (Rice et al., 1995), lifetime personal history of a diagnosed DSM-IV psychiatric disorder (Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al., 2001)), significant alcohol/substance use (>10 lifetime alcoholic drinks or >2 drinks/occasion, >5 uses of marijuana, any other drug use, or >4 cigarettes per day) (Customary Drinking and Drug Use Record) (Brown et al., 1998), neurological illness, significant head trauma (loss of consciousness >2 minutes), serious medical problems, mental retardation or learning disability, prenatal exposure to drugs or alcohol (Structured Clinical Interview) (Brown et al., 1994), left-handedness (Edinburgh Handedness Inventory) (Oldfield, 1971), irremovable metal, and pregnancy.
Family History of Alcohol Use Disorders
The FHAM (Rice et al., 1995) was used to assess DSM-IV criteria for substance abuse and dependence in first and second degree relatives during a structured telephone interview. Based on the information provided by youth and parents on the FHAM, youth were categorized as either FHP or FHN. Youth were considered FHP if a history of alcohol abuse and/or dependence was reported for at least one biological parent, or two or more second degree relatives on either the maternal or paternal side of the family. Individuals with a total absence of alcohol abuse/dependence among relatives were considered FHN. Classification of individuals based on first, or first and second degree relatives with an AUD has been shown to be a robust measure of substance abuse vulnerability (Stoltenberg et al., 1998). In addition, FHD scores were computed for each participant; parents and second degree relatives with a history of an AUD were given a score based on their familial relatedness to the participant. Parents received 0.5, grandparents 0.25, and maternal and paternal aunts and uncles received a weighted ratio of 0.25 divided by the total number of siblings on that side of the family (Zucker et al., 1994). Scores were summed across relatives, resulting in FHD scores that ranged from 0.25 to 1.0 in the current sample.
fMRI Tasks
A modified block design spatial 2-back task was used to assess SWM (Nagel et al., 2007). The task included 4 blocks each of an alternating experimental SWM 2-back condition and a vigilance condition with brief presentations of fixation between block conditions. Blocks of a verbal 2-back condition were also interspersed during the task, but are described in a previous report (Cservenka et al., 2012). In the SWM condition, white alphabetical letters were presented in various locations on a black screen, and participants were told to “Press for the same LOCATION as 2 screens before” (Figure 1). The SWM condition included 63 trials, 20 of which were a 2-back spatial letter repeat. In the vigilance condition, gray and white dots appeared in random locations on the screen, and subjects were told to “Press the button when a gray dot appears” (Figure 1). The vigilance condition had 32 trials, with 12 requiring a button press. The vigilance condition was collected to control for visual, attentional, and simple motor processes involved during the SWM condition. In each condition, stimuli were presented on the screen for 500 ms, with an inter-trial stimulus interval of 1500 ms.
Figure 1.
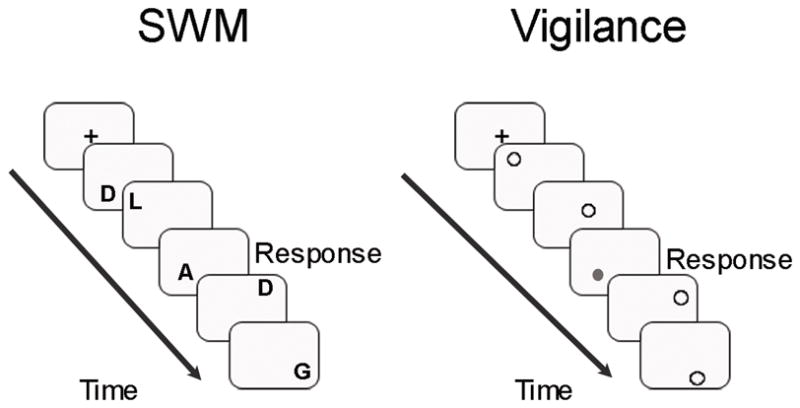
Spatial working memory 2-back and vigilance task used in the fMRI scan. Spatial 2-back task is shown on the left. Participants were instructed to press a button when they saw a letter presented in the same location as it was two screens before. The vigilance task is shown on the right. For this task, participants were instructed to press a button whenever they saw a grey dot. “Response” indicates when a participant should have made a button press. Each block of trials was preceded by a fixation cross.
Participant Characterization
Youth were administered the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) to provide an estimate of overall intellectual functioning (IQ), grade point average was obtained via youth self-report, and the Hollingshead Index of Social Position was administered to parents to assess socioeconomic status (Hollingshead, 1975). Pubertal maturation was evaluated by having individuals complete the self-rating Pubertal Development Scale (Petersen et al., 1988), with scores translated into Tanner Stages (Carskadon and Acebo, 1993).
