Abstract
Individuals with Tourette syndrome (TS) exhibit deficits in inhibitory information processing which may reflect impaired neural mechanisms underlying symptoms and can be detected using a negative priming (NP) task. NP is the normal reduction of performance when identifying target stimuli that appears where non-target stimuli appeared previously. TS subjects exhibit diminished NP and their NP levels predict their response to behavioral therapy. Here we review relevant literature on this issue and also report a novel rat NP task. In the latter, rats respond to target stimuli (continuous light) while ignoring non-target stimuli (blinking light). Each trial was preceded by a prime in which target and non-target stimuli were briefly presented. Performance was challenged by shortening prime duration and by administering amphetamine. During the short prime challenge, rats exhibited lower accuracy in NP vs. baseline trials, indicative of inhibitory information processing. Modulation by amphetamine administration indicates that this drug had rate-dependent effects. Evidence is provided of individual differences in NP and response to the drug, with priming being reduced in high NP rats, while it was increased in low NP subjects. The rat NP task represents a novel and suitable tool for investigating the neural bases of inhibitory information processing and its dysfunction in TS.
Keywords: visuospatial priming, negative priming, information processing, Tourette syndrome, rats
1. Introduction
The capacity to automatically inhibit or modify motor responses to sensory stimuli is crucial for proper goal-directed behavior. Conversely, impairments in inhibitory information processing, defined as the ability to suppress reactions to irrelevant or inappropriate information, may contribute to symptoms of conditions characterized by repetitive or otherwise disordered stimulus-driven behaviors. For example, such inhibitory deficits may underlie both the premonitory urges and the motor and vocal tics in Tourette syndrome (TS). Deficient inhibitory information processing is also apparent in other neuropsychiatric disorders, such as schizophrenia and obsessive-compulsive disorder, and may contribute to cognitive and behavioral abnormalities in these conditions.
Unlike motor and vocal tics, information processing can be operationally assessed in specific laboratory measures. For example, a visuospatial priming task measures the ability of a subject to respond to a target stimulus while ignoring a non-target distractor stimulus. Performance during a given “probe” trial is evaluated in the context of the location of cue stimuli in the preceding “prime” trial. When the target stimulus in a probe is presented in the same location as the non-target stimulus in the preceding prime (“negative priming trial”), healthy subjects exhibit slower reaction times and lower accuracy compared to probes in which target location is unrelated to non-target location during the prime (“baseline trial”) (Tipper, 1985, 2001). Possible explanations of this negative priming (NP) phenomenon include the activation of inhibitory processes that suppress attention to the location of the non-target stimulus; in an NP trial, the individual must then activate a previously inhibited strategy in order to attend to, and respond in, the location that previously held the non-target stimulus. An alternative theory postulates the formation of a “memory trace” of the location holding the non-target stimulus marking this location as inappropriate for responding; in an NP trial, presentation of the target stimulus in this location during the probe triggers retrieval of this “do not respond” memory trace, which impairs response in this location (Tipper, 1985, 2001).
Negative visuospatial priming is significantly reduced in TS subjects (Deckersbach et al., 2006; Swerdlow et al., 1996), and the severity of NP deficits in TS patients correlates significantly with the therapeutic impact of habit reversal therapy (Deckersbach et al., 2006), an effective behavioral treatment for TS (Azrin and Peterson, 1988; Piacentini et al., 2010). This finding suggests that NP deficits may reflect neural mechanisms that are intrinsically linked to TS symptoms (Wright et al., 2006; Wright et al., 2005), and that NP may be useful for predicting the therapeutic potential of interventions, including pharmacological treatments, for TS. Conceivably, treatments that normalize NP might also target other disorders characterized by impaired inhibitory processing and reduced NP, such as schizophrenia (Elkins and Cromwell, 1994).
No rodent paradigm measuring negative visuospatial priming has been reported. Such a paradigm would facilitate the systematic, controlled investigation of the neural mechanisms underlying inhibitory information processing and enable the generation of animal models of inhibitory deficits relevant to TS. A predictive animal model of NP might also allow for the development of novel TS medications; conceivably, NP-enhancing medications might directly reduce TS symptoms, or – based on the relationship reported by Deckersbach et al. (2006) –enhance the therapeutic impact of behavioral therapies. In this study, we aimed to describe the characteristics and heuristic values of a novel rat negative visuospatial priming paradigm (Figure 1), based on a well-established human visuospatial priming task (Swerdlow et al., 1996; Tipper, 1985). Because dysfunction of dopaminergic signaling has been implicated in both the pathophysiology of TS (Albin et al., 2003; Leckman et al., 2010) and the mediation of visuospatial priming (Swerdlow et al., 1997; Wylie and Stout, 2002; Yamaguchi and Kobayashi, 1998), we also report on the impact on NP of modulation of dopaminergic signaling through an acute challenge with the indirect dopamine agonist, d-amphetamine.
