Abstract
Elastic fibers are critical connective tissue components providing elasticity and resilience to skin and other tissues. These fibers are composed of elastin and a number of elastin-associated microfibrillar proteins that assemble in a complex fiber network in a multi-step process. Multiple cellular processes, including mitochondrial function, specific molecules in the secretory pathways, and temporally and spatially ordered production of elastic fiber components, are required for the biogenesis of functional elastic fibers. Abnormalities in these processes can lead to loss of functional elastic fibers manifesting phenotypically as a skin disease. The paradigm of elastic fiber diseases affecting the skin is cutis laxa, a clinically and genetically heterogeneous group of disorders characterized by loose and sagging skin, frequently associated with extracutaneous manifestations in the lungs and the arterial blood vessels. The complexity of cutis laxa is emphasized by the fact that as many as 10 distinct genes can harbor mutations in this and related disorders. Understanding of the pathomechanistic pathways involved in perturbed elastic fiber assembly in cutis laxa provides information potentially helpful for development of molecular strategies towards treatment of these, currently intractable, diseases.
Keywords: Elastic fiber assembly, elastin, microfibrillar proteins, cutis laxa, heritable skin diseases
Introduction
The elastic fiber system of connective tissues forms a network that is responsible for resilience and elasticity of various organs. The relative concentration of the elastic fibers in different tissues is highly variable, their relative concentrations being highest in the lungs, aorta and the arterial blood vessels (for review on elastic fibers, see refs. (1-3). Elastic fibers represent ~2-4% of the total dry weight of the dermis in adult, sun-protected area of skin. Elastin is synthesized in arterial blood vessels by vascular smooth muscle cells, and the cell type responsible for elastin synthesis in the skin is the fibroblast. These notions are based on observations that vascular smooth muscle cells and dermal fibroblasts in culture actively synthesize this protein (4).
The critical role of elastic fibers in providing elasticity and resilience to a number of tissues, particularly to the skin, is attested by observations that in a number of diseases alteration in elastic fibers is associated with loose and sagging skin with loss of recoil (5, 6). This overview will discuss one of such entities, cutis laxa (CL), as a paradigm of disorders attesting to the complexity of elastic fiber assembly.
The structural and molecular features of elastic fibers in the skin
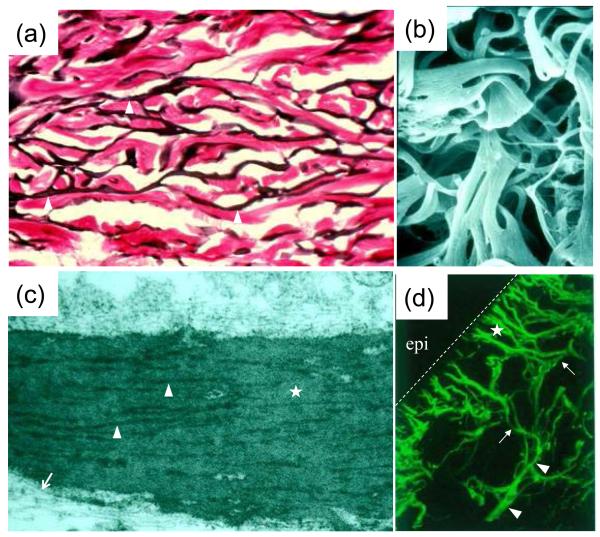
Examination of the elastic fibers in human dermis either by histopathologic means, by immunostaining or by ultrastructural analysis reveals an interconnecting network of fibers (Fig. 1a). The intricate, interwoven network structure can also be demonstrated by examination of the insoluble elastic fibers that have been isolated from tissues by techniques which result in dissolution of the cellular elements and other extracellular matrix components, primarily collagen, leaving insoluble elastin intact (Fig. 1b). Transmission electron microscopy of elastic fibers in the skin demonstrates the presence of two distinct components: (a) elastin, a well-characterized connective tissue protein, and (b) elastin-associated microfibrils (Fig. 1c). The fiber network in the reticular dermis is oriented parallel to the skin surface. In the upper reticular dermis there are finer fiber structures, so-called elaunin fibers, and extending from these fibers perpendicular to the skin surface are fine elastic fiber structures, so-called oxytalan fibers, which terminate just below the dermal-epidermal basement membrane (Fig. 1d). The epidermis does not appear to have elastic fibers, although cultured keratinocytes have been shown to express the elastin gene at a low level (7). The presence of an intact elastic fiber network is critical for elasticity of the skin, as attested by the fact that chronic sun exposure results in destruction of the fiber architecture, accompanied by loss of elasticity of the skin (8).
Figure 1. Morphologic characteristics of elastic fibers in human skin.
(a) Histopathologic staining with Verhoeff von Giesson stain demonstrates interconnecting elastic fibers running parallel to the skin surface in the reticular dermis (arrowheads); (b) Scanning electron microscopy of insoluble elastic fibers isolated from human skin by dissolution of cellular elements and other extracellular matrix components; (c) Transmission electron microscopy of a mature elastic fiber in reticular dermis reveals an elastin core (uniform gray background; asterisk), surrounded by bundles of electron-dense microfibrils (arrowheads) or individual microfibrils (arrows); (d) Immunofluorescent analysis of elastic fibers with an elastin antibody reveals broad elastic fibers in reticular dermis (arrowheads) from which smaller fibers, elaunin (arrows) and oxytalan (asterisk) fibers extend vertically towards the dermal-epidermal junction delineated by a dashed line. Note that epidermis (epi) is devoid of elastic fibers. (The original magnifications: a, 400x; b, 5,000x; c, 16,000x; d, 200x).