Imaging Procedures
Images were acquired on a 3.0 Tesla Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) with a twelve channel head coil at OHSU’s Advanced Imaging Research Center. Whole-brain, high-resolution structural anatomical images were acquired in the sagittal plane using a T1 weighted MPRAGE scanning sequence (TI = 900ms, Flip Angle = 10°, TE = 3.58 ms, TR = 2300 ms, acquisition matrix = 256×240, resolution = 1mm × 1mm × 1.1mm). Whole-brain functional images were collected in the axial plane oblique to the anterior-posterior commissure, using a T2*-weighted echo planar BOLD sequence (TR = 2000 ms, TE = 30 ms, FOV= 240 mm, flip-angle = 90°, 33 slices no gap, resolution = 3.75 mm × 3.75mm × 3.8mm). The participants were able to see the task stimuli through a mirror mounted on the head coil and make responses through an MRI compatible optical button box.
Image Processing
Analysis of Functional NeuroImages (AFNI) (Cox, 1996) software was used for data processing and analyses. Preprocessing included slice timing correction, motion correction, co-registration of functional to anatomical images, and spatial smoothing using a Gaussian filter (full-width half maximum = 6 mm kernel). To reduce motion artifact in the data, only data with less than 1.5 mm root mean squared (RMS) of within-run motion, across the six motion parameters, were included in further analyses. Furthermore, time repetitions that showed greater than 2.5 mm or 2.5° in any of the 3 rotational or 3 displacement parameters were removed from the subsequent analyses. Although the groups were not significantly different in the number of time points censored (t(32) = 0.11, p = 0.92), the groups were significantly different in average RMS values (FHN: mean (M) = 0.36, standard deviation (SD) = 0.29; FHP: M = 0.21, SD = 0.14, t(32) = 2.05, p = 0.049). Movement was therefore used as a covariate in all fMRI analyses. Next, functional masks were created to mask out non-brain areas, and then time series data were normalized to its mean, resulting in images scaled by percent signal change. Using a deconvolution process, time series data were then correlated with a vector representing the task design in light of the delay of the hemodynamic response, while covarying for motion and linear trends (Cohen, 1997). The fit coefficients derived from fitting the time series data to the model represent the BOLD response, which was then contrasted between the SWM and vigilance, SWM and baseline, and vigilance and baseline conditions. Functional data sets were resampled into 3 mm3 voxels and were transformed into standard Talairach coordinates (Talairach and Tournoux, 1988).
Group Analyses
Demographic and Behavioral Data
Statistical analyses were performed in PASW Statistics 18 (PASW, Chicago, Illinois). Demographic data were analyzed using independent samples t-tests and task-related data were analyzed using repeated measures analysis of variance (ANOVA), with Task (SWM, Vigilance) as a within-subjects factor and Group (FHP, FHN) as a between-subjects factor. Exploratory repeated measures ANOVAs, with Task (SWM, Vigilance) as a within-subjects factor and Sex (Male, Female) as a between-subjects factor, were analyzed for performance data.
Imaging Data
All analyses below include movement as a covariate. Task-specific activation was examined separately for each group using an analysis of covariance (ANCOVA) for the contrast of SWM compared to vigilance. In order to best represent task-related activity for both FHP and FHN participants, individual group maps were thresholded at p < 0.05 and then combined to form a map of task-related brain activity for the entire sample. Between group analysis consisted of an ANCOVA for the task-related brain activity for the contrast of SWM compared to vigilance. Additionally, a whole-brain regression of FHD on the contrast of SWM compared to vigilance was performed for the FHP sample only. Only FHP adolescents were included in this analysis, to maintain a continuous and distributed range of FHD scores. Using AlphaSim (Cox, 1996), Monte Carlo simulations were performed using both a voxel and cluster threshold (Forman et al., 1995). For all analyses, a voxel-wise threshold of p < 0.025 and a whole-brain α < 0.01 was used. Given each analysis had different degrees of freedom, the number of required 3 mm3 contiguous voxels to be considered significant at an α < .01 was 83 for the group analysis, 63 for analyses with only FHN participants, and 80 for analyses only with FHP participants. For significant clusters exceeding 2,000 voxels, the cluster was subjected to increasing voxel-wise and cluster-wise thresholds until the cluster fragmented into smaller parts (Andrews-Hanna et al., 2011). The peaks for these smaller clusters are listed in Table 2. Significant clusters in regions susceptible to drop out and movement (based on the Main Effect of Movement in the above ANCOVAs) were examined to ensure adequate coverage across participants. If five or more participants in either group did not have full coverage, this cluster was excluded.
