Abstract
Upper gastrointestinal neoplasia in the esophagus, stomach and pancreas is associated with the formation of pre-neoplastic metaplasias. We have previously reported the up-regulation of Human epididymis protein 4 (HE4) in all metaplasias in the stomach of humans and mice. We have now sought to evaluate the expression of HE4 in metaplasias/pre-neoplastic precursors and cancers of the human stomach, pancreas and esophagus. Tissue microarrays for gastric cancers, pancreatic cancers and esophageal adenocarcinoma were stained with antibodies against HE4. Immunostaining was quantified by digital imaging and the results were evaluated to assess expression in metaplasias, expression in cancer pathological subtypes and the effects of expression on survival in cancer patients. In gastric cancer patients from Korea, HE4 was detected in 74% of intestinal and 90% of diffuse cancers, while in a gastric cancer cohort from Johns Hopkins HE4 was detected 74% of intestinal type and 92% of diffuse cancers. Nevertheless, in both cohorts there was no impact of HE4 expression on overall survival. In the esophagus, we observed expression of HE4 in scattered endocrine cells within Barrett’s esophagus samples, but Barrett’s columnar metaplasias and HE4 was detected in only 2% of esophageal adenocarcinomas. Finally, in the pancreas, HE4 expression was not observed in pancreatic intraepithelial neoplasia (PanIN) lesions, but 46.8% of pancreatic adenocarcinomas expressed HE4 expression. Still, we did not observe any influence of HE4 expression on survival. The results suggest that HE4 is up-regulated during gastric and pancreatic carcinogenesis.
Keywords: WFDC2, HE4, metaplasia, adenocarcinoma
INTRODUCTION
Upper gastrointestinal adenocarcinomas carry an especially poor prognosis. Between 1999 and 2005, only 17% of esophageal cancer patients, 26% of stomach cancer patients, and 6% of pancreatic cancer patients survived 5 years after identification of the disease.[1] While many aspects of these cancers are likely distinct, all of these adenocarcinomas display one common feature: association with pre-neoplastic metaplastic lineages. We have previously demonstrated that pre-neoplastic lineages in the human stomach demonstrate up-regulation of specific protein markers.[2, 3] In particular, in both human stomach metaplasias and in metaplasia models in mice, we have demonstrated a marked up-regulation of Human epididymis protein 4/Whey-acidic protein four-disulfide core domain protein 2 (HE4/WFDC2).[3] HE4/WFDC2 is a whey-acidic protein (WAP) four domain protein and is a member of a larger family of WAP domain-containing secreted putative extracellular protease inhibitor proteins.[4, 5] Originally identified in human epididymis, HE4 has recently received a great deal of attention as a serum marker for ovarian cancer.[4, 5] Ovarian cancer patients show early up-regulation of detectable protein in the serum and HE4 screening is under extensive evaluation as a serum screening test for ovarian cancer or a method for follow-up of resected patients.[6–8]
We and others have found that HE4 is undetectable in the normal human stomach.[3, 4] Thus, the up-regulation of HE4 in metaplasias makes HE4 an important candidate marker of the metaplastic process. Nevertheless, it is unclear whether HE4 is upregulated in other upper gastrointestinal metaplasias and cancers. We have now evaluated the expression of HE4 in metaplasias/pre-neoplastic precursors and adenocarcinomas from stomach, esophagus and pancreas. Our studies have revealed that HE4 is up-regulated in gastric and pancreatic ductal adenocarcinomas, but not in esophageal adenocarcinoma. The results, therefore, indicate that up-regulation of HE4 expression is characteristic of carcinogenesis in the pancreas and stomach, but it is not a universal marker of metaplastic processes in the upper gastrointestinal tract.