Elastin
Elastin is a well characterized connective tissue protein which is initially synthesized as a linear polypeptide of ~70 kDa, known as tropoelastin. The precise amino acid sequence of human tropoelastin has been deciphered from cloning of full-length cDNA and the corresponding gene (ELN) (9, 10). The primary sequence of elastin is characterized by alternating hydrophobic domains and so-called crosslink domains rich in alanine and lysine (Fig. 2). During elastin fibrillogenesis, newly synthesized tropoelastin polypeptides become juxtaposed, and their association becomes stabilized by formation of covalent intermolecular crosslinks, desmosines. Desmosines are unique to elastin among mammalian tissues, and the content of desmosines in various elastin preparations can therefore be assayed as a quantitative measure of the elastin content (11, 12). The synthesis of desmosines is initiated by oxidative deamination of lysyl residues by lysyl oxidases, copper dependent enzymes synthesized and secreted by smooth muscle cells and fibroblasts (13). Subsequently, three of the resulting aldehydes and an unmodified epsilon-amino group of lysine non-enzymatically fuse to form a stable desmosine crosslink. Progressive addition of desmosines to different parts of the molecule links additional tropoelastins to form highly interconnected elastin polymer.
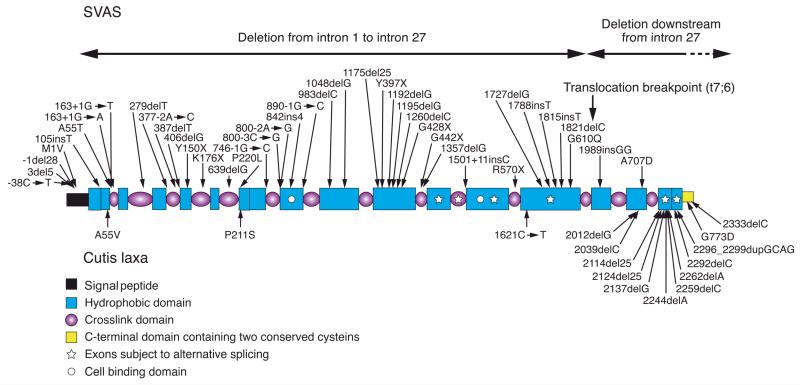
Figure 2. Schematic representation of the elastin domain organization.
The polypeptide consists of modules as defined on the lower left. The C-terminal region has also been described as a cell binding domain (74). Positions of mutations in the autosomal dominant cutis laxa (below) and in supravalvular aortic stenosis (SVAS) (above the molecule) are indicated by arrows.
The human elastin gene, ELN, is localized to the long arm of chromosome 7 and consists of 34 exons spanning ~45 kb of the genome (14). These exons correspond to a full-length cDNA of ~3.5 kb which encodes a tropoelastin polypeptide of ~800 amino acids. An interesting feature of the human elastin gene is that the initially synthesized pre-mRNA undergoes extensive alternative splicing, resulting in synthesis of an ensemble of tropoelastin polypeptides with slightly variable primary sequences (Fig. 2). Currently, the physiological or developmental significance of the alternative splicing in elastin is unknown, but these observations suggest that small insertions or deletions in the gene, if in-frame, are unlikely to result in significant pathology. Indeed, sequencing of control individuals has revealed some benign, insertion-deletion variants in exons encoding hydrophobic domains with peptide repeats (15). Inter-species comparison of elastin gene sequences has uncovered loss of whole exons and expansion or contraction of hydrophobic peptide repeats consistent with rapid evolution of elastin in vertebrate lineages driven by variable exon use and insertion-deletion variation (16, 17). Stressors relevant to skin aging, such as UV and heat exposure, can alter the alternative splicing pattern of elastin (18), and population-specific differences in elastin mRNA splicing have also been uncovered (19).
Elastin turnover
The metabolic turnover of elastin is slow, with a half-life comparable to human lifespan (20). Thus, unlike collagen fibers, the function of elastic fibers is not thought to depend on constant remodeling and turnover. However, pathological conditions, such as inflammation, sun damage of the skin and cigarette smoke exposure of the lungs, can lead to degradation of elastic fibers. This process is dependent on elastolytic enzymes, elastases, which are present in a number of tissues and secreted by various cell types, including polymorphonuclear leukocytes, monocyte/macrophages, as well as by smooth muscle cells and fibroblasts (21-23).
Elastin-associated microfibrils
The microfibrillar component of the elastic fibers is composed of a number of proteins contributing to the physiological properties of elastic fibers (Table 1); among them are fibrillins 1 and 2 (FBN1 and 2), fibulins 1, 2, 3, 4 and 5 (FBLN1, 2, 3, 4, 5) as well as a number of less well characterized microfibril-associated proteins (24,26). While some of these proteins are characterized by molecular cloning, their precise physiological roles in elastic fiber assembly are currently unknown.
Table 1.
