Abstract
Purpose
After conventional radiotherapy for head and neck cancer, xerostomia has traditionally been the major effector of patient-reported quality of life (QOL), and recent publications suggest that dysphagia has an even stronger effect. We hypothesized that IMRT aiming to spare the salivary glands and swallowing structures reduced, or eliminated, the effects of these toxicities on QOL.
Methods and Materials
Prospective longitudinal study: 72 patients with Stage III-IV oropharyngeal cancer treated uniformly with definitive chemo-IMRT sparing the salivary glands and swallowing structures. Overall QOL was assessed by summary scores of the Head Neck QOL (HNQOL) and University of Washington QOL (UWQOL) questionnaires, as well as HNQOL “Overall Bother” question. QOL, observer-rated toxicities (CTCAE v2), and objective evaluations (videofluoroscopy assessing dysphagia and saliva flow rates assessing xerostomia) were recorded pre-therapy through 2 years post-therapy. Correlations between toxicities/objective evaluations and overall QOL were assessed using longitudinal repeated measures of analysis and Pearson correlations.
Results
All observer-rated toxicities and QOL scores worsened 1-3 months after therapy and improved through 12 months, with minor further improvements through 24 months. At 12 months, dysphagia grades 0-1, 2, and 3, were observed in 95%, 4%, and 1% of patients, respectively. Using all post-therapy observations, observer-rated dysphagia was highly correlated with all overall QOL measures (p<0.0001), while xerostomia, mucosal, and voice toxicities were significantly correlated with some, but not all, overall QOL measures, with lower correlation coefficients than dysphagia. Late overall QOL (≥6 or ≥12 months post-therapy) was primarily associated with observer-rated dysphagia, and to a lesser extent with xerostomia. Videofluoroscopy scores, but not salivary flows, were significantly correlated with some of the overall QOL measures.
Conclusion
After chemo-IMRT, while late dysphagia was on average mild, it was still the major correlate of QOL. Further efforts to reduce swallowing dysfunction are likely to yield additional gains in QOL.
Keywords: quality of life, dysphagia, xerostomia, head neck cancer
Introduction
Health-related quality of life (QOL) encompasses the physical, psychological, and social dimensions of the mental and functional well-being, related to the effects of both tumor and therapy side-effects. This concept is particularly important in assessing the outcomes of treatment for head and neck cancer (HNC) patients given the high rates of post-treatment side effects. Several authors have previously looked at the relationship of treatment-related toxicities and QOL for HNC patients, with xerostomia reported most often to be the primary determinant of long-term QOL in survivors (1). More recently, Langendijk et al. reported that dysphagia was the strongest determinant of overall QOL after conventional radiotherapy (2).
In comparison to these studies, decreased xerostomia (3-6) and dysphagia (7) have been reported using IMRT aiming to reduce these toxicities. We hypothesized that following the reduction of severe xerostomia and dysphagia by IMRT, they would cease to be the main determinants of post-treatment QOL.
In this paper we report an analysis based on the results of a prospective study in advanced oropharyngeal cancer patients treated with concurrent chemotherapy and IMRT that aimed to spare the salivary glands and swallowing structures. The goal of this analysis was to assess which treatment-related toxicities affected significantly QOL, using longitudinal evaluations of observer-rated toxicities and objective measurements of salivary and swallowing dysfunction, and correlating these outcomes with patient-reported overall QOL. Overall QOL was scored by two commonly applied methods: Summary scales of whole validated QOL questionnaires, as well as a single question from a validated questionnaire which aims to capture global QOL (8).
Materials and Methods
From May 22, 2003 to February 23, 2008, 72 patients with stage III-IV oropharyngeal cancer were treated with definitive chemoradiation in a prospective study approved by the XXX Review Board. All patients received concurrent weekly chemotherapy with carboplatin (AUC=1) and paclitaxel (30mg/m2). None received induction or adjuvant chemotherapy. IMRT prescription doses were 70Gy to the primary tumor and involved lymph nodes, 59-63Gy to high risk nodal regions and the anatomical compartments around the gross tumor volumes, and 56-59Gy to low risk nodal regions, all delivered in 35 fractions. Priorities were given during planning to spare the non-involved parts of the major salivary glands, including both parotid glands and the contralateral submandibular glands when contralateral neck level I was not a target, the oral cavity, pharyngeal constrictor muscles, larynx (glottic and supraglottic), and esophagus, to minimize late xerostomia and dysphagia. Details of the radiation treatment planning and the dosimetric and functional results in these patients have previously been published (7, 9-10).