Table 2.
Contrast of the spatial 2-back task compared to the vigilance task. Only clusters with > 20 voxels are presented in this table. All voxels are significant at a voxel-wise p = .025 and whole-brain corrected α < .01. For clusters > 2,000 voxels, the cluster was subjected to increasing voxel-wise and cluster-wise thresholds until the cluster fragmented into smaller parts (Andrews-Hanna et al., 2011). Local peaks within these clusters are presented below the larger cluster. Peak coordinates for the clusters are presented in Talairach coordinates.
| Brain Region | BA | Voxels | x | y | z | t- statistic |
|---|---|---|---|---|---|---|
| FHN | ||||||
| Spatial 2-back > Vigilance | ||||||
| Bilateral Frontal-Parietal Cluster | -- | 8,781 | 1.5 | 25.5 | −21.5 | 3.70 |
| R and L Precuneus, SPL, IPL | 7, 40, 39 | -- | 1.5 | −67.5 | 53.5 | 5.04 |
| R DLPFC, MFG, dorsal ACC | 9/46, 6, 8, 10, 24 | -- | 34.5 | 55.5 | −9.5 | 5.11 |
| L MFG | 6 | -- | −28.5 | 1.5 | 56.5 | 4.90 |
| L MFG, SFG | 10 | -- | −28.5 | 58.5 | 5.5 | 4.88 |
| L DLPFC | 9/46 | -- | −46.5 | 22.5 | 32.5 | 4.69 |
| R Cerebellum | -- | 147 | 34.5 | −28.5 | −30.5 | 3.13 |
| L Cerebellum | -- | 68 | −37.5 | −34.5 | 33.5 | 2.86 |
| Vigilance > Spatial 2-back | ||||||
| L Cuneus | 18 | 565 | −4.5 | −85.5 | 26.5 | 3.39 |
| L Lingual Gyrus | 18, 19 | 109 | −13.5 | −49.5 | 2.5 | 3.12 |
| FHP | ||||||
| Spatial 2-back > Vigilance | ||||||
| Bilateral Parietal Cluster | -- | 4,223 | 1.5 | −61.5 | 56.5 | 4.45 |
| R and L Precuneus, SPL, IPL | 7, 40, 39 | 2,318 | 1.5 | −61.5 | 56.5 | 5.46 |
| Bilateral Frontal Cluster | -- | 3,477 | 28.5 | 4.5 | 59.5 | 3.96 |
| R DLPFC, MFG | 6, 8, 9 | 1,283 | 28.5 | 4.5 | 59.5 | 5.19 |
| R MFG | 10 | 97 | 34.5 | 61.5 | 5.5 | 4.54 |
| L Cerebellum | -- | 471 | −34.5 | −37.5 | −36.5 | 3.25 |
| R Cerebellum | -- | 336 | 43.5 | −58.5 | −36.5 | 3.26 |
| L MFG | 10 | 241 | −22.5 | 64.5 | −3.5 | 3.37 |
| L MTG, Fusiform Gyrus, Middle OCC | 20, 19, 37 | 222 | −43.5 | −73.5 | −9.5 | 2.94 |
| Corpus Callosum | -- | 124 | 1.5 | −25.5 | 26.5 | 3.18 |
| R MTG, Fusiform Gyrus, Middle OCC | 21, 19, 37 | 101 | 52.5 | −52.5 | −9.5 | 3.10 |
| L Thalamus | -- | 100 | −1.5 | −7.5 | −6.5 | 2.96 |
| R Insula | 13 | 99 | 31.5 | 22.5 | 2.5 | 3.48 |
| L Insula | 13 | 87 | −28.5 | 22.5 | 2.5 | 3.58 |
| Vigilance > Spatial 2-back | ||||||
| Bilateral Posterior Cluster | -- | 2,850 | −10.5 | −91.5 | 29.5 | 3.71 |
| R and L Cuneus, Lingual Gyrus R Parahippocampal Gyrus |
18, 17 36 |
708 | −10.5 | −91.5 | 29.5 | 4.86 |
| L pCC, Lingual Gyrus | 30, 18, 19 | 181 | −7.5 | −52.5 | 8.5 | 4.48 |
| L Medial Frontal Gyrus, ACC | 9, 10, 32 | 663 | −1.5 | 61.5 | 11.5 | 3.20 |
| L Precentral Gyrus | 4 | 234 | −40.5 | −13.5 | 59.5 | 3.18 |
| L IPL, Postcentral Gyrus | 40 | 214 | −64.5 | −22.5 | 20.5 | 2.89 |
| R MTG | 21 | 198 | 55.5 | 13.5 | −12.5 | 2.95 |
| L IFG | 47 | 152 | −46.5 | 25.5 | −12.5 | 3.31 |
| L STG, Parahippocampal Gyrus | 34 | 143 | −31.5 | 7.5 | −15.5 | 3.06 |
| R Precentral Gyrus | 6 | 89 | 40.5 | −13.5 | 59.5 | 3.22 |
| FHN > FHP* | ||||||
| Spatial 2-back > Vigilance | ||||||
| R MFG, DLPFC | 10, 9/46 | 1444 | 4.5 | 22.5 | −21.5 | 2.80 |
BA = Brodmann Area. DLPFC = dorsolateral prefrontal cortex, MFG = middle frontal gyrus, ACC = anterior cingulate cortex, SFG = superior frontal gyrus, IFG = inferior frontal gyrus, SPL = superior parietal lobule, IPL = inferior parietal lobule, pCC = posterior cingulate cortex, MTG = middle temporal gyrus, OCC = occipital cortex, STG = superior temporal gyrus.