Components of the elastic fibers
| Characteristic Features | |
|---|---|
| ELASTIN | Structural protein of the elastin core |
| MICROFIBRILLAR PROTEINS | |
| Fibrillins | |
| FBN1 | Structural protein of microfibrils |
| FBN2 | Structural protein of microfibrils |
| FBN3 | Structural protein of microfibrils |
| Fibulins | |
| FBLN1 | Associated with elastin, required for endothelial development |
| FBLN2 | Located at the elastin-microfibril interface |
| FBLN3 | Binds tissue inhibitor of metalloproteases 3 (TIMP3) |
| FBLN4 | (EFEMP2) Binds LOX and FBN1 |
| FBLN5 | Binds ELN, FBN1, LTBP2, LOXL1 and integrins |
| Latent TGF-β binding proteins | |
| LTBP1 | Binds microfibrils, fibronectin and latent TGFβ |
| LTBP2 | Binds FBLN5, microfibrils and elastic fibers |
| LTBP3 | Binds microfibrils and latent TGFβ |
| LTBP4 | Binds microfibrils, fibronectin and latent TGFβ |
| Microfibril-associated glycoproteins | |
| MAGP1 (MFAP2) | Binds FBN1 |
| MAGP2 (MFAP5) | Localized to microfibrils |
| Microfibril-associated proteins | |
| MFAP1 | Localized to microfibrils |
| MFAP3 | Localized to microfibrils |
| MFAP4 | Localized to microfibrils |
| Other components | |
| TGFBI (MP78/70, βig-h3) | Localized to elastic fiber/collagen fiber interface |
| LOX | Binds FBLN4, microfibril-elastin interface, crosslinks ELN |
| LOXL1 | Binds FBLN5, crosslink ELN |
| EMILIN1 | Localized to microfibril-elastin interface |
| EMILIN2 | Localized to microfibril-elastin interface |
| VCAN | Binds FBN1 |
| Heparan sulfate | Interaction with LTBP4 |
Heterogeneity of cutis laxa
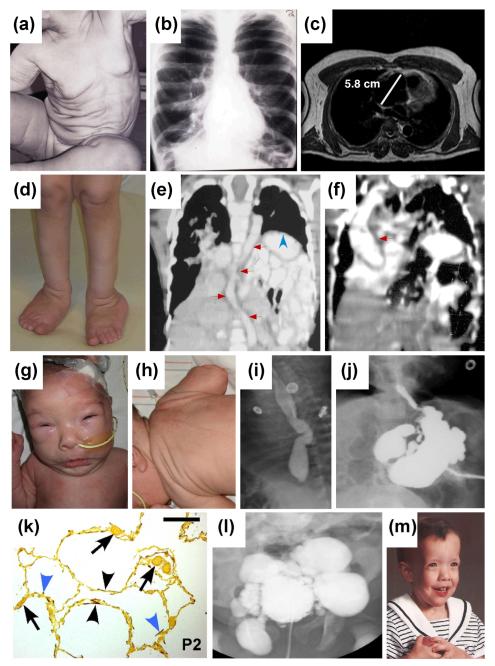
A group of disorders resulting from abnormal elastic fibers is CL, characterized by loose, redundant skin with loss of elasticity and recoil (Fig. 3) (for review on clinical features of CL, see ref. (27)). The redundant skin most often affects the entire body area (Fig 3a) and can be seen on the face creating a prematurely aged appearance. The inheritance of CL may be autosomal dominant, autosomal recessive or X-linked recessive. In addition, cases considered to be acquired, demonstrating late onset skin involvement often associated with preceding inflammatory reactions or with hematological malignancies have been reported (28, 29). The precise classification of CL is somewhat difficult due to the paucity of cases in each category and significant overlap in the clinical phenotypes, but certain subtypes with a particular form of inheritance have been identified in association with specific genes (Table 2) (27).
Figure 3. The spectrum of clinical manifestations of cutis laxa.
Patients with ADCL, caused by ELN mutations, manifest with generalized skin laxity (a), pulmonary emphysema (b), and an aortic root aneurysm (c). A patient with ARCL1A, caused by a homozygous mutation in the FBLN4 gene, shows skin and joint laxity (d), aortic tortuosity (e, red arrows), diaphragmatic hernia (e, blue arrowhead), and an ascending aortic aneurysm (f, red arrow). Patients with ARCL1C (Urban-Rifkin-Davis syndrome), caused by mutations in the LTBP4 gene, demonstrate hypertelorism, periorbital fullness, microretrognathia and long philtrum (g), redundant skin on the neck and back (h), esophageal tortuosity (i), gastric diverticula (j), developmental emphysema with underdeveloped alveolar septa (k, blue arrowheads), abnormal localization of elastic fibers (k, black arrowheads), thickened capillary walls (k, arrows) and bladder diverticula (l). Characteristic craniofacial manifestations of a patient with ARCL2A, caused by mutations in ATP6V0A2, include broad forehead, bitemporal narrowing, hypertelorism, downward-slanting palpebral fissures, and broad, flat nasal bridge (m). Magnification bar in (k) is 200 μm. Reproduced with permission from the following publications: (a) Callewaert et al. (30); (b) Urban et al. (73); (c, e, f, m) Berk et al. (27); (g-l) Urban et al. (39).
Table 2.