MATERIALS AND METHODS
Tissue Analysis
All esophageal tissue samples,including 12 archived cases of Barrett’s epithelium, were obtained from the archives of the Department of Pathology at Vanderbilt University (Nashville, TN, USA). The use of specimens from the archival tissue repository was approved by the Institutional Review Board. A tissue microarray (TMA) that contained 75 tumors of lower esophageal or gastro-esophageal junction origin adenocarcinomas was constructed with each tumor sample on the TMA represented by three tissue cores (1 mm core size). Adjacent normal gastric and esophageal tissues were available for some cases for comparisons. Histopathological diagnosis of the esophageal adenocarcinomas was verified according to the Vienna classification of gastrointestinal epithelial neoplasia.[9] The adenocarcinomas ranged from moderately-differentiated to poorly-differentiated, stages II to IV based on AJCC 7th edition staging manual[10], and were all of the intestinal type.
For analysis of gastric cancer, two sets of TMAs were utilized. One set was obtained from Johns Hopkins Medical Institutions containing 481 tissue cores (1.5 mm) constructed from 131 patients with gastric carcinomas resected between 1985 and 1995.[11] A second set of TMAs contained 450 gastric carcinomas (2.0 mm cores) resected at the Seoul National University Hospital in 2004 (SNUH-2004-GC).[2]
For analysis of pancreatic lesions, two sets of tissue arrays were used from Johns Hopkins Medical Institutions. To determine the staining in PanIN lesions, we evaluated a tissue array constructed with samples from 55 PanIN lesions including 16 PanIN 1A, 18 PanIN 1B, 14 PanIN 2, and 7 PanIN 3 lesions.[12] To assess staining in pancreatic adenocarcinomas, we evaluated an array set containing samples from 673 tissue cores constructed from 172 patients who had pancreatic ductal adenocarcinomas.
Immunohistochemistry
Paraffin-embedded TMA sections were heated at 60°C for 30 minutes and cooled to room temperature. Deparaffinization and rehydration of tissue occurred in Histoclear (National Diagnostics, Atlanta, GA), graded concentrations of ethanol, and distilled water. Antigen retrieval was performed using Target Retrieval Solution (Dako North America Inc, Carpinteria, CA) at 120°C for 15 minutes, and cooled afterwards at 4°C. Sections were blocked with 5% Normal Goat Serum provided with the Vectastain kit (Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Primary antibody application was performed overnight at 4°C. The primary antibody used was an anti-rabbit human HE4 at 1:1,000 [5]. The next day sections were incubated with biotinylated rabbit IgG (1:200) (Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature, followed by incubation with Avidin/Biotin Complex for 15 minutes at room temperature. Slides were developed with 3,3-diaminobenzidine (DAB) or alkaline phosphatase using a kit from Vector Laboratoriesand counterstained with Mayer’s Hematoxylin (Sigma-Aldrich Corp., St. Louis, MO) solution, dehydrated, and mounted.
Immunofluorescence
For double staining with anti-HE4 and anti-Chromogranin A, deparaffinizedsections were blocked with protein blocking (Dako) and incubated with rabbit IgGanti-HE4 (1:1000) and mouse IgG anti-Chromogranin A (1:25, AbDSerotec, Raleigh, NC) at the same time overnightat 4°C, followed by incubation with Cy3-labeled goat anti-rabbit IgG (Jackson ImmunoResearch) and Alexa488 goat anti-mouseIgG(Invitrogen). After washing withPBS, sections were mounted using ProLong Gold Antifade Reagent with 4,6-diamino-2-phenylindole(DAPI) (Invitrogen) as a nuclear counterstain.
Quantitative Immunohistochemistry Analysis
Stained TMA slides were analyzed using the Ariol SL-50 automated slide scanner (Applied Imaging, San Jose, CA) to quantitate the amount of positive staining for each tissue core. TMA slides stained for HE4 were analyzed using a MultistainHighRes script trained to the desired intensity and color for the VectorRed staining. Before analysis, areas of non-epithelial cells were removed from the cores. After analysis, percent epithelium that was positive for HE4 was determined based on the area of VectorRed staining subtracted from the total area of epithelium.
Statistical analyses
For each set of data, three groups were analyzed based on percent HE4 staining. The first group encompassed those with no staining (for the pancreatic cancer set this was staining equal to zero, while for the other two sets staining less than 0.1 percent was considered as zero). The non-zero staining cores were then stratified as low staining and high staining based on the median level of staining for all those with more than zero staining.