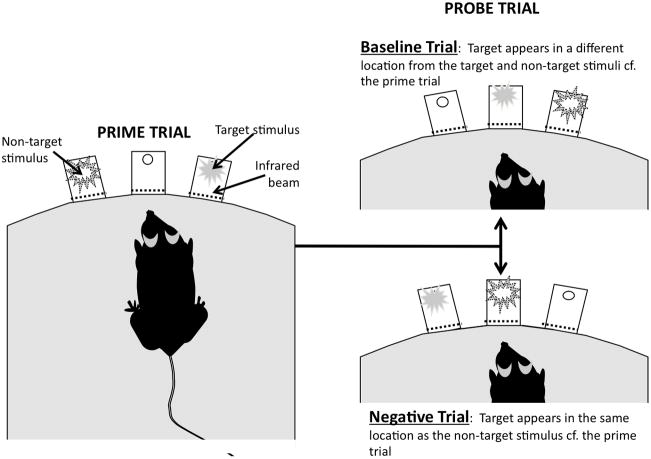
Figure 1.
Task schematic for the negative priming task exemplifying the different trial types. Training and testing are conducted in 9-hole operant testing chambers enclosed in ventilated sound-attenuating chambers (Med Associates Inc., St. Albans, VT and Lafayette Instrument Company, Lafayette, IN). Each testing chamber contains a curved rear wall with nine contiguous apertures. Metal inserts cover six of the apertures, leaving open apertures 3, 5, and 7, so that the rodent faces a curved wall with three equidistant apertures. Each aperture contains an infrared beam at the entrance to detect nosepoke responses and a LED stimulus light at the rear to present stimuli. Liquid reinforcement in the form of strawberry milkshake (Nesquik® plus non-fat milk, 40 μl) can be delivered into a magazine located in the opposite wall via peristaltic pump; an infrared beam detects head entries into the magazine. A house light is located in the middle of the chamber ceiling. The control of stimuli and recording of responses is managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates Inc., St. Albans, VT) using custom programming.
During the prime trial, the target stimulus (continuous light) and the non-target stimulus (5 Hz flashing light) are briefly presented simultaneously in pseudorandom locations. After a short period of time (interstimulus interval), a probe trial follows. In the case of a baseline trial, the location of the target stimulus in the probe trial is unrelated to the location of either stimulus in the prime trial. In the case of a negative priming trial, the target stimulus in the probe trial is located in the same aperture that contained the non-target stimulus during the preceding prime trial.
2. Behavioral Procedure
Male Long-Evans rats (Charles River Laboratories, Wilmington, MA) were housed two per cage on a 12 h:12 h reversed light-dark cycle (lights off at 7:00 am). All behavioral testing was conducted during the animals’ dark cycle. Rats were allowed to reach a body weight of at least 300 g before initiation of food restriction and behavioral training. Food restriction was calibrated to keep rats at 90% of their free-feeding weight and continued throughout behavioral testing. Water was available ad libitum at all times except during testing. Animals were treated in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals. All experiments were approved by the Animal Care and Use Committee of the University of California San Diego.
Rats were trained to retrieve the liquid reward from the magazine for two consecutive days in 10 min sessions, during which a 40 μl increment of strawberry milkshake was delivered noncontingently into the magazine every 15 s (Habituation 1). Delivery of the reward was accompanied by illumination of the magazine light. Head entries into the magazine to consume the reward led to extinction of the magazine light until the delivery of the next reward.
Next, the rats were trained to nosepoke for the reward during daily 30-minute sessions, in which all three apertures were illuminated until a nosepoke response occurred (Habituation 2). A nosepoke into any aperture resulted in extinction of all aperture lights and delivery of a reward into the food magazine. Head entries into the magazine initiated a 4 s intertrial interval (ITI), after which all apertures were again illuminated. To prevent the establishment of positional biases, a streak of five consecutive responses into the same aperture resulted in this aperture no longer being illuminated or responsive until the rat nosepoked at least once into each of the two remaining apertures.