a region of ventral medial prefrontal cortex was also significant in the analyses, but was not included due to insufficient coverage across participants in this region.
Results
Demographic and Behavioral Data
The FHP and FHN groups did not differ on demographic factors and estimated IQ. See Table 1 for group characteristics. There was a Main Effect of Task on accuracy, F(1,32) = 23.24, p < 0.001, with both groups demonstrating greater accuracy on vigilance than SWM, and there was a trend towards a Main Effect of Task on reaction time (RT), with both groups demonstrating slower responses on SWM than vigilance, F(1,32) = 2.95, p = 0.096. There were no other main effects or interactions for accuracy (all p’s > 0.27) or RT (all p’s > 0.22) (Table 1). Exploratory analyses revealed a trend of Sex on accuracy, F(1,32) = 2.91, p = 0.097. There were no other main effects for or interactions of Sex on accuracy or RT (all p’s > 0.59).
Spatial Working Memory
Individually, both groups demonstrated an expected network of frontal and parietal activation for SWM, including DLPFC, anterior frontal, dorsal anterior cingulate cortex (dACC), and superior parietal regions. Both groups also demonstrated greater activation of posterior regions for vigilance compared to SWM. For the FHN group, this was restricted to the cuneus and lingual gyrus, whereas the FHP group demonstrated activation of a more diffuse network of regions including the cuneus, posterior cingulate cortex, and parahippocampal gyrus. Additionally, only the FHP group demonstrated activation of medial and inferior frontal regions for vigilance compared to SWM (Table 2).
Areas of group difference were examined only in regions that demonstrated task-specific activation for SWM, as compared to the control vigilance condition. A region of anterior middle frontal gyrus (MFG; Brodmann Area (BA) 10) extending into right DLPFC (BA 9/46) showed significantly greater activation for the FHN than FHP group (Table 2 and Figure 2). The average percent signal change for the contrasts of SWM compared to fixation and vigilance compared to fixation was extracted for the right MFG cluster for each group separately, and values were entered into a 2 (Group: FHP, FHN) × 2 (Task: SWM, Vigilance) repeated measures ANOVA. The Group x Task interaction was significant, F(1,32) = 11.00, p = 0.002. Follow-up simple main effect analyses of within-group activation revealed the FHN group demonstrated greater activation of right MFG for SWM than vigilance, F(1,32) = 40.65, p < 0.001. The FHP group did not demonstrate significant differential activation between the conditions, but did demonstrate a trend towards greater activation of the right MFG for SWM than vigilance, F(1,32) = 3.71, p = 0.063. Additionally, when examining between-group activation, the FHP and FHN groups did not show differential activation for SWM in the right MFG, F(1,32) = 0.31, p = 0.58, but the FHN group showed significantly less activation for vigilance than the FHP group, F(1,32) = 13.00, p = 0.001 (Figure 2). FHD was not related to activation for SWM or vigilance in the right MFG in the FHP group (all p’s > 0.74).
Figure 2.