Molecular genetics of heritable forms of cutis laxa and related disorders
| Disease* | Distinguishing Clinical Features | Mutated Genes | References |
|---|---|---|---|
| ADCL | Pulmonary and cardiovascular manifestations absent, milder or later onset |
ELN | 30, 31 |
| ARCL1A | Arterial tortuosity, lethal pulmonary hypertension, bone fragility |
FBLN4 | 35-37 |
| ARCL1B | Supravalvular aortic stenosis, lethal developmental emphysema |
FBLN5 | 38 |
| ARCL1C/ URDS |
Severe gastrointestinal and urinary malformations, lethal developmental emphysema mild cardiovascular involvement |
LTBP4 | 39 |
| ARCL2A | Growth and developmental delay, abnormal glycosylation of serum proteins |
ATP6V0A2 | 52, 53 |
| ARCL2B | Growth and developmental delay, triangular face, normal glycosylation |
PYCR1 | 55, 56 |
| XLCL | Occipital exostoses, pili torti | ATP7A | 60-62 |
| DBS/ ARCL3 |
Corneal clouding, athetoid movements | ATP6V0A2, PYCR1, ALDH18A1 |
53, 56-58 |
| GO | Bone fragility, short stature | GORAB | 59 |
| MACS | Macrocephaly, alopecia, scoliosis | RIN2 | 63 |
| ATS | Triangular face, arterial tortuosity, normal lungs |
SLC2A10 | 65, 66 |
ADCL, autosomal dominant cutis laxa; ARCL, autosomal recessive cutis laxa; URDS, Urban-Rifkin-Davis syndrome; XLCL, X-linked cutis laxa; DBS, DeBarsy syndrome; GO, geroderma osteodysplasticum; MACS, macrocephaly-alopecia-cutis laxa-scoliosis syndrome; ATS, arterial tortuosity syndrome.
Autosomal dominant cutis laxa
The phenotypic variability in CL is reflected by the underlying mutations in the causative genes. For example, among the inherited forms, the autosomal dominant CL (ADCL), which may present predominantly with cutaneous findings but can also be associated with systemic manifestations in the cardiac and pulmonary systems, are mostly due to frame-shift mutations in the 3′-end of the ELN gene which results in extended missense peptide sequence at the carboxy-terminal end of the newly synthesized tropoelastin (Fig. 2). These mutant tropoelastin polypeptides, which have reduced fibrillin-binding capacity, are incorporated into the wild-type elastic structures leading to fibers with increased compliance and reduced stiffness of tissues (30, 31). Thus, ELN mutations in ADCL act in a dominant negative manner with respect to tissue mechanics. It is of interest to note that ~30% of the patients with the ADCL have been suggested to result from de novo dominant mutations in ELN with no family history.
While the mutations in ADCL reside predominantly in the 3′-end of the ELN gene, another allelic condition, supravalvular aortic stenosis (SVAS), is caused by mutations broadly distributed in the same gene (Fig. 2). The repertoire of mutations in SVAS includes missense, nonsense, 1-bp insertion or deletion and splice-site mutations, and cases with large deletions encompassing portions of the ELN gene have also been reported (Fig. 2). These mutations lead to loss of function either by activating the nonsense-mediated mRNA decay pathway (32) or by producing elastin precursors with impaired self-association/polymerization properties (33). Therefore, the disease mechanism in SVAS is functional haploinsufficiency. It should be noted that SVAS can be part of the Williams syndrome, a constellation of developmental and central nervous system problems. The Williams syndrome is a contiguous gene deletion syndrome on chromosome 7q, and because the deleted segment of genome includes ELN, SVAS is part of the clinical presentation (34).
Autosomal recessive cutis laxa type 1
The autosomal recessive forms of cutis laxa (ARCL) are divided into two types based on difference in phenotypes, and reflecting the underlying mutations in different genes (Table 2) (27). The ARCL1 is defined as a disease with severe, often lethal cardiovascular and pulmonary manifestations, including developmental emphysema, diaphragmatic defects, arterial aneurysms or stenoses (Fig. 3d-1). ARCL1A is caused by the fibulin-4 (FBLN4/EFEMP2) gene mutations and is associated with arterial tortuosity and aneurysms as well as bone fragility as distinguishing manifestations (35-37). Mutations in fibulin-5 (FBLN5) gene cause ARCL1B, and several of these patients have supravalvular aortic stenosis, not found in other types of cutis laxa (38). Patients with ARCL1C, also known as Urban-Rifkin-Davis syndrome (URDS), commonly have severe gastrointestinal and urinary tract involvement and harbor mutations in the LTBP4 gene encoding a latent TGFβ binding protein which facilitates the localization of latent TGFβ to the microfibrils (39).
All three proteins, i.e., fibulins 4 and 5, and LTBP4, involved in ARCL1 are required for proper assembly of elastic fibers. Specifically, fibulin-5 interacts with integrin receptors (40), tropoelastin (41), fibrillin-1 (42), lysyl oxidase-like-1 (43) and LTBP2 (44), and facilitates the deposition of tropoelastin onto the microfibrillar scaffold (45). Fibulin-4 can also bind tropoelastin, lysyl oxidase (46) and fibrillin-1 (42). LTBP4, in addition to serving as a regulator of TGFβ signaling, is also required for elastic fiber assembly (47), perhaps through its ability to interact with fibrillin-1 (48), fibronectin and heparan sulphate (49). Competitive interactions between fibulins, LTBPs and fibrillins (42) may contribute to the hierarchical, cell directed process of elastic fiber assembly (50, 51).
Autosomal recessive cutis laxa type 2 and related conditions
ARCL2 is defined as cutis laxa with growth and developmental delay and is subdivided into A and B subtypes. ARCL2A patients demonstrate microcephaly and delayed closure of the anterior fontanelles and characteristic craniofacial features (Fig. 3m). An important diagnostic test in ARCL2A is assay for serum protein glycosylation, which shows combined N- and O-glycosylation defects (52). This subtype of CL is caused by mutations in the ATP6V0A2 gene, which encodes a subunit of a proton pump located in the Golgi apparatus and vesicles of the secretory pathway (53). As a consequence, vesicular trafficking is impaired resulting in the accumulation of the elastin precursor, tropoelastin, in the Golgi vesicles (54).