Once rats had performed >60 responses for two consecutive sessions, they were trained to distinguish the target and non-target visual stimuli (Visual Discrimination). All trials were initiated by a head entry into the food magazine. An initial noncontingent liquid reward was delivered into the magazine at the start of each session to facilitate initiation of the first trial. After a 4 s ITI, the target stimulus (a continuous light) and the non-target distractor stimulus (a 5 Hz flashing light) were presented in two of the response apertures. The location of the target and non-target stimuli varied pseudorandomly between trials, so that each location contained the stimuli an equal number of times per session, but their location was not predictable on any given trial. A nosepoke in the aperture containing the target stimulus within a limited hold period (correct response) resulted in the delivery of a reward into the food magazine, along with illumination of the magazine light. Nosepokes into the aperture containing the non-target stimulus (false alarm) or into an unlit aperture (incorrect response) were punished by a 4 s timeout, marked by illumination of the house light and no delivery of the reward. Nosepokes in any aperture made before presentation of the target and non-target stimuli (premature responses) likewise resulted in a timeout and no reward. At the end of the timeout, the house light was extinguished and the magazine light illuminated to prompt the animal to initiate the next trial via a head entry into the magazine. The magazine light was extinguished after removal of the head from the magazine. Each session lasted 30 min. During the initial sessions, the target and non-target stimuli were continuously illuminated until a nosepoke occurred. Once rats had performed at least 60 correct responses, the target and non-targets stimulus durations were limited to 10 s. No response within the stimulus presentation resulted in recording of an omission error and punishment of rats with a timeout and no reward. Rats were trained until they performed at least 60 correct responses per session and selected the target stimulus with >60% accuracy. On average, rats required around 15 sessions to reach criterion performance.
Rats were then trained on the negative visuospatial priming (NP) task. See Figure 1 for a task schematic and Figure 2 for a flowchart of task performance. Again, all trials were initiated by a head entry into the magazine. After a 4 s ITI, the animal was presented with the prime, consisting of a 0.5 s presentation of the target and non-target stimuli. Nosepoke responses to the prime in any location were recorded, but had no consequence. The stimuli were then extinguished, and a 0.3 s interstimulus interval (ISI) followed. After the ISI, the animal was presented with the probe, consisting of the target and non-target stimuli presented in different locations. In half of the trials, the target stimulus during the probe was located in the aperture that had contained no visual stimulus during the prime (baseline trials). In the remaining trials, the target stimulus during the probe was located in the aperture that had contained the non-target stimulus during the prime (negative priming or NP trials). Baseline and NP trials alternated pseudorandomly, so that that each trial type was presented equally often during each session, but the trial type was not predictable on any given trial. Premature responses during the ITI before the onset of the prime stimuli were punished with a timeout period and no reward; responses during the ISI (i.e. between the prime and probe stimuli presentations) were recorded, but had consequence. Each session lasted 30 min or until the animal had completed 120 trials, whichever occurred first. The duration of the target and non-target stimuli during the probe was initially set at 10 s; responses had to be performed during the presentation of the stimuli or within a 1 s limited hold after extinction of the stimuli to avoid registration of an omission. Once an animal reached criterion performance (>60 correct responses and >60% accuracy), the duration of the probe stimuli was decreased stepwise until rats were no longer able to reach criterion performance. The final probe stimulus duration achieved was 0.5 s for seven rats, 0.75 s for three rats, 1 s for one rat, and 1.5 s for two rats. Three of 16 rats failed to develop stable performance in the task and were excluded, leading to a final n of 13 rats. On average, around 20 sessions were required to train rats to perform at their final stimulus duration.
Figure 2.