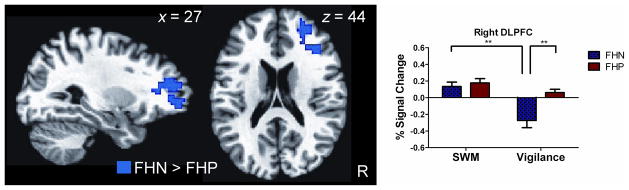
Greater activation by adolescents with no family history of alcohol abuse/dependence (FHN) than adolescents with a family history of alcohol abuse/dependence (FHP) during spatial working memory (SWM) as compared to vigilance. Blue indicates greater activation by the FHN than FHP group in right middle frontal gyrus (MFG) and dorsolateral prefrontal cortex (DLPFC). Graphs to the right show average percent signal change extracted for SWM compared to fixation (SWM) and vigilance compared to fixation (Vigilance) for the cluster shown to the left in the FHP and FHN groups separately. ** p < 0.001.
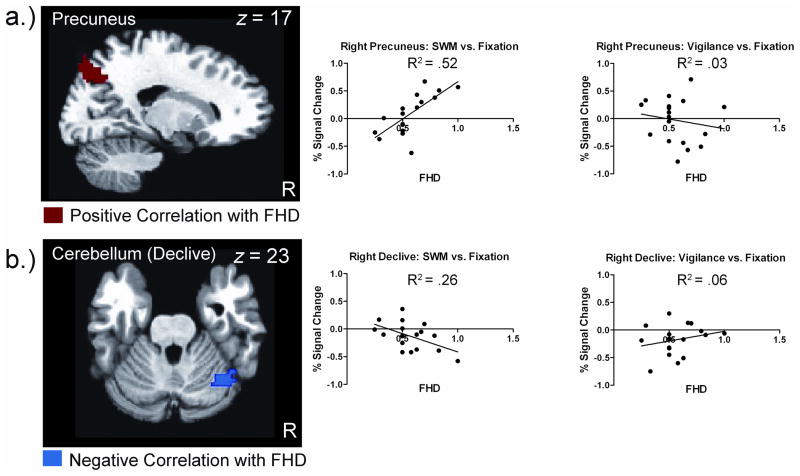
Family History Density
Two brain regions were significantly correlated with the whole-brain regression of FHD on the contrast of SWM compared to vigilance in the FHP group, including right precuneus (BA 7; peak: x = 16.5, y = −73.5, z = 44.5; 99 voxels) and right cerebellar declive (peak: x = 43.5, −58.5, −18.5; 94 voxels). The average percent signal change for the contrasts of SWM compared to fixation and vigilance compared to fixation was extracted for each of the significant clusters and correlated with FHD. Percent signal change for SWM compared to fixation was positively correlated with FHD for the right precuneus (r = 0.71, p = 0.001), such that greater activation during SWM was associated with higher FHD. In contrast, percent signal change for SWM compared to fixation was negatively correlated with FHD for the right cerebellar declive (r = −0.52, p = 0.027), with greater activation during SWM being associated with lower FHD. There was no correlation between FHD and percent signal change for vigilance compared to fixation in either the right precuneus (r = −0.16, p = 0.51) or right cerebellar declive (r = 0.24, p = 0.34) (Figure 3).
Figure 3.
Whole-brain regression of family history density (FHD) on the contrast of spatial working memory (SWM) compared to vigilance in adolescents with a family history of abuse/dependence (FHP). Red indicates greater FHD associated with greater activation and blue indicates greater FHD associated with less activation. Scatter plots to the right display the correlation between FHD and average percent signal change extracted for SWM compared to fixation and vigilance compared to fixation for the cluster shown to the left. Each dot represents a single participant. A) Greater activation of right precuneus is associated with greater FHD. This correlation is only significant for SWM activity. B) Less activation of right declive is associated with greater FHD. This correlation is only significant for SWM activity.
Discussion
This study investigated whether FHP adolescents demonstrate altered brain activation when performing a SWM task compared to FHN adolescents. In addition to alterations at a group level, the study also examined whether individual differences in FHD were associated with brain response. Contrary to our initial hypothesis, both FHN and FHP adolescents activated right MFG and DLPFC during SWM. However, only the FHN adolescents demonstrated differential activation of right MFG and DLPFC for SWM compared to vigilance, which was driven by decreased activation during vigilance for the FHN group. At the individual level, FHD was associated with activation during SWM in the right superior parietal cortex and cerebellum, as opposed to the hypothesized association between FHD and basic vigilance-related neural processing.