Head and neck cancer and treatment-related QOL was assessed by 2 validated instruments: the Head Neck QOL (HNQOL) questionnaire and the University of Washington QOL (UWQOL) questionnaire, detailed elsewhere (6-7, 11). Patients were given the HNQOL and UWQOL questionnaires at six time points: prior to the initiation of treatment, and at 3, 6, 12, 18 and 24 months post-treatment. The scaling of scores for each question was modified to range from 0 to 100,with higher scores corresponding to worse condition, as detailed elsewhere (6, 11). After scaling, each item score was added, and the average sum, including all the items in the questionnaire, was transformed linearly to produce the summary score, with 0 the best and 100 the worst score. Three measures of overall QOL were used in this study: The summary scores from the HNQOL and UWQOL instruments, as has previously been detailed (6, 10), and in addition, the question “Overall Bother” from the HNQOL instrument was also used as a measure of overall QOL.
Observer-rated toxicities were recorded weekly throughout treatment and at each 2-month follow-up appointment for two years, using the Common Toxicity Criteria Adverse Effects (CTCAE) scale, version 2.
Objective assessments of dysphagia and xerostomia were made by modified barium swallow (videofluoroscopy, VF) and by measurements of the salivary output from the major salivary glands, respectively. Details of VF performed at our institution are provided elsewhere (7). VF was performed pre-therapy and at 3, 12, and 24 months post-therapy. The results of VF were graded using the Swallowing Performance Scale (VF) score, with a score of 1 indicating a normal swallow, 2-3 indicating mild dysfunction, 4 indicating mild/moderate dysfunction, 5 requiring a modified diet, and scores of 6-7 requiring enteral feeding (12). Stimulated and unstimulated saliva measurements were performed pretreatment and at 1 month, 3 months, 6 months, 12 months, 18months, and 24 months post-treatment. Selective measurements of salivary flow rates were made from each major gland (parotid and submandibular) as previously described (10). For correlation purposes with QOL, the sum of the stimulated output from the four major salivary glands was taken into account at each time point.
Statistical analyses were performed to assess associations between clinical factors and overall QOL, and between specific toxicities (using observer- rated CTCAE scores), or objective measures (VF scores and salivary flow rates), and overall QOL, measured using Overall Bother, summary HNQOL score, or summary UWQOL score. Pearson correlations between specific toxicities, or objective measures, and overall QOL were calculated to quantify the degree of correlation. To test for a presence of significant correlation, longitudinal data regression models were fit that included the grade of toxicity and a time-dependent intercept term. An auto-regressive [AR(1)] correlation structure was used to account for the correlation within subjects over time. Parameter estimates from the regression models are reported, and can be interpreted as the average change in QOL per one unit increase in toxicity scores (or one unit worsening of objective measure such as VF or saliva output). Analyses were performed using SAS/STAT for Windows version 9.2 [SAS Institute Inc. 2008. SAS/STAT® 9.2 User's Guide. Cary, NC: SAS Institute Inc].
Results
Patient Characteristics and QOL and Toxicity Trends
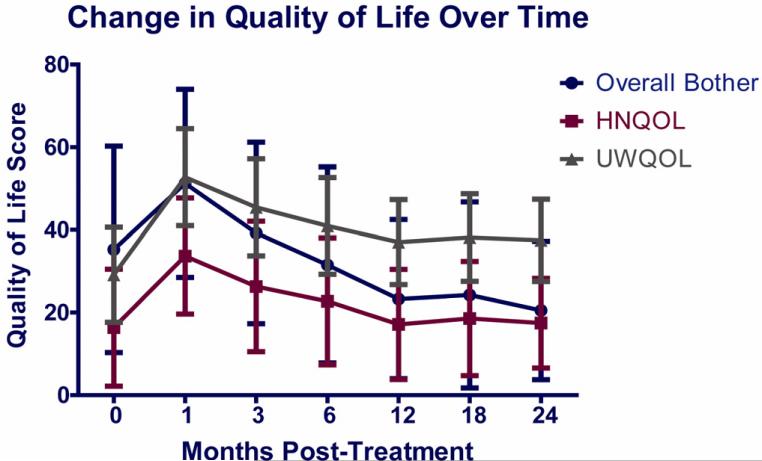
Patient and tumor characteristics are provided in Table 1. Overall QOL worsened significantly 1-3 months after therapy and then improved significantly through 12 months, with minor further improvements through 24 months (Figure 1). These trends over time were similar for the three overall QOL measures.