The ARCL2B subtype is associated with progeroid features and triangular face, but with presentation otherwise similar to ARCL2B, and results from mutations in the PYCR1 gene involved in proline metabolism in the mitochondria (55, 56). PYCR1 deficient cells have altered mitochondrial morphology, and increased sensitivity to oxidative stress, but the molecular mechanisms of altered elastic fiber function in ARCL2A remain to be investigated.
DeBarsy syndrome (DBS), also known as ARCL3, forms a phenotypic continuum with ARCL2A and ARCL2B and is characterized by progeroid appearance, reduced subcutaneous adipose tissue, and bilateral corneal opacities. Mutations in patients diagnosed with DBS have been found in ATP6V0A2 (53), in PYCR1 (56), and in the gene for another mitochondrial enzyme in the proline biosynthetic pathway, ALDH18A1 (57, 58).
Geroderma osteodysplasticum (GO) is an autosomal recessive disorder related to ARCL2, characterized by markedly short stature and severe bone fragility. The gene responsible for GO is GORAB, which encodes a binding partner of the Rab6 G-protein involved in vesicular transport from the Golgi to the plasma membrane (59).
The X-linked cutis laxa
The X-linked variant of cutis laxa, XLCL, was previously classified as the Ehlers-Danlos syndrome type IX. However the cutaneous findings, hyperextensible and wrinkled skin, with associated features of bladder diverticula and inguinal hernias, are more consistent with the cutis laxa phenotype. XLCL has also been described as occipital horn syndrome, since these patients demonstrate downward-pointing exostoses on the occipital bones (60). XLCL results from copper deficiency and can be diagnosed by serum copper and ceruloplasmin level determinations. The copper deficiency is a result of mutations in the ATP7A gene which is also mutated in the Menkes syndrome (61). However, the XLCL patients mainly demonstrate connective tissue problems, while patients with the Menkes syndrome also have severe neurological defects, often leading to premature demise within the first few years of age. The connective tissue manifestations in XLCL are likely to be caused by reduced lysyl oxidase enzyme activity resulting in deficient cross-linking of elastin and collagen (62).
Additional conditions with cutis laxa-like phenotypes
A limited number of syndromes with skin laxity and associated systemic involvement have been described, with identification of distinct causative genes. One of them is known as MACS syndrome, described in a family with age-dependent redundant skin, droopy eyelids and joint hypermobility, in association with a number of connective tissue problems, as well as macrocephaly, alopecia and scoliosis (63). This family was shown to harbor mutations in the RIN2 gene which encodes a protein serving as an exchange factor for Rab5, a guanosine triphosphatase that regulates membrane and protein trafficking. Patients with MACS syndrome share features with both cutis laxa and the Ehlers-Danlos syndromes and show abnormalities of both the elastic and collagen fibers (64).
Arterial tortuosity syndrome (ATS), an autosomal recessive condition with skin laxity, is associated with elongation and tortuosity of major arteries leading to vascular aneurysms, dissections and stenosis, with death in early childhood (65). This syndrome is associated with inactivating mutations in the SLC2A10 gene which encodes GLUT10, a glucose transporter family member that has been localized to mitochondria and shown to participate in vitamin C transport (66).
Patients with excessively slack and redundant skin with pseudoxanthoma elasticum (PXE)-like cutaneous finding have recently been described and suggested to consist of a combination of cutis laxa and PXE (67, 68). These patients also demonstrated vitamin K-dependent coagulation factor deficiency. While the cutaneous features in these patients are more severe than in the classic forms of PXE, an ectopic mineralization disorder caused by mutations in the ABCC6 gene (69), the ocular findings, including angioid streaks, which are characteristic in PXE are very mild in these patients. Also, histopathology of affected skin shows characteristic findings of PXE, i.e., ectopic mineralization of degenerated pleiomorphic elastotic structures in mid dermis, rather than loss and fragmentation of the elastic fibers as in CL (67, 68). This disorder was shown to be caused by mutations in the GGCX gene encoding γ-glutamyl carboxylase which catalyzes carboxylation of Gla-proteins, such as vitamin K-dependent coagulation factors and matrix Gla protein, an anti-mineralization factor in tissues (67, 68, 70).
Conclusions and translational perspective
The cutis laxa-related genes highlight three major pathways required for elastic fiber biogenesis. Secreted proteins, such as ELN, FBLN4, FBLN5 and LTBP4, serve either as structural building blocks or facilitators of a complex, cell-directed assembly process. The downstream effect of mutations in several of these genes is up-regulation of TGFβ signaling (30, 31, 37, 39). Thus pharmacological inhibitors acting either directly or indirectly on the TGFβ pathway may be promising agents to test as experimental therapeutics.
The secretory pathway is also affected by multiple gene defects related to cutis laxa (ATP6V0A2, RIN2, GORAB). The products of these genes are active in the Golgi apparatus and subsequent sorting of molecules destined for secretion. Specific connections between individual components of the secretory machinery and cargo molecules are beginning to emerge. For example, ATP6V0A2 is required for the efficient secretion of tropoelastin (54), and RIN2 deficiency affects the production of microfibrils and fibulin-5 (63). Intracellular sorting and pre-assembly of elastic fiber components may be essential for efficient extracellular production of fibers. The use of molecular chaperones would be a possible approach for the treatment elastic fiber dysfunction related to secretory defects.
Surprisingly, several genes important in mitochondrial function were found to be mutated in cutis laxa-related conditions (PYCR1, ALDH18A1, and SLC2A10/GLUT10). Mitochondrial dysfunction and increased sensitivity to oxidative stress has been shown in either PYCR1 (56) or GLUT10 deficiency (66, 71, 72). Proline or vitamin C supplementation or antioxidant treatment targeted to the mitochondria may be therapeutic approaches to explore in these conditions.