While there were no group differences in accuracy, both FHN and FHP adolescents were less accurate on SWM than vigilance. This is consistent with other developmental studies of SWM (Luciana et al., 2005; Schweinsburg et al., 2005). While some studies have shown worse accuracy for attentional and visuospatial tasks (Corral et al., 1999; 2003) in FHP youth, other studies using block fMRI designs have not shown differences in accuracy between FHP and FHN youth on executive functioning tasks (Cservenka et al., 2012; Herting et al., 2011). A limit of using a block design is that you cannot remove error trials and associated error-related brain response. By using an accuracy threshold, we reduced the contribution of confounding error response to observed brain activation. However, it is possible that use of a threshold restricts the variability in responding for both groups. Taken together, these results suggest group differences in brain response are not due to differential task difficulty between FHP and FHN youth or error-related brain processes.
In addition to showing comparable task accuracy, FHP and FHN adolescents also did not demonstrate differences in speed of responding for SWM or vigilance. This is in contrast to studies demonstrating FHP adolescents respond slower than FHN adolescents on SWM tasks (e.g., Corral et al., 1999). There are many possible explanations for this lack of difference. First, the block design of the fMRI studies may help reinforce a top-down attentional set (Dosenbach et al., 2006), and therefore performance differences are less robust than in larger, behavioral studies. In a previous block design fMRI study of SWM, Tapert and colleagues (2004) found only trend level differences in performance between adolescents with an AUD and controls. Secondly, the lack of differences could be related to the difficulty of the task itself. Our previous fMRI study of verbal working memory (Cservenka et al., 2012) found slower response times for FHP than FHN adolescents, but the verbal working memory and vigilance tasks were equally difficult for participants (as indexed by no difference in accuracy between the tasks). Lastly, previous studies demonstrating performance differences between FHP and FHN youth have included younger samples (Corral et al., 1999; 2003) than our study. Given continued performance improvement on spatial working memory tasks over the course of adolescence (Luciana et al., 2005), performance differences may not be as apparent in older youth. Given the SWM task was harder for both groups, differences in response may not be as pronounced. However, the lack of significant difference in performance between FHN and FHP adolescents indicates that the observed differences in brain activation patterns are more likely related to changes in behavioral strategy or compensatory mechanisms than performance differences. Similar suggestions have been made with regard to developmental brain changes in the frontal-parietal network associated with working memory that are not performance related (Kwon et al., 2002; Schweinsburg et al., 2005).
Contrary to our predictions, there were no differences between FHP and FHN adolescents for SWM task-related activation; however, differences were found in vigilance task activation. Although both groups displayed an expected pattern of frontal and parietal activation for SWM (Kwon et al., 2002; Schweinsburg et al., 2005; Spadoni et al., 2008), only the FHN adolescents demonstrated greater activation of the right anterior MFG and DLPFC during SWM as compared to vigilance. FHP adolescents positively activated these frontal regions for both task conditions, whereas the FHN adolescents only demonstrated positive activation during SWM (with deactivation during vigilance). In addition to being implicated in updating information in working memory (for review, see Wager and Smith, 2003), MFG and DLPFC have been shown to be involved in cognitive control (e.g., Banich et al., 2000) and task-positive networks (Dosenbach et al., 2006). As briefly mentioned earlier, using a block design and restricting accuracy allows us to examine brain regions involved in top-down cognitive control. MFG and DLPFC have been associated with proactive cognitive control, which biases towards task-relevant goals and information (Banich et al., 2000; Braver et al., 2007). This may suggest that FHP adolescents need to use more proactive, top-down cognitive control than FHN adolescents even for a simpler vigilance task. Vigilance, which requires directing and maintaining attention, may be more difficult and less automatic for the FHP adolescents, and therefore FHP adolescents need compensatory recruitment of frontal regions for this task. This is consistent with neuropsychological findings of deficits in performance on attentional tasks in both FHP adolescents and adolescents with AUDs (Corral et al., 1999; Tapert and Brown, 2000). Also, two other studies examining working memory in FHP adolescents found similar patterns of activation during control, vigilance task conditions (Cservenka et al., 2012; Spadoni et al., 2008), albeit in more medial and inferior frontal regions than those shown here. In contrast to the more lateral prefrontal regions we demonstrate as different between FHP and FHN youth, medial frontal regions are associated with response evaluation, or reactive control (Banich et al., 2000; Braver et al., 2007), which is likely recruited more during error processing. The anatomical disparity between our and previous working memory study of FHP adolescents may be due to the fact that no accuracy threshold was used in these prior studies, and therefore more response evaluation and error-related neural processes were likely present.