Estimated median survival times for each group were obtained using Kaplan-Meier estimates. The log-rank test was used to test for a difference between the groups for Korean dataset. The two Johns Hopkins data sets had multiple cores per subject, so a Wald test statistic was used to test differences in survival between the groups, these test statistics were obtained using the robust sandwich estimate of Lin and Wei [13] for the covariance matrix that describes the correlation between cores within subjects.
RESULTS
Analysis of Cohorts of Gastric Cancer Resections
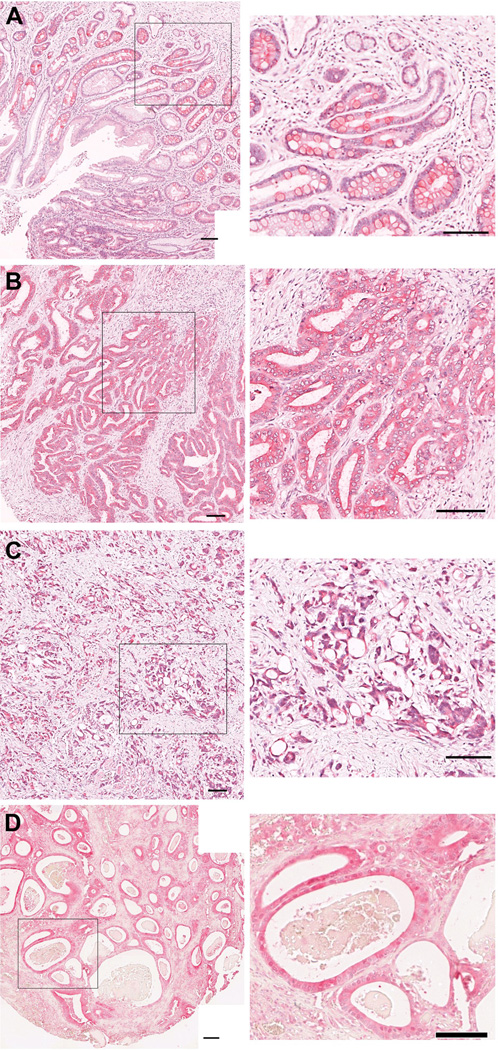
We evaluated the expression of HE4 in two large cohorts of gastric cancer specimens, one from Johns Hopkins Medical Institutions and the other from Seoul National University in Korea. As previously demonstrated [3], intestinal metaplasia showed strong HE4 staining (Figure 1A). The Korean set contained 433 different patient resections that had complete data for both HE4 staining and survival. The majority of the cases in this cohort were Stages 1 or 2. The cancers were annotated according to their Lauren classification as intestinal type, diffuse or mixed cancers (Table 1). Twenty-five percent of intestinal type gastric cancers showed high expression for HE4, compared with 46% with low levels of expression and 29% that displayed no detectable expression (Figure 1B). 61% of diffuse type gastric cancersshowed high expression for HE4, compared with 29% with low levels of expression and 10% that displayed no detectable expression (Figure 1C). In comparison, among mixed tumors, 28% showed high levels of HE4, 47% had low levels of expression and 25% showed no HE4 expression. There was a statistically significant difference between staining pattern and the Lauren grades for intestinal, diffuse and mixed (the χ2 test statistic resulted in p <0.001); these differences were found predominately in the high staining for diffuse type and low staining for intestinal type. The second set of gastric cancer specimens obtained from John Hopkins contained mostly Stage 3 or 4 cancers, with multiple cores from 121 patients (Table 2). These cancers were annotated based on Lauren classification as intestinal type or diffuse. Of the 200 cancer patient cores, 34% of theintestinal type gastric cancers showed high expression for HE4, compared with 40% with low levels of expression and 26% that displayed no detectable expression (Figure 1D). Among the diffuse type gastric cancers, 74% showed high expression for HE4, compared with 18% with low levels of expression and 8% that displayed no detectable expression. These patterns were similar to those found in the Korean specimens, and were also statistically significantly different (p < 0.001).
Figure 1. Immunohistochemical staining of HE4 in intestinal metaplasia and gastric cancers.