Table 1.
Patient Characteristics
| Characteristic | Patients |
|---|---|
| Age, median | 55 years (range 50-78) |
| Sex | 64 (89%) Men 8 (11%) Female |
| Tumor Site | 34 (47%) Tonsil 38 (53%) Base of Tongue |
| Tumor Stage | 9 (13%) T1 29 (40%) T2 16 (22%) T3 18 (25%) T4 |
| Nodal Stage | 5(7%) N0 6(8%) N1 55 (76%) N2 6 (8%) N3 |
| AJCC Stage | 8 (11%) Stage III 58 (81%) Stage IVA 8 (8%) Stage IVB |
| Smoking Status | 26 (36%) Never 30 (42%) Previous 16 (22%) Current |
Figure 1.
Mean scores of three overall quality of life measures: Overall Bother scores, Head and Neck QOL (HNQOL) questionnaire summary scores, and University of Washington QOL questionnaire (UWQOL) summary scores, before therapy and through 24 months after therapy.
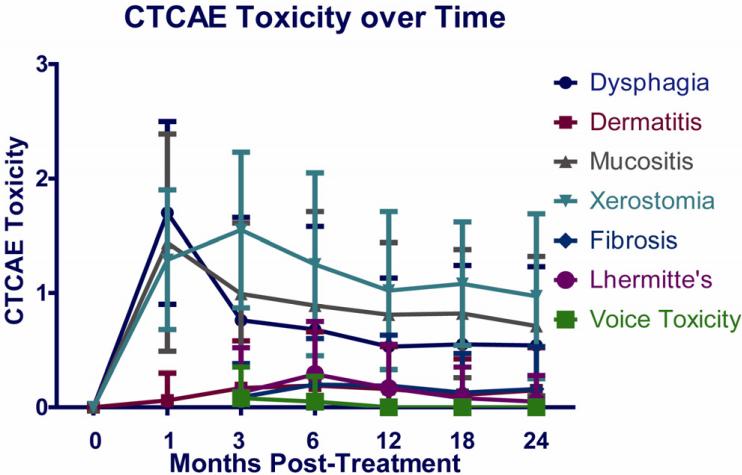
Figure 2 shows the trend over time for CTCAE toxicity scores for dysphagia, mucositis, saliva, dermatitis, fibrosis, Lhermitte sign, and voice. Most toxicities worsened significantly 1-3 months after therapy and then improved through 12 months, with only minimal further improvement through 24 months. CTCAEDysphagia grades were 0-1 in 88% of the patients by 6 months and in 95-96% by 12-24 months, while grade 2 (altered diet) was observed in 6% at 6 months and 2-4% at 12-24 months. Grade 3 dysphagia (feeding tube requirement) was observed in 6% at 6 months, and in one patient (1-2%) thereafter. Xerostomia mean scores were highest at 3 months, at 1.5, and improved to 1.0 by 12 months (Figure 2). Grade 1 voice toxicity was observed in a small number of patients early on (5 patients at 3 months and 3 patients at 6 months) with no voice toxicity observed beyond 6 months.
Figure 2.
Mean observer-rated toxicity scores (Common Toxicity Criteria Adverse Effects, CTCAE) before therapy and through 24 months after therapy
Influence of Clinical Factors and Toxicities on overall QOL
Of the clinical factors examined (T stage, N stage, tumor site, age, gender, smoking status, and neck dissection), T-Stage was the only clinical factor significantly associated with all three overall QOL measures in univariate analysis. In multivariate analysis, T stage retained significant correlation with HNQOL and UWQOL, while approaching significance (p=0.09) for “Overall Bother”.