Given that cognitive control includes the ability to guide behavior towards a task-relevant process (Banich et al., 2000), alterations in these processes may have many implications for decision-making in FHP adolescents. As demands and contextual factors increase, these deficits in cognitive control are likely to become more pronounced. Previous studies have found that emotional arousal, presence of reward, and peers alter decision-making in normal adolescents (for review, see Steinberg, 2007). FHP adolescents may therefore be at an increased risk of making poor decisions because they have greater difficulty maintaining a goal in the face of distracting information. Examples might include maintaining the intention of not drinking around peers or considering the long-term consequences of binge-drinking on the weekends in the face of the positive, short-term gains.
In addition to group level differences between FHP and FHN adolescents, we also found associations between FHD and brain activation within FHP adolescents. Although there was no difference in SWM activation between groups, SWM-specific activation was associated with FHD. Specifically, increased activation in the right superior parietal cortex and less activation in the right cerebellum was associated with greater FHD. This region of superior parietal cortex (precuneus; BA 7) has been frequently implicated in SWM in children, adolescents (Kwon et al., 2002; Schweinsburg et al., 2005), and adults (for review, see Cavanna and Trimble, 2006), as well as spatial processing (Cavanna and Trimble, 2006). Therefore, adolescents with higher FHD may need to recruit additional regions associated with spatial processing and working memory to perform the task at the same level as those with a lower FHD. The association of greater FHD with less activation in right cerebellum, specifically the declive, during the SWM task, is consistent with previous studies of working memory in FHP youth (Spadoni et al., 2008), adolescents with an AUD (Tapert et al., 2004), and adults with an AUD (Park et al., 2011). Decreased connectivity in fronto-cerebellar circuitry and decreased volume of the cerebellum have also been found in FHP youth and adults and adolescents with AUDs (De Bellis et al., 2005; Herting et al., 2011; Hill et al., 2007; Sullivan, 2003). Although decreased cerebellar volume has often been associated with motor or gait problems in alcoholics (e.g., Sullivan et al, 2010), it has also been associated with cognitive deficits (Sullivan, 2003). Developmental studies have found the cerebellum is involved in higher order cognition, attention, visuospatial functioning, and processing speed (for review, see Steinlin, 2008). Therefore, our findings provide further support for the suggestion that alterations in the cerebellum may be a risk-factor for future development of an AUD, as opposed to solely being the result of alcohol-consumption. The extent of family history loading might reflect genetic contributions, making alterations in these brain regions particularly promising biomarkers for familial AUD risk.
There are some limitations to the study that warrant mention. Given the sample size, we were not able to explore the effect of gender on brain activation. Previous research with FHP youth (Silveri et al., 2011) and adolescents with an AUD (Caldwell et al., 2005) has found different patterns of activation in response to cognitive tasks for males and females. Furthermore, studies of adults with an AUD have found differential relationships with cerebellar volume in males and females (Sullivan et al, 2010). Thus, it is important for gender effects to be examined in future studies. Additionally, results related to altered prefrontal activation will need to be replicated in other tasks requiring cognitive control in order to further support our interpretation. Furthermore, it is not possible to determine whether altered basic visuospatial processing may be driving FHD associations with superior parietal activation, as FHP youth demonstrate visuospatial processing deficits (e.g., Corral et al., 1999). Inclusion of additional visuospatial processing measures in future studies is necessary to address this issue. Also of note, only one parent provided family history information, which is less reliable than information from multiple sources. Additionally, although a family history of psychosis and bipolar disorder, two highly heritable disorders, was exclusionary, familial history of other Axis I disorders was not assessed and thus could contribute to observed differences. Lastly, longitudinal studies will need to be conducted to fully determine whether alterations in brain activation observed in this study are predictive of future alcohol use or AUD.
In conclusion, this study suggests that FHP adolescents demonstrate alterations in activation of prefrontal regions that are related to the general maintenance of top-down, cognitive control, as well as alterations in parietal and cerebellar regions that are specific to spatial working memory. Atypical brain activity related to cognitive control was observed in FHP adolescents, but was not dependent on FHD. In contrast, alterations in parietal and cerebellar regions that are SWM-specific were related to FHD, and therefore may present a possible biomarker of genetic loading for AUD risk. Future research should explore whether the atypical activity in brain regions subserving cognitive control extends to other tasks and whether altered activation that is FHD-related has the potential to predict future alcohol abuse.
Acknowledgments
Support:
The authors were supported by the following grants from the National Institute on Alcohol Abuse and Alcoholism, R01 AA017664 (BJN), T32 AA007468-24 (KMS), F31-AA019866 (MMH), and an OHSU Graduate Research Scholarship (AC).