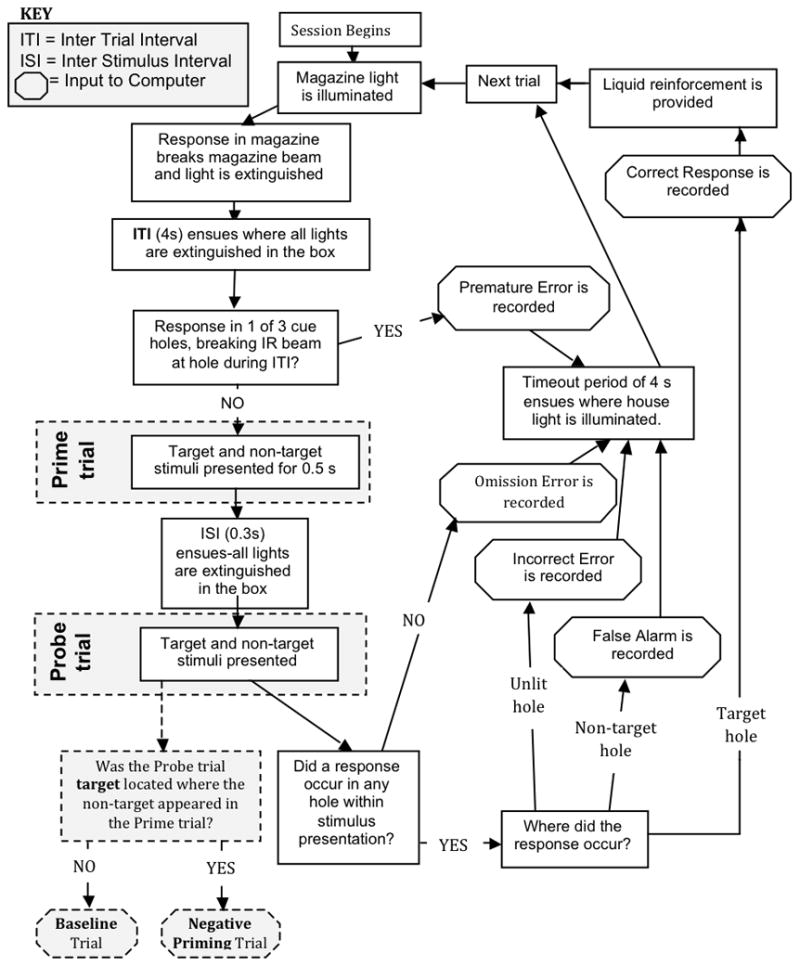
Flowchart of negative priming (NP) task performance. Every trial begins with the illumination of the magazine (reward delivery area) light. The magazine light is extinguished once the rat performs a head entry into the magazine. Exiting the magazine initiates a 4 s intertrial interval. Nosepoking in any aperture during this interval (i.e., before presentation of the stimuli) results in a premature response being recorded and the error sequence (see below) being initiated. Otherwise, at the end of the intertrial interval, the prime trial begins, during which the target stimulus (continuous light) and the non-target stimulus (flashing light) are simultaneously presented for 0.5 s. No responses are required during the prime trial; any nosepokes performed by the rat are recorded but have no consequence. The stimuli are then extinguished for a 0.3 s interstimulus interval. The probe trial then begins, in which the target and non-target stimuli are again simultaneously presented in different locations. If the target stimulus is presented in the aperture that held the non-target stimulus during the preceding prime trial, the current trial constitutes an NP trial; otherwise, it is a baseline trial (see Fig. 1). Any following computer inputs are tallied separately for baseline and NP trials. If the rat nosepokes into the aperture containing the target stimulus, a correct response is recorded and a liquid reward (strawberry milkshake) is delivered into a now illuminated magazine. Head entry into the magazine to consume the reward again extinguishes the magazine light, and exiting initiates a new trial and intertrial interval. Alternatively, if the rat nosepokes into the aperture containing the non-target stimulus, a false alarm is recorded; if it nosepokes into an unlit aperture, an incorrect response is recorded; if it fails to perform a nosepoke during the duration of stimulus presentation, an omission is recorded. All of these errors initiate the error sequence: all stimulus lights are extinguished and the house light is illuminated for a 6 s timeout, and no food reward is delivered.
After completion of the timeout interval, the house light is extinguished and the magazine light illuminated so that the next trial can begin.
The following measures were calculated to assess task performance:
Accuracy: number of correct responses divided by the sum of correct responses, incorrect responses, and false alarms [# correct responses/(# correct responses + # incorrect responses + # false alarms)]. Accuracy was only computed if correct responses + incorrect responses + false alarms totaled 10 or more.
Latency to correct response: time from the onset of the light stimulus to the performance of a correct nosepoke response.