Longitudinal regression models using all post-therapy time points demonstrated a highly significant relationship between observer-rated dysphagia and all three overall QOL measures (p<0.0005 for each measure), while correlations between xerostomia or voice toxicity and QOL were less strong and only significant for some of the overall QOL measures (p = 0.02 to 0.06). Other CTCAE toxicities were not significantly correlated with the QOL measures.
Pearson’ Correlation Coefficients, their statistical significance for each toxicity vs. each of the overall QOL measures, and parameter estimates (the average change in QOL per one unit increase in toxicity scores), are detailed in Table 2. When all post-therapy data points were taken into account, the magnitude of correlation with overall QOL was the largest for dysphagia: its correlation coefficients with the three overall QOL measures in the range of 0.49-0.56. The correlation coefficients of xerostomia with each of the three overall QOL measures were lower: 0.26-0.35, and their parameter estimates were lower as well. The correlation coefficients for mucosal toxicity were similar to those of xerostomia when all post-therapy time points were taken into account, and were lower for the rest of the toxicities (Table 2).
Table 2.
Association between Observer-rated (CTCAE) Toxicities or objective measures (VF scores or stimulated saliva) and overall patient-reported QOL: for all post-therapy and for late (≥6 and ≥12 months) data
| Variable | Pearson Correlation Coefficient for all data | p-value for Correlation for all data | Parameter Estimate for all data | Pearson Correlation Coefficient for ≥ 6 month data | p-value for Correlation for ≥ 6 month data | Parameter Estimate for ≥ 6 month data | Pearson Correlation Coefficient for ≥ 12 month data | p-value for Correlation for ≥ 12 month data | Parameter Estimate for ≥ 12 month data |
|---|---|---|---|---|---|---|---|---|---|
| “Overall Bother” | |||||||||
| CTCAEDysphagia | 0.49 | 0.0005 | 3.0 | 0.44 | <0.00001 | 4.0 | 0.33 | 0.01 | 3.0 |
| CTCAEXerostomia | 0.26 | 0.02 | 2.0 | 0.25 | 0.10 | 1.0 | 0.23 | 0.01 | 3.0 |
| CTCAEVoice | 0.06 | NS | 3.0 | 0.06 | NS | 3.0 | 0 | n/a | 0 |
| CTCAEmucosa | 0.35 | 0.01 | 2.0 | 0.33 | 0.009 | 3.0 | 0.19 | 0.05 | 3.0 |
| CTCAEskin | 0.03 | 0.005 | 4.0 | 0.21 | 0.01 | 3.0 | 0.15 | 0.03 | 4.0 |
| CTCAEfibrosis | 0.19 | 0.02 | 4.0 | 0.19 | 0.02 | 4.0 | 0.19 | 0.03 | 4.0 |
| CTCAEL'hermitte's | 0.17 | NS | 2.0 | 0.17 | NS | 2.0 | 0.20 | NS | 1.0 |
| VF score | 0.16 | NS | 2.0 | 0.16 | NS | 2.0 | 0.16 | NS | 2.0 |
| Total | -0.23 | NS | -4.0 | -0.17 | NS | -4.0 | -0.15 | NS | -5.0 |
| Stimulated Saliva | |||||||||
| Summary HNQOL Score | |||||||||
| CTCAEDysphagia | 0.53 | <0.0001 | 5.2 | 0.51 | <0.0001 | 5.0 | 0.50 | <0.0001 | 7.3 |
| CTCAEXerostomia | 0.31 | 0.02 | 2.3 | 0.30 | 0.01 | 2.9 | 0.26 | <0.001 | 4.8 |
| CTCAEVoice | 0.18 | 0.04 | 7.0 | 0.09 | NS | 3.3 | 0 | n/a | 0 |
| CTCAEmucosa | 0.32 | 0.01 | 2.4 | 0.30 | NS | 2.2 | 0.23 | 0.01 | 4.4 |
| CTCAEskin | 0.02 | NS | 0.4 | 0.17 | NS | 2.9 | 0.04 | NS | 1.7 |
| CTCAEfibrosis | 0.08 | NS | 0.7 | 0.14 | NS | 2.1 | 0.09 | NS | 1.9 |
| CTCAEL'hermitte's | 0.12 | NS | 2.5 | 0.18 | NS | 3.3 | 0.16 | NS | 3.0 |
| VF score | 0.37 | <0.0001 | 4.4 | 0.29 | 0.01 | 3.5 | 0.29 | 0.01 | 3.5 |
| Total | -0.20 | NS | -4.3 | -0.12 | NS | -3.8 | -0.12 | NS | -5.4 |
| Stimulated Saliva | |||||||||
| Summary UWQOL Score | |||||||||
| CTCAEDysphagia | 0.56 | <0.0001 | 3.5 | 0.53 | <0.001 | 3.3 | 0.57 | <0.0001 | 5.6 |