Degenerative elastic fiber dysfunction in inherited diseases, such as PXE and related conditions, points to the existence of active cellular processes required for the protection of elastic fibers from ectopic calcification and degradation of elastic fibers. Further understanding of the molecular mechanisms of these disorders will provide therapeutic targets to protect elastic fibers from functional deterioration as a result of genetic or environmental influences or aging.
Acknowledgements
Carol Kelly assisted in manuscript preparation. The authors’ original studies have been supported in part by NIH/NIAMS grant R01AR28450 to J.U., by NIH/NHLBI grant R01HL090648 to Z.U., and by March of Dimes grant #FY09-556 to Z.U. Dr. Li is the recipient of a Dermatology Foundation Career Development Award.
Abbreviations
- CL
cutis laxa
- ADCL
autosomal dominant cutis laxa
- ARCL
autosomal recessive cutis laxa
- XLCL
X-linked cutis laxa
- SVAS
supravalvular aortic stenosis
- GO
geroderma osteodysplasticum
- ATS
Arterial tortuosity syndrome
- PXE
pseudoxanthoma elasticum
Footnotes
Author Contributions
All authors contributed to the research and preparation of the manuscript, and the final version has been read and approved by all of them.
Conflict of Interest
The authors have declared no conflicting interests.
References
- 1.Christiano AM, Uitto J. Molecular pathology of the elastic fibers. The Journal of investigative dermatology. 1994;103:53S–57S. doi: 10.1111/1523-1747.ep12399044. [DOI] [PubMed] [Google Scholar]
- 2.Ramirez F. Pathophysiology of the microfibril/elastic fiber system: introduction. Matrix Biol. 2000;19:455–456. doi: 10.1016/s0945-053x(00)00098-6. [DOI] [PubMed] [Google Scholar]
- 3.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81:229–240. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- 4.Pasquali-Ronchetti I, Baccarani-Contri M. Elastic fiber during development and aging. Microsc Res Tech. 1997;38:428–435. doi: 10.1002/(SICI)1097-0029(19970815)38:4<428::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: part I. Increased elastic tissue and solar elastotic syndromes. Journal of the American Academy of Dermatology. 2004;51:1–21. doi: 10.1016/j.jaad.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: Part II. decreased elastic tissue. Journal of the American Academy of Dermatology. 2004;51:165–185. doi: 10.1016/j.jaad.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Hirano E, Okamoto K, Matsubara Y, Takehana M, Kobayashi S, Tajima S. Elastin expression in cultured human keratinocytes: exon 26A of elastin primary transcript is always included in terminally differentiated keratinocytes. Arch Dermatol Res. 2001;293:430–433. doi: 10.1007/s004030100247. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein EF, Uitto J. The effect of photodamage on dermal extracellular matrix. Clin Dermatol. 1996;14:143–151. doi: 10.1016/0738-081x(95)00149-a. [DOI] [PubMed] [Google Scholar]
- 9.Fazio MJ, Olsen DR, Kauh EA, et al. Cloning of full-length elastin cDNAs from a human skin fibroblast recombinant cDNA library: further elucidation of alternative splicing utilizing exon-specific oligonucleotides. The Journal of investigative dermatology. 1988;91:458–464. doi: 10.1111/1523-1747.ep12476591. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbloom J, Bashir M, Yeh H, et al. Regulation of elastin gene expression. Ann N Y Acad Sci. 1991;624:116–136. doi: 10.1111/j.1749-6632.1991.tb17012.x. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein RA, Starcher BC. Urinary excretion of elastin peptides containing desmosin after intratracheal injection of elastase in hamsters. J Clin Invest. 1978;61:1286–1290. doi: 10.1172/JCI109045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viglio S, Annovazzi L, Luisetti M, Stolk J, Casado B, Iadarola P. Progress in the methodological strategies for the detection in real samples of desmosine and isodesmosine, two biological markers of elastin degradation. J Sep Sci. 2007;30:202–213. doi: 10.1002/jssc.200600260. [DOI] [PubMed] [Google Scholar]
- 13.Maki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24:651–660. doi: 10.14670/HH-24.651. [DOI] [PubMed] [Google Scholar]
- 14.Fazio MJ, Olsen DR, Kuivaniemi H, et al. Isolation and characterization of human elastin cDNAs, and age-associated variation in elastin gene expression in cultured skin fibroblasts. Laboratory investigation; a journal of technical methods and pathology. 1988;58:270–277. [PubMed] [Google Scholar]
- 15.Urban Z, Zhang J, Davis EC, et al. Supravalvular aortic stenosis: genetic and molecular dissection of a complex mutation in the elastin gene. Hum Genet. 2001;109:512–520. doi: 10.1007/s00439-001-0608-z. [DOI] [PubMed] [Google Scholar]
- 16.Piontkivska H, Zhang Y, Green ED, Elnitski L. Multi-species sequence comparison reveals dynamic evolution of the elastin gene that has involved purifying selection and lineage-specific insertions/deletions. BMC Genomics. 2004;5:31. doi: 10.1186/1471-2164-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo Z, Levi-Minzi SA, Christiano AM, et al. Sequential loss of two neighboring exons of the tropoelastin gene during primate evolution. J Mol Evol. 1999;49:664–671. doi: 10.1007/pl00006587. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Shin MH, Moon YJ, et al. Modulation of elastin exon 26A mRNA and protein expression in human skin in vivo. Experimental dermatology. 2009;18:378–386. doi: 10.1111/j.1600-0625.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- 19.Urban Z, Agapova O, Hucthagowder V, Yang P, Starcher BC, Hernandez MR. Population differences in elastin maturation in optic nerve head tissue and astrocytes. Invest Ophthalmol Vis Sci. 2007;48:3209–3215. doi: 10.1167/iovs.07-0107. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro SD, Campbell EJ, Welgus HG, Senior RM. Elastin degradation by mononuclear phagocytes. Ann N Y Acad Sci. 1991;624:69–80. doi: 10.1111/j.1749-6632.1991.tb17007.x. [DOI] [PubMed] [Google Scholar]