A. HE4 staining in intestinal metaplasia. B, C. HE4 staining in gastric cancer specimens from Korean patient arrays. D. HE4 staining in intestinal type gastric cancer specimen from Johns Hopkins arrays. Higher magnification views are shown at right. All scale bars are 100 µm.
Table 1.
HE4 immunostaining in tissue microarray from Korean patients.
| LAUREN CLASS | HE4 High N=181 |
HE4 Low N=167 |
HE4 None N=85 |
Total Cases N=433 |
|---|---|---|---|---|
| Intestinal | 40 (25%) | 73 (46%) | 46 (29%) | 159 |
| Diffuse | 101 (61%) | 47 (29%) | 17(10%) | 165 |
| Mixed | 19 (28%) | 32 (47%) | 17 (25%) | 68 |
| Undetermined | 0 | 2 (100%) | 0 | 2 |
| Unclassified/Missing | 21 (54%) | 13 (33%) | 5 (13%) | 39 |
This table displays data from only those cores with both staining and survival data (433/480 cores).
Table 2.
HE4 immunostaining in Johns Hopkins gastric cancer tissue array.
| Lauren Class | HE4 High N=97 |
HE4 Low N=64 |
HE4 None N=39 |
Total Cases N=200 |
|---|---|---|---|---|
| Intestinal | 44 (34%) | 51 (40%) | 33 (26%) | 128 |
| Diffuse | 53 (74%) | 13 (18%) | 6 (8%) | 72 |
The data in the table reflect removal of 10 cores (from the 210 used in the survival analysis) that were missing Lauren grade. Note that all of the analyzed cores were from cancers, so they do not include adjacent normal or other gastrointestinal tissue cores that were included on the tissue array.
HE4 in the Esophagus
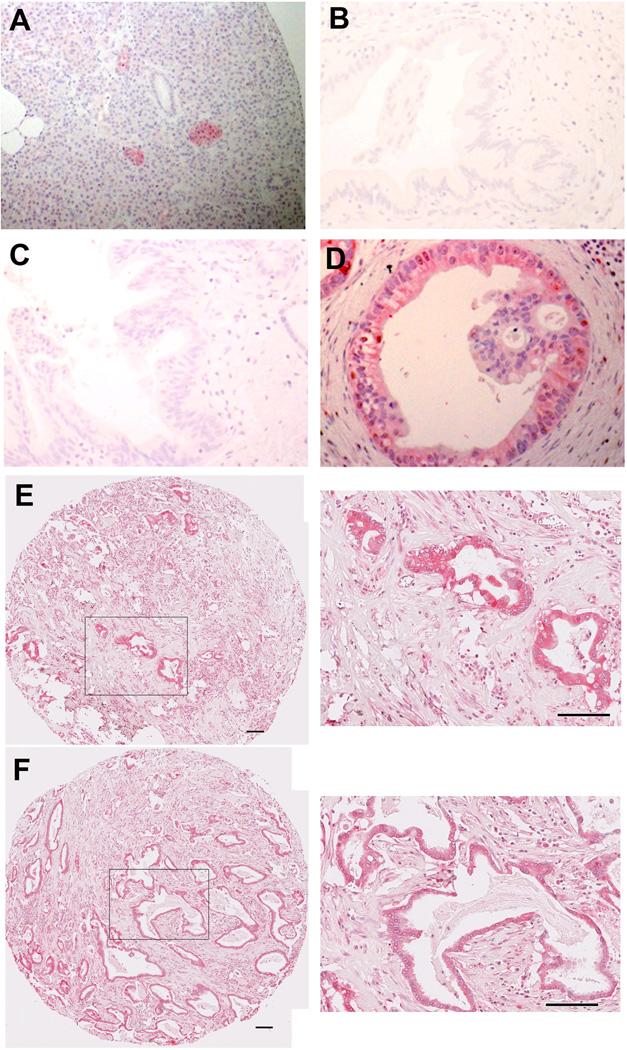
No previous investigations have considered HE4 expression in esophagus. In normal esophagus, no immunohistochemical positivity for protein expression of HE4 in the squamous epithelium of the esophagus was observed (Figure 2A). We also stained sections from 12 patient cases containing Barrett’s metaplasia in the esophagus. Of the 12 sections stained, 8 of the sections showed no staining for HE4 in Barrett’s intestinal metaplasia (Figure 2B). However, 4 resections showed immunohistochemical staining for HE4 protein expression in small triangular cells usually deep in the glands of the Barrett’s epithelium (Figure 2C–E). Dual staining of these cells with chromogranin A antibodies demonstrated that the HE4-expressing cells in the Barrett’s epithelium were enteroendocrine cells (Figure 2E,F). It is of note that not all of the chromogranin positive endocrine cells were also stained for HE4.