Accuracy priming value: difference in accuracy between baseline and NP trials (average accuracy during baseline trials - average accuracy during NP trials). Accuracy priming values > 0 reflect a negative priming effect, indicating that rats performed with higher accuracy in baseline compared to NP trials.
Correct response latency priming value: difference in correct response latency between baseline and NP trials (average correct response latency during baseline trials - average correct response latency during NP trials). Correct response latency priming values < 0 reflect a negative priming effect, indicating that rats performed with shorter correct response latencies in baseline compared to NP trials.
3. Findings
3.1. Effects of Prime Duration Challenges on NP
3.1.1. Increased Prime Duration Challenge
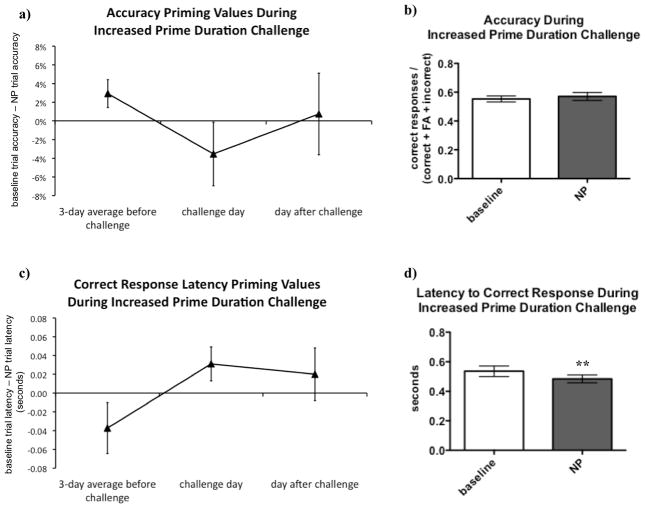
To determine whether lengthening the prime stimuli would increase the influence of the prime, leading to more robust negative priming, rats were challenged by increasing the duration of the prime stimuli from 0.5 s to 1 s after establishment of stable performance in the negative visuospatial priming task.
There was a nonsignificant tendency for accuracy priming values to become less positive on the challenge day (see Figure 3a), suggesting a diminished negative priming effect. No significant difference in accuracy between baseline and NP trials was observed on the day of the challenge (see Figure 3b). Similarly, on the day of the challenge, the correct response latency priming values became less negative (see Figure 3c), again indicating a lessened negative priming effect, reflected by shorter latencies in NP trials compared to baseline trials (see Figure 3d).
Figure 3.
Decreasing the duration of the prime stimulus disrupted negative priming (NP). On the day of the increased prime duration challenge, accuracy priming values became less positive (A), reflecting less NP, although this effect did not reach statistical significance [F(2, 24) = 1.06, p > 0.1]; no significant difference between accuracy in baseline and NP trials [t(12) = 0.94, p > 0.1] was detected on the day of the challenge (B). Likewise, there was a trend for correct latency priming values to become less negative on the day of the challenge [F(2, 24) = 2.78, p = 0.08], also reflecting less NP (C); correct response latencies during NP trials were actually shorter than during baseline trials (the opposite pattern from that predicted by NP) during the challenge [t(12) = 3.34, p < 0.01] (D).
Data were analyzed using repeated-measures analyses of variance (ANOVA) to compare the priming values during challenge to the sessions before and after the challenge. When statistically significant effects were found in the ANOVAs, post hoc comparisons among means were conducted using Newman-Keuls tests. Paired two-tailed t-tests were used to compare performance during baseline and NP trials on the duration challenge days. The level of significance was set at 0.05 throughout. Data were analyzed using GraphPad Prism® (GraphPad, San Diego, CA) and Sigmaplot® (Systat Software Inc., San Jose, CA, USA). Values are expressed as mean ± SEM. Asterisks (**p < 0.01) denote significant differences compared with baseline trials.