| CTCAEXerostomia | 0.35 | <0.001 | 2.4 | 0.32 | NS | 0.9 | 0.28 | 0.01 | 2.4 |
| CTCAEVoice | 0.17 | 0.02 | 5.3 | 0.11 | NS | 5.1 | 0 | n/a | 0 |
| CTCAEmucosa | 0.43 | <0.0001 | 3.2 | 0.38 | NS | 0.9 | 0.37 | 0.02 | 2.9 |
| CTCAEskin | 0.04 | NS | 1.6 | 0.14 | 0.05 | 2.4 | 0.03 | NS | 2.4 |
| CTCAEfibrosis | 0.08 | NS | 1.3 | 0.14 | NS | 2.1 | 0.09 | NS | 1.5 |
| CTCAEL'hermitte's | 0.05 | NS | 1.4 | 0.11 | NS | 2.1 | 0.10 | NS | 0.5 |
| VF score | 0.44 | 0.0001 | 3.0 | 0.41 | 0.0001 | 4.4 | 0.41 | <0.0001 | 4.4 |
| Total | -0.21 | NS | -3.2 | -0.15 | NS | -2.4 | -0.14 | NS | -2.7 |
| Stimulated |
When the analysis was limited to long-term QOL, ≥6 or ≥12 months post-treatment, the correlation coefficients did not change substantially for most toxicities and the overall trends were maintained (Table 2). Dysphagia retained the highest correlation coefficients and highest statistical significance of all toxicities, with similar, or slightly lower, correlation coefficients compared with those observed for dysphagia when all post-therapy data were taken into account. The correlation coefficients for other toxicities at ≥6 months or ≥ 12 months remained weaker, with xerostomia and mucosal toxicities being the only other toxicities retaining statistically significant correlations with most overall QOL measures.
Association between Objective Studies and Overall QOL
Taking into account all post-therapy data points, VF scores were significantly correlates with the HNQOL and UWQOL summary scores (p<0.0001) but not with “Overal Bother” scores Table 2). When only late (≥ 6 months) data points were analyzed, the high significance and high Pearson correlation coefficients were retained for summary UWQOL scores but were less significant (p=0.01), with lower correlation coefficients and parameter estimates, for summary HNQOL scores. Analyses taking into account ≥ 12 months data points were similar (Table 2). The association of summary HNQOL score with VF scores is illustrated in Figure 3. Total stimulated saliva did not correlate significantly with any of the overall QOL scores (Table 2). Due to the multiple correlation tests performed (54 tests detailed in Table 2), a Bonferroni correction was employed, requiring p≤ 0.0009 to be regarded as statistically significant. This correction left CTCAEdysphagia and the VF scores as the only factors that consistently had statistically significant correlations with most overall QOL measures.
Figure 3.
Summary HNQOL Scores as a function of Swallow Summary (videofluoroscopy, VF) Scores
Discussion
This study demonstrated that despite the absence of, or mild, observer- rated dysphagia in the large majority of patients, it still had the highest correlation with all overall QOL measures. VF scores, serving as objective measures of swallowing dysfunction, were also highly correlated with many overall QOL measures, supporting this finding. Our study, conducted in a homogeneous patient population receiving uniform chemo-IMRT, reached similar conclusions as those reached by Langendijk et al., who previously showed that RTOGdysphagia was the strongest determinant of post-treatment QOL in a very heterogeneous group of patients treated with 3D radiation therapy, mostly without concurrent chemotherapy, where no effort was made to spare the swallowing structures or salivary glands. Our findings that even the mild dysphagia observed after chemo-IMRT was still highly associated with patient-reported QOL suggest that efforts to reduce late dysphagia even further have the potential to improve patient-reported QOL.