Figure 2. HE4 in normal esophageal tissue and Barrett’s epithelium.
HE4 immunostaining was performed in human esophageal tissues.(A) Normal esophageal squamous epithelium showed no staining for HE4. (B) Barrett’s epithelium without HE4 staining. C–E. Barrett’s epithelium with HE4 staining in the bases of some glands. F. Dual immunofluorescence staining for HE4 and chromograninA in Barrett’s epithelium. HE4 immunoreactive cells in the Barrett’s epithelium were also immunoreactive for chromogranin A (dual label merged image at right: HE4 pseudocolored red and chromogranin A pseudocolored green with direct overlap seen as yellow), indicating that they are enteroendocrine cells. All scale bars are 100 µm.
To assess the possible association of HE4 with esophageal carcinogenesis, we stained tissue microarrays containing 133 samples of esophageal adenocarcinomas. Only three cancer cores showed significant staining in cancer cells, although we were able to identify scattered triangular HE4 staining cells in association with cancer, consistent with enteroendocrine cells. All of these results indicate that HE4 expression is not significantly associated with either Barrett’s intestinal metaplasia in the esophagus or esophageal adenocarcinoma.
HE4 in Pancreas
Pancreatic adenocarcinoma also develops in the setting of non-invasive PanIN lesions. We examined HE4 expression in normal, PanIN and adenocarcinoma specimens from patients at John Hopkins. No HE4 staining was observed in normal acinar cells or pancreatic ducts. However, prominent HE4 staining was found in pancreatic islets (Figure 3A). Importantly, we did not observe any staining in either early or late PanIN lesions in the pancreas (Figure 3B,C). Nevertheless, strong staining was observed in pancreatic ductal adenocarcinoma in patients without staining in adjacent PanIN lesions (Figure 3D). Strong HE4 staining was observed in pancreatic cancers with varying levels of differentiation (Figure 3E,F). Among 220 pancreatic cancer samples (representing 172 individual patients) analyzed in this cohort, 103 (46.8%) stained for HE4 in >5% of the neoplastic epithelium.
Figure 3. Immunohistochemical staining of HE4 in normal pancreas, PanIN and pancreatic cancer.
A. HE4 staining in normal pancreas. Note strong staining in a pancreatic islet. B. HE4 staining in a low grade PanIN lesion. C. HE4 staining in a high grade PanIN lesion. D. HE4 staining in pancreatic adenocarcinoma from same patient with PanIN lesion in C. E,F. HE4 staining in pancreatic adenocarcinomas. Higher magnification views are shown at right. All scale bars are 100 µm.
Survival Determination Based on HE4 Expression
We utilized patient outcome data for both the pancreatic and gastric cancer tissue arrays to evaluate whether expression of HE4 could influence patient survival. Staining patterns were stratified into three groups; Tumors without any staining, and then those with staining were stratified into either high or low staining based on the median level in the samples. The median percent staining for the Johns Hopkins pancreatic cancer cohort was 11%, 7% for Johns Hopkins the gastric cancer cohort, and 4% for the Korean cohort.