3.1.2. Decreased Prime Duration Challenge
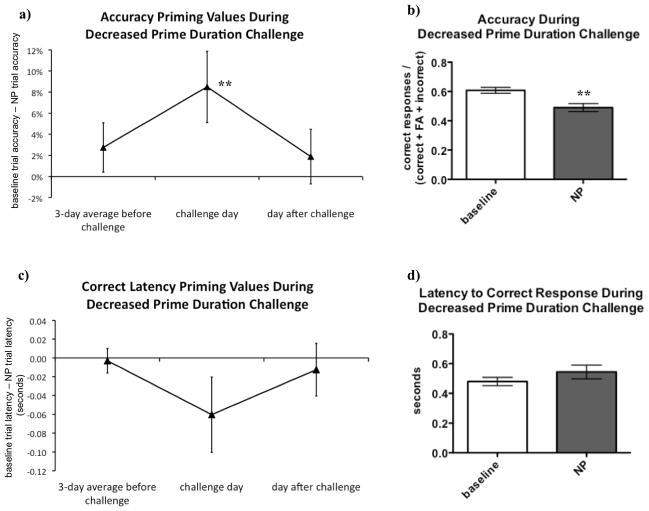
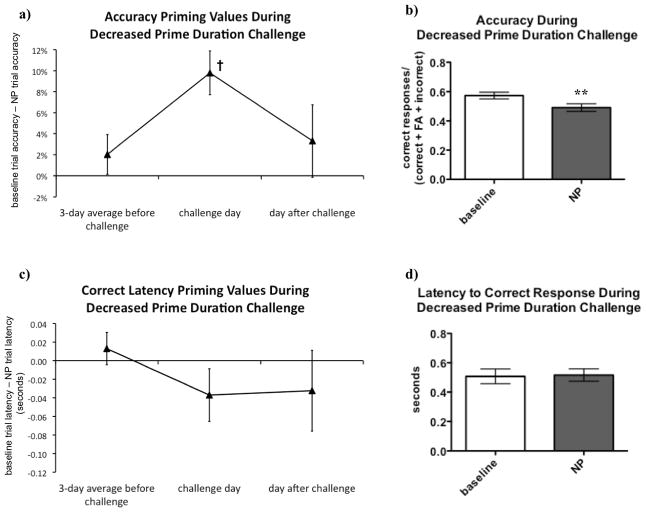
Because the longer prime stimuli were in fact associated with reduced negative priming, rats were next challenged with a decrease in the duration of prime stimuli. 14 sessions after the increased prime duration challenge, during which rats were trained under normal task parameters, rats were challenged by decreasing the duration of the prime stimuli from 0.5 s to 0.25 s. After five days of normal training sessions, this short prime duration challenge was repeated.
Shorter prime duration resulted in accuracy priming values becoming more positive compared to the days before and after the challenge, indicating a stronger NP effect (see Figures 4a and 5a). On the days of the challenge, rats exhibited significantly lower accuracy in NP trials compared to baseline trials (see Figures 4b and 5b), confirming a robust NP effect. These findings were consistent during both repetitions of the challenge.
Figure 4.
Decreasing the duration of the prime stimulus enhanced negative priming (NP). On the day of the decreased prime duration challenge, accuracy priming values became more positive, reflecting more NP (A). Comparison of accuracy priming values before, during, and after the challenge showed a significant main effect of day [F(2, 24) = 5.35, p < 0.05]. Post hoc testing confirmed that accuracy priming values on the challenge day were significantly larger compared to both the three days preceding the challenge (p < 0.05) and the day after the challenge (p < 0.05). Rats exhibited significantly higher accuracy in baseline trials compared with NP trials [t(12) = 4.29, p < 0.01] on the day of the challenge (B). Similarly, correct latency priming values became more negative on the day of the challenge, also reflecting more NP (C), although this effect did not reach statistical significance [F<1, ns] and there was no significant difference between correct response latencies in baseline and NP trials [t(12) = 0.91, p > 0.1] on the day of the challenge (D).
Data were analyzed using repeated-measures analyses of variance (ANOVA) to compare the priming values during challenge to the sessions before and after the challenge. When statistically significant effects were found in the ANOVAs, post hoc comparisons among means were conducted using Newman-Keuls tests. Paired two-tailed t-tests were used to compare performance during baseline and NP trials on the duration challenge days. Values are expressed as mean ± SEM. Asterisks (**p < 0.01) denote significant differences compared with days before and after the challenge (A) or compared with baseline trials (B).
Figure 5.