How can we achieve further improvements in the rate of post-treatment dysphagia? Dose-effect relationships for the swallowing structures have been published in recent years (13). While the patients participating in this study and their dysphagia results served to produce some of these relationships (9), they were not yet known yet at the time of the study. Adherence to the published dose-effect relationships, reducing the doses to the swallowing structures as much as possible without affecting target doses, is likely to further improve dysphagia. Importantly, several current studies are currently assessing the safety of reduced-intensity treatment for good-prognosis patients with Human Papilloma Virus- associated oropharyngeal cancer. If these studies will demonstrate that high tumor control rates are preserved, future reduced-intensity treatment in these patients is expected to reduce late dysphagia and improve QOL.
Additional ways to reduce late dysphagia include specific efforts to reduce acute mucositis. Our previous study of MRI before and 3 months after chemoradiation demonstrated anatomical and structural changes in the pharyngeal constrictors, notably thickening and increased T2 signal, which were not found in other muscles receiving high doses (14). This finding suggests a consequential effect of acute mucositis on the submucosal constrictors, an effect which was not observed in other muscles (which are not close anatomically to the mucosa). Reducing acute mucositis using novel, effective agents, is therefore likely to reduce late dysfunction of the swallowing-related organs. Efforts in this direction are ongoing (15). Other avenues include intensive nutritional support (16) and recent reports suggesting that prophylactic swallowing exercises during chemo-radiotherapy may reduce subsequent dysphagia (17). Such interventions may further reduce dysphagia in patients whose swallowing organs dosimetry has improved as much as possible using IMRT.
The high correlation between the generally mild late observer-rated dysphagia and the overall QOL found in our study may be explained by our previous findings that observer-rated grade 1 CTCAEDysphagia which persists late after therapy (≥12 months) is associated with VF scores that characterize higher-grade dysphagia (18). Thus, even mild observer-rated dysphagia, which does not require a change in diet, may be associated with objective VF abnormalities and affect patient-reported QOL.
Other observer-rated toxicities, notably xerostomia and mucosal toxicities, were associated with some, but not the majority, of overall QOL measures, and the strength of these correlations were lower than those found for dysphagia. A lack of correlation between xerostomia or salivary output and overall QOL has been reported by few investigators (19-20), however, a strong correlation was found by others following conventional radiotherapy (1), as well as our previous study in which only the parotid glands were spared (10). It is possible that more recent improvements in reducing xerostomia made in the patients who participated in the current study, such as efforts to spare the minor salivary glands within the oral cavity and the contralateral submandibular glands, in addition to sparing the parotid glands (10), may have reduced xerostomia effect on QOL.
The limits of the study include the lack of a gold-standard for assessment of overall health-related QOL, prompting us to test three different measures which represent the two common methods to assess overall QOL (8). Two measures in our study consisted of summary scores of whole validated questionnaires, and one measure consisted of a single question aiming to capture global QOL (“Overall Bother”). CTCAEdysphagia was highly statistically significantly correlated with all the three overall QOL measures, strengthening our most important conclusions. A more detailed analysis of the correlations between QOL and specific toxicities such as dysphagia and xerostomia may include analyses of correlations of each of these toxicities with the specific domains of the QOL instruments, which was outside the scope of this investigation. Rather than just a correlate with overall QOL, dysphagia likely had a causal relationship with QOL, fulfilling the criteria of cause-effect relationships of symptoms and QOL described by Fayers et al (8).
In conclusion, modern chemo-IMRT aimed at reducing xerostomia and dysphagia was successful in achieving no, or mild, late observer-rated dysphagia and xerostomia, however, this study suggests that patients still perceive dysphagia as a sequel that affect their overall QOL. Further efforts, beyond dosimetric ones, to reduce late dysphagia, are therefore likely to yield additional gains in QOL.
In a prospective longitudinal study of oropharyngeal cancer patients receiving concurrent chemo-IMRT aiming to spare the swallowing structures and salivary glands, almost all late dysphagia toxicities were CTCAE grade 0-1, however, dysphagia and objective assessments of swallowing were still the primary correlates of overall QOL. These data support additional efforts to reduce dysphagia in order to further improve QOL.