In the pancreatic cancer group, there was no impact of staining for HE4 on survival (Figure 4A). In the gastric cancer cohort from Korea, there was also no effect of survival based on HE4 expression (Figure 4B). In the Johns Hopkins gastric cancer patients, who predominantly were resected with high stage tumors, the group without HE4 staining showed an early survival advantage, but this difference was not statistically significant overall (p=0.175; Figure 4C). Differences in the survival patterns in the two gastric cohorts was also reflected in the proportion of the samples with no, low, and high levels of staining with 24%, 38%, 38%, respectively, for the Korean sample, and 19%, 31%, 50%, respectively, for the Johns Hopkins sample. The Johns Hopkins pancreatic cohort had almost equal numbers of subjects within each staining level, 34% with no staining, 35% with low staining, and 29% with high staining. The test statistics for the John Hopkins cohorts (both gastric and pancreatic) were adjusted to account for multiple cores per subject. The degree of need for this adjustment can be evaluated by examining the ratio in the standard errors for the parameter estimates based on adjusted (robust) versus non-adjusted estimation. As expected (because of the number of cores for each subject), the pancreatic set had a larger degree of difference (highest ratio was between no staining and low staining group at 1.3, meaning the standard error was 30% larger using the robust estimation). The gastric set had ratios very close to one (with less than 1% difference between the two estimates).
Figure 4. Outcomes in pancreatic and gastric adenocarcinoma patients associated with HE4 expression.
Samples in all groups were classified based on no detectable HE4 staining or low or high staining based on the median staining observed among positively stained samples. Kaplan-Meier survival statistics were calculated for all groups. A. Survival in pancreatic cancer patients identified at Johns Hopkins. B. Survival in gastric cancer patients identified at Seoul National University, Korea. C. Survival in gastric cancer patients resected at Johns Hopkins.
DISCUSSION
Carcinogenesis in the upper gastrointestinal tract is associated with distinct mucinous metaplastic lineages. The presence of these metaplastic lineages suggests that development of metaplasia is a key event in the evolution of adenocarcinoma in the stomach, esophagus and pancreas. Despite this general association of adenocarcinoma with pre-existing metaplasia, there has been surprisingly little concordance in biomarker expression within these cancers. We have demonstrated here that up-regulation of HE4 is a common feature in at least gastric and pancreatic cancers. However, the patterns for the timing of up-regulation appear to be different. Thus, while HE4 is up-regulated in all of metaplastic lineages in the stomach, we did not observe any expression of HE4 in any PanIN lineages in the pancreas. Nevertheless, HE4 expression appeared elevated in the majority of pancreatic adenocarcinomas to a similar extent as in gastric adenocarcinomas. Up-regulation of HE4 did not appear to be a significant part of carcinogenesis in the esophagus. These results indicate that the up-regulation of HE4 is associated with tissue-specific carcinogenesis at variable points in neoplastic progression.
HE4 is a member of a broader family of secreted putative protease inhibitors, all containing 2–4 whey acidic protein (WAP) domains.[4, 5, 14] The exact function of these proteins is poorly understood, but their status as likely extracellular protease inhibitors suggests that they may be involved in the regulation of extracellular matrix, cell migration and cell invasion. This type of role could also explain different tissue-specific patterns of expression in metaplasias. The metaplasias in the stomach are organized into distinct glandular structures that require migration of cells bidirectionally towards the lumen or gland bases.[15] In contrast, metaplasias in the pancreas are organized into simpler ductular structures, which are lined by mucinous metaplastic lineages.[16] Thus, in the case of pancreatic cancers, up-regulation of HE4 may be more indicative of changes towards more invasive phenotypes.
The patterns of HE4 up-regulation may also reflect common processes for the derivation of cancerous lineages in the stomach and the pancreas. Thus, increasing evidence suggests that initial preneoplastic lesions in both the stomach and the pancreas are derived from transdifferentiation of zymogenic cells in stomach or pancreas into mucinous metaplasias.[17, 18] It should also be noted that metaplasias of fimbrial secretory cells may also give rise to serous ovarian cancers.[19, 20] Thus, the process of up-regulation of HE4 expression may reflect a pattern of cell derivation from acinar cell lineages. The actual lineage of origin for adenocarcinoma through Barrett’s epithelium remains obscure, but there is no evidence for transdifferentiation of an acinar type cell in the genesis of Barrett’s metaplasia or esophageal adenocarcinoma. Indeed, since different patterns for HE4 expression in esophageal and gastric adenocarcinoma would support different pathways for the evolution of these two types of mucosal cancer.