- 22.Reid PT, Sallenave JM. Neutrophil-derived elastases and their inhibitors: potential role in the pathogenesis of lung disease. Curr Opin Investig Drugs. 2001;2:59–67. [PubMed] [Google Scholar]
- 23.Hornebeck W, Emonard H. The cell-elastin-elastase(s) interacting triade directs elastolysis. Front Biosci. 2011;16:707–722. doi: 10.2741/3714. [DOI] [PubMed] [Google Scholar]
- 24.Kielty CM, Wess TJ, Haston L, Ashworth JL, Sherratt MJ, Shuttleworth CA. Fibrillin-rich microfibrils: elastic biopolymers of the extracellular matrix. J Muscle Res Cell Motil. 2002;23:581–596. [PubMed] [Google Scholar]
- 25.Yanagisawa H, Davis EC. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int J Biochem Cell Biol. 2010;42:1084–1093. doi: 10.1016/j.biocel.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craft CS, Broekelmann TJ, Zou W, Chappel JC, Teitelbaum SL, Mecham RP. Oophorectomy-induced bone loss is attenuated in MAGP1-deficient mice. J Cell Biochem. 2012;113:93–99. doi: 10.1002/jcb.23331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berk DR, Bentley DD, Bayliss SJ, Lind A, Urban Z. Cutis Laxa: A Review. Journal of the American Academy of Dermatology. 2012;66:842.e841–842.e817. doi: 10.1016/j.jaad.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 28.McCarty MJ, Davidson JM, Cardone JS, Anderson LL. Cutis laxa acquisita associated with multiple myeloma: a case report and review of the literature. Cutis. 1996;57:267–270. [PubMed] [Google Scholar]
- 29.Timmer DEML, Broekhuijsen VANHDM, Oldhoff JM, DB DEG, Sigurdsson V, Pasmans SG. Acquired cutis laxa in childhood Sweet’s syndrome. Pediatric dermatology. 2009;26:358–360. doi: 10.1111/j.1525-1470.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 30.Callewaert B, Renard M, Hucthagowder V, et al. New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations. Human mutation. 2011;32:445–455. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Q, Shifren A, Sens C, et al. Mechanisms of emphysema in autosomal dominant cutis laxa. Matrix Biol. 2010;29:621–628. doi: 10.1016/j.matbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban Z, Michels VV, Thibodeau SN, et al. Isolated supravalvular aortic stenosis: functional haploinsufficiency of the elastin gene as a result of nonsense-mediated decay. Hum Genet. 2000;106:577–588. doi: 10.1007/s004390000285. [DOI] [PubMed] [Google Scholar]
- 33.Wachi H, Sato F, Nakazawa J, et al. Domains 16 and 17 of tropoelastin in elastic fibre formation. Biochem J. 2007;402:63–70. doi: 10.1042/BJ20061145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ewart AK, Morris CA, Atkinson D, et al. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet. 1993;5:11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- 35.Dasouki M, Markova D, Garola R, et al. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143A:2635–2641. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- 36.Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78:1075–1080. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renard M, Holm T, Veith R, et al. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet. 2010;18:895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loeys B, Van Maldergem L, Mortier G, et al. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Human molecular genetics. 2002;11:2113–2118. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- 39.Urban Z, Hucthagowder V, Schurmann N, et al. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet. 2009;85:593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura T, Lozano PR, Ikeda Y, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 41.Yanagisawa H, Davis EC, Starcher BC, et al. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 42.Ono RN, Sengle G, Charbonneau NL, et al. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. The Journal of biological chemistry. 2009;284:16872–16881. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 44.Hirai M, Horiguchi M, Ohbayashi T, Kita T, Chien KR, Nakamura T. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. Embo J. 2007;26:3283–3295. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu Q, Loeys BL, Coucke PJ, et al. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Human molecular genetics. 2006;15:3379–3386. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- 46.Horiguchi M, Inoue T, Ohbayashi T, et al. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci U S A. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dabovic B, Chen Y, Choi J, et al. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity. J Cell Physiol. 2009;219:14–22. doi: 10.1002/jcp.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isogai Z, Ono RN, Ushiro S, et al. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. The Journal of biological chemistry. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- 49.Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-beta binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res. 2008;314:2488–2500. doi: 10.1016/j.yexcr.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Czirok A, Zach J, Kozel BA, Mecham RP, Davis EC, Rongish BJ. Elastic fiber macro-assembly is a hierarchical, cell motion-mediated process. J Cell Physiol. 2006;207:97–106. doi: 10.1002/jcp.20573. [DOI] [PubMed] [Google Scholar]