References
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS ONE. 2011;6:e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Barad V, Gullett D, Shah C, Brown C. Prefrontal regions play a predominant role in imposing an attentional “set”: evidence from fMRI. Cognitive Brain Res. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varities of working memory variation: dual mechanisms of cognitive control. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in Working Memory. Oxford University Press; Oxford: 2007. pp. 76–106. [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Appl Prev Psychol. 1994;3:61–73. [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Rohlfing T, Pfefferbaum A, Sullivan EV. Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010;35:1868–1878. doi: 10.1038/npp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Reich T, Bohman M. Inheritance of risk to develop alcoholism. NIDA Res Monogr. 1986;66:86–96. [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Corral M, Holguin SR, Cadaveira F. Neuropsychological characteristics of young children from high-density alcoholism families: a three-year follow-up. J Stud Alcohol. 2003;64:195–199. doi: 10.15288/jsa.2003.64.195. [DOI] [PubMed] [Google Scholar]
- Corral MM, Holguin SR, Cadaveira F. Neuropsychological characteristics in children of alcoholics: familial density. J Stud Alcohol. 1999;60:509–513. doi: 10.15288/jsa.1999.60.509. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug Alcohol Depend. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Narasimham A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wegner KK, Kang HC, Burgund ED, Grimes AL, Schlagger BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Martin CS, Tarter PE, Pelham WE, Moss HB. Executive cognitive functioning and aggressive behavior in preadolescent boys at high risk for substance abuse/dependence. J Stud Alcohol. 1996;57:352–359. doi: 10.15288/jsa.1996.57.352. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Petersen JB, Pihl RO. Risk for alcoholism, antisocial behavior, and response perseveration. J Clin Psychol. 1993;49:423–428. doi: 10.1002/1097-4679(199305)49:3<423::aid-jclp2270490317>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Grant BF. Estimates of US children exposed to alcohol abuse and dependence in the family. Am J Public Health. 2000;90:112–115. doi: 10.2105/ajph.90.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden PW, Pihl RO. Cognitive function, cardiovascular reactivity, and behavior in boys at high risk for alcoholism. J Abnorm Psychol. 1995;104:94–103. doi: 10.1037//0021-843x.104.1.94. [DOI] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci U S A. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Dev. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Ohannessian A, Cummins K. Performance dissociation during verbal and spatial working memory tasks. Percept Mot Skills. 2007;105:243–250. doi: 10.2466/pms.105.1.243-250. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ozkaragoz TS, Noble EP. Neuropsychological functioning in sons of active alcoholic, recovering alcoholic, and social drinking fathers. Alcohol. 1997;14:31–37. doi: 10.1016/s0741-8329(96)00084-5. [DOI] [PubMed] [Google Scholar]
- Park M-S, Sunju S, Park JE, Kim S-H, Yu IK, Sohn J-H. Brain functions associated with verbal working memory tasks among young males with alcohol use disorders. Scand J Psychol. 2011;52:1–7. doi: 10.1111/j.1467-9450.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Scaife JC, Duka T. Behavioural measures of frontal lobe function in a population of young social drinkers with binge drinking pattern. Pharmacol Biochem Behav. 2009;93:354–362. doi: 10.1016/j.pbb.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005;11:631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during stroop performance. Alcohol Clin Exp Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: new perspectives from brain and behavioral science. Curr Dir Psychol Sci. 2007;16:55–59. [Google Scholar]
- Steinlin M. Cerebellar disorders in childhood: cognitive problems. Cerebellum. 2008;7:607–610. doi: 10.1007/s12311-008-0083-3. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping. Psychopharmacology. 2010;208:279–290. doi: 10.1007/s00213-009-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-dimensional coplanar stereotaxic atlas of the human brain proportional system: an approach to cerebral imaging. Thieme; New York: 1988. [Google Scholar]
- Tapert SF, Brown GG. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Jacob T, Bremner DL. Specific cognitive impairment in sons of early onset alcoholics. Alcohol Clin Exp Res. 1989;13:786–789. doi: 10.1111/j.1530-0277.1989.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corp; San Antonio, TX: 1999. [Google Scholar]
- Zucker RA, Ellis DA, Fitzgerald HE. Developmental evidence for at least two alcoholisms: I. Biopsychosocial variation among pathways into symptomatic difficulty. In: Barbor TF, Hesselbrock VM, Meyer RE, Shoemaker W, editors. Types of alcoholics: evidence from clinical, experimental, and genetic research. New York Academy of Sciences; New York: 1994. pp. 134–146. [DOI] [PubMed] [Google Scholar]