Nevertheless, we have also observed an association of HE4 expression with some endocrine cell populations. HE4 was expressed in islet cells and endocrine cells within Barrett’s epithelium. Previous investigations have noted that endocrine cells are often present in Barrett’s epithelium.[21] In contrast with the results in esophageal metaplasia and pancreas, we did not observe HE4 expression in enteroendocrine cells in either the human or mouse gastric mucosa. The function of HE4 in these cells therefore remains unclear.
In summary, we have demonstrated in these studies that up-regulation of the extracellular protease inhibitor HE4 is associated with carcinogenesis in the stomach and pancreas. Although we did not discern a significant impact of HE4 expression on patient outcomes, HE4 may represent an important marker of the neoplastic process in the stomach and pancreas.
ACKNOWLEDGEMENTS
These studies were supported by grants to J.R.G. from RO1 DK071590, and ARRA Supplemental Funding from RO1 DK071590-S1 and a Merit Review Award from the Department of Veterans Affairs and funding to R.D. from U01 CA152990. This work was supported by core resources of the Vanderbilt Digestive Disease Center (P30 DK058404), the Vanderbilt SPORE in Gastrointestinal Cancer Tissue Core (P50 CA095103), the Vanderbilt Epithelial Biology Center and the Vanderbilt-Ingram Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflicts of interest in the pursuit of this work.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CACancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Lee HJ, Nam KT, Park HS, et al. Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology. 2010;139:213–225. doi: 10.1053/j.gastro.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–521. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingle L, Singleton V, Bingle CD. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene. 2002;21:2768–2773. doi: 10.1038/sj.onc.1205363. [DOI] [PubMed] [Google Scholar]
- 5.Drapkin R, von Horsten HH, Lin Y, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 6.Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40–46. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore RG, Miller MC, Eklund EE, Lu KH, Bast RC, Jr, Lambert-Messerlian G. Serum levels of the ovarian cancer biomarker HE4 are decreased in pregnancy and increase with age. Am J Obstet Gynecol. 2011;206:349.e1–349.e17. doi: 10.1016/j.ajog.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore RG, Miller MC, Steinhoff MM, et al. Serum HE4 levels are less frequently elevated than CA125 in women with benign gynecologic disorders. Am J Obstet Gynecol. 2011;206:351.e351–351.e358. doi: 10.1016/j.ajog.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlemper RJ, Kato Y, Stolte M. Diagnostic criteria for gastrointestinal carcinomas in Japan and Western countries: proposal for a new classification system of gastrointestinal epithelial neoplasia. J Gastroenterol Hepatol. 2000;15(Suppl):G49–G57. doi: 10.1046/j.1440-1746.2000.02266.x. [DOI] [PubMed] [Google Scholar]
- 10.Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 11.Leys CM, Nomura S, LaFleur BJ, et al. Expression and prognostic significance of prothymosin-alpha and ERp57 in human gastric cancer. Surgery. 2007;141:41–50. doi: 10.1016/j.surg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–912. doi: 10.1097/01.MP.0000086072.56290.FB. [DOI] [PubMed] [Google Scholar]
- 13.Lin DY, Wei LJ. The robust inference for the proportional hazards model. J Amer Statistical Assoc. 1989;84:1074–1078. [Google Scholar]
- 14.Clauss A, Ng V, Liu J, et al. Overexpression of elafin in ovarian carcinoma is driven by genomic gains and activation of the nuclear factor kappaB pathway and is associated with poor overall survival. Neoplasia. 2010;12:161–172. doi: 10.1593/neo.91542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: Time for re-evaluation of metaplasias and the origins of gastric cancer. Gastroenterolgy. 2010;139:213–225. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest. 2011;121:4572–4578. doi: 10.1172/JCI57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Means AL, Meszoely IM, Suzuki K, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Abushahin N, Pang S, et al. Tubal origin of 'ovarian' low-grade serous carcinoma. Mod Pathol. 2011;24:1488–1499. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- 20.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–5293. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton K, Chiappori A, Olson S, Sawyers J, Johnson D, Washington K. Prevalence and prognostic significance of neuroendocrine cells in esophageal adenocarcinoma. Mod Pathol. 2000;13:475–481. doi: 10.1038/modpathol.3880081. [DOI] [PubMed] [Google Scholar]