- 51.Kozel BA, Rongish BJ, Czirok A, et al. Elastic fiber formation: a dynamic view of extracellular matrix assembly using timer reporters. J Cell Physiol. 2006;207:87–96. doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- 52.Morava E, Lefeber DJ, Urban Z, et al. Defining the phenotype in an autosomal recessive cutis laxa syndrome with a combined congenital defect of glycosylation. Eur J Hum Genet. 2008;16:28–35. doi: 10.1038/sj.ejhg.5201947. [DOI] [PubMed] [Google Scholar]
- 53.Kornak U, Reynders E, Dimopoulou A, et al. Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat Genet. 2008;40:32–34. doi: 10.1038/ng.2007.45. [DOI] [PubMed] [Google Scholar]
- 54.Hucthagowder V, Morava E, Kornak U, et al. Loss-of-function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival. Human molecular genetics. 2009;18:2149–2165. doi: 10.1093/hmg/ddp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guernsey DL, Jiang H, Evans SC, et al. Mutation in pyrroline-5-carboxylate reductase 1 gene in families with cutis laxa type 2. Am J Hum Genet. 2009;85:120–129. doi: 10.1016/j.ajhg.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reversade B, Escande-Beillard N, Dimopoulou A, et al. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet. 2009;41:1016–1021. doi: 10.1038/ng.413. [DOI] [PubMed] [Google Scholar]
- 57.Bicknell LS, Pitt J, Aftimos S, Ramadas R, Maw MA, Robertson SP. A missense mutation in ALDH18A1, encoding Delta1-pyrroline-5-carboxylate synthase (P5CS), causes an autosomal recessive neurocutaneous syndrome. European journal of human genetics: EJHG. 2008;16:1176–1186. doi: 10.1038/ejhg.2008.91. [DOI] [PubMed] [Google Scholar]
- 58.Skidmore DL, Chitayat D, Morgan T, et al. Further expansion of the phenotypic spectrum associated with mutations in ALDH18A1, encoding Delta(1)-pyrroline-5-carboxylate synthase (P5CS) American journal of medical genetics Part A. 2011;155A:1848–1856. doi: 10.1002/ajmg.a.34057. [DOI] [PubMed] [Google Scholar]
- 59.Hennies HC, Kornak U, Zhang H, et al. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nature genetics. 2008;40:1410–1412. doi: 10.1038/ng.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kennerson ML, Nicholson GA, Kaler SG, et al. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am J Hum Genet. 2010;86:343–352. doi: 10.1016/j.ajhg.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moller LB, Bukrinsky JT, Molgaard A, et al. Identification and analysis of 21 novel disease-causing amino acid substitutions in the conserved part of ATP7A. Human mutation. 2005;26:84–93. doi: 10.1002/humu.20190. [DOI] [PubMed] [Google Scholar]
- 62.Byers PH, Siegel RC, Holbrook KA, Narayanan AS, Bornstein P, Hall JG. X-linked cutis laxa: defective cross-link formation in collagen due to decreased lysyl oxidase activity. N Engl J Med. 1980;303:61–65. doi: 10.1056/NEJM198007103030201. [DOI] [PubMed] [Google Scholar]
- 63.Basel-Vanagaite L, Sarig O, Hershkovitz D, et al. RIN2 deficiency results in macrocephaly, alopecia, cutis laxa, and scoliosis: MACS syndrome. Am J Hum Genet. 2009;85:254–263. doi: 10.1016/j.ajhg.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Syx D, Malfait F, Van Laer L, et al. The RIN2 syndrome: a new autosomal recessive connective tissue disorder caused by deficiency of Ras and Rab interactor 2 (RIN2) Hum Genet. 2010;128:79–88. doi: 10.1007/s00439-010-0829-0. [DOI] [PubMed] [Google Scholar]
- 65.Coucke PJ, Willaert A, Wessels MW, et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nature genetics. 2006;38:452–457. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- 66.Lee YC, Huang HY, Chang CJ, Cheng CH, Chen YT. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: mechanistic insight into arterial tortuosity syndrome. Human molecular genetics. 2010;19:3721–3733. doi: 10.1093/hmg/ddq286. [DOI] [PubMed] [Google Scholar]
- 67.Vanakker OM, Martin L, Gheduzzi D, et al. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. The Journal of investigative dermatology. 2007;127:581–587. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- 68.Li Q, Schurgers LJ, Smith AC, Tsokos M, Uitto J, Cowen EW. Co-existent pseudoxanthoma elasticum and vitamin K-dependent coagulation factor deficiency: compound heterozygosity for mutations in the GGCX gene. The American journal of pathology. 2009;174:534–540. doi: 10.2353/ajpath.2009.080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. The Journal of investigative dermatology. 2010;130:661–670. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Q, Grange DK, Armstrong NL, et al. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. The Journal of investigative dermatology. 2009;129:553–563. doi: 10.1038/jid.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willaert A, Khatri S, Callewaert BL, et al. GLUT10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGFbeta signaling. Human molecular genetics. 2012;21:1248–1259. doi: 10.1093/hmg/ddr555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohamed M, Kouwenberg D, Gardeitchik T, Kornak U, Wevers RA, Morava E. Metabolic cutis laxa syndromes. J Inherit Metab Dis. 2011;34:907–916. doi: 10.1007/s10545-011-9305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Urban Z, Gao J, Pope FM, Davis EC. Autosomal dominant cutis laxa with severe lung disease: Synthesis and matrix deposition of mutant tropoelastin. The Journal of investigative dermatology. 2005;124:1193–1199. doi: 10.1111/j.0022-202X.2005.23758.x. [DOI] [PubMed] [Google Scholar]
- 74.Bax DV, Rodgers UR, Bilek MM, Weiss AS. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3. J Biol Chem. 2009;284:28616–28623. doi: 10.1074/jbc.M109.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]