Acknowledgments
Supported by NIH grant PO1 CA59827 and the Newman Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflict of interest. No copyrighted information or patient photos are used.
References
- 1.Bjordal K, Kaas S, Matekaasa A. Quality of life in patients treated for head and neck cancer. Int J Rad Onc Biol Phys. 1994;28:847–855. doi: 10.1016/0360-3016(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 2.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008 Aug 1;26(22):3770–6. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 3.Pow EHN, Kwong DLW, McMillan AS, et al. Xerostomia and quality of life after intensity modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal cancer. Int J Rad Onc Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011 Feb;12(2):127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007 Nov 1;25(31):4873–9. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 6.Jabbari S, Kim HM, Feng M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head and neck cancer: initial report. Int J Radiat Oncol Biol Phys. 2005;63(3):735–31. doi: 10.1016/j.ijrobp.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 7.Feng FY, Kim HM, Lyden TH, et al. Intensity modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol. 2010;28:2732–8. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayers PM, Hand DJ, Bjordal K, Groenvold M. Causal indicators in quality of life research. Quality of Life Res. 1997;6:393–406. doi: 10.1023/a:1018491512095. [DOI] [PubMed] [Google Scholar]
- 9.Eisbruch A, Kim HM, Feng FY, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011 Nov 1;81(3):e93–9. doi: 10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little M, Schipper M, Feng FY, et al. Reducing Xerostomia After Chemo-IMRT for Head-and-Neck Cancer: Beyond Sparing the Parotid Glands. Int J Radiat Oncol Biol Phys. 2012 Jul 1;83(3):1007–14. doi: 10.1016/j.ijrobp.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin AL, Kim HM, Terrell JE, et al. Quality of life after parotid-sparing IMRT for head and neck cancer: a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2003;57(1):61–70. doi: 10.1016/s0360-3016(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 12.Karnell MP, MacCracken E. A database information storage and reporting system for videoflurographic oropharyngeal motility swallowing evaluations. Am J Speech Language Pathol. 1994;3:54–60. [Google Scholar]
- 13.Rancati T, Schwartz M, Allen AM, et al. Radiation dose-volume effects in the larynx and pharynx. Int J Rad Onc Biol Phys. 2010;76(3 Suppl):S64–9. doi: 10.1016/j.ijrobp.2009.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popovtzer A, Cao Y, Feng FY, et al. Anatomical changes in the pharyngeal constrictors after chemo-irradiation of head and neck cancer and their dose-effect relationships: MRI-based study. Radiother Oncol. 2009 Dec;93(3):510–5. doi: 10.1016/j.radonc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le QT, Kim HE, Schneider CJ, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011 Jul 10;29(20):2808–14. doi: 10.1200/JCO.2010.32.4095. Epub 2011 Jun 13. [DOI] [PubMed] [Google Scholar]
- 16.Silander E, Nyman J, Bove M, et al. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer-randomized study. Head Neck. 2012;34:1–9. doi: 10.1002/hed.21700. [DOI] [PubMed] [Google Scholar]
- 17.Carnaby-Mann G, Crary MA, Schmalfuss I, Amdur R. “pharyngocise”: randomized controlled trial of preventive exercises to maintain muscle structure and swallowing function during head and neck chemoradiotherapy. Int J Rad Onc Biol Phys. 2012;83:210–9. doi: 10.1016/j.ijrobp.2011.06.1954. [DOI] [PubMed] [Google Scholar]
- 18.Gluck I, Feng FY, Lyden T, et al. Evaluating and reporting dysphagia in trials of chemoirradiation for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010 Jul 1;77(3):727–33. doi: 10.1016/j.ijrobp.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scrimger R, Kanji A, Parliament M, et al. Correlation between saliva production and quality of life in head and neck cancer patients treated with IMRT. Am J Clin Oncol. 2007;30:271–277. doi: 10.1097/01.coc.0000258081.70643.3d. [DOI] [PubMed] [Google Scholar]
- 20.Ringash J, Warde P, Lockwood G, et al. Postradiotherapy quality of life for head and neck cancer patients is independent of xerostomia. Int J rad onc Biol Phys. 2005;61:1403–7. doi: 10.1016/j.ijrobp.2004.08.001. [DOI] [PubMed] [Google Scholar]