The effect of the decreased prime stimulus challenge to enhance negative priming (NP) was sustained upon repeated challenge. On the day of the second decreased prime duration challenge, accuracy priming values again became more positive, reflecting more NP (A), with a very strong trend towards differences between the accuracy priming values obtained before, during, and after the challenge [F(2, 24) = 3.40, p = 0.05]. Rats again exhibited significantly higher accuracy in baseline trials compared with NP trials [t(12) = 3.66, p < 0.01] on the day of the challenge (B). Although there was a tendency for correct latency priming values to become more negative on the day of the challenge, also reflecting more NP (C), this tendency persisted on the day after the challenge, and did not result in statistically significant differences in response latency priming values on the different days [F(2, 24) = 2.19, p > 0.1]. There was no significant difference between correct response latencies in baseline and NP trials [t(12) = 0.38, p > 0.1] on the day of the challenge (D).
Data were analyzed using repeated-measures analyses of variance (ANOVA) to compare the priming values during challenge to the sessions before and after the challenge. When statistically significant effects were found in the ANOVAs, post hoc comparisons among means were conducted using Newman-Keuls tests. Paired two-tailed t-tests were used to compare performance during baseline and NP trials on the duration challenge days. Values are expressed as mean ± SEM. Dagger symbol (†) denotes trend toward difference between with days before, during, and after the challenge; asterisks (**p < 0.01) denote significant differences compared with baseline trials.
Response latency priming values also appeared to become more negative on the days of the challenge, again suggesting a stronger NP effect (see Figures 4c and 5c). However, this effect did not reach statistical significance, and during the repeated challenge, latency priming values remained negative on the day after the challenge. Likewise, although rats exhibited longer correct response latencies in NP trials compared to baseline trials on the challenge day (see Figure 4d), no significant differences were observed.
Individual accuracy priming values during the two decreased prime duration challenges were significantly positively correlated (see Figure 6), indicating that animals exhibiting a strong NP effect during the first challenge were likely to also exhibit a strong NP effect during the second challenge.
Figure 6.
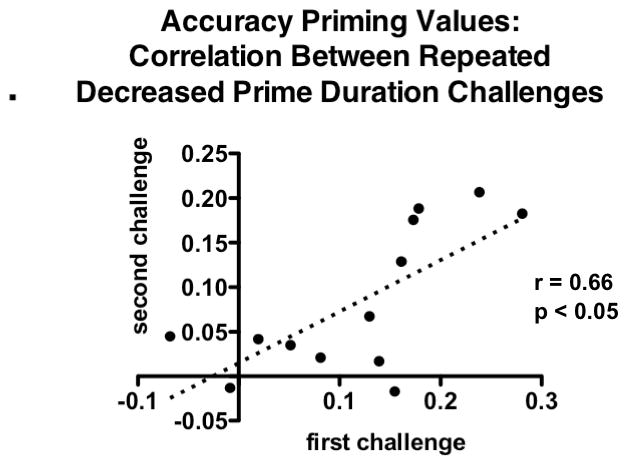
Positive correlations of performance between first and second short prime duration challenges. Individual accuracy priming values for each animal from the two short prime duration challenges were compared using Spearman correlations. Values from the first and second decreased prime duration challenges were significantly positively correlated (r = 0.66, p < 0.05), indicating that rats exhibiting high rates of negative priming (NP) in the first challenge also did so during the second challenge, and likewise for rats exhibiting low rates of NP.
3.2. Effects of Amphetamine on NP
D-Amphetamine sulfate (Sigma, St. Louis, MO, USA) was dissolved in 0.9% saline solution and administered by subcutaneous injection in a volume of 1 ml/kg at doses of 0.25, 0.5, or 1.0 mg/kg. These doses have been reported to produce selective effects on performance of similarly complex cognitive tasks without inducing stereotypical movements in rats (Grilly and Loveland, 2001; Ko and Evenden, 2009; Paterson et al., 2011). Pilot studies in our laboratory in a different cohort indicated that higher doses (2.0 mg/kg) profoundly and nonspecifically disrupted performance of the task, to the point where measurement of visuospatial priming was impossible (data not shown).
Rats were habituated to the injection procedure with a subcutaneous saline injection on the day before initiation of the experiment. Rats then received 0, 0.25, 0.5, or 1 mg/kg D-amphetamine 10 min before being tested in the negative visuospatial priming task. Because the shortened 0.25 s prime stimulus duration produced the most robust negative priming in the previous experiments, this prime duration was used during the amphetamine challenge days. Each rat received each dose of amphetamine following a Latin square design (Dénes and Keedwell, 1974), which counterbalanced the order of all doses to minimize the risk of confounding order effects. Drug administrations were separated by 5–6 drug-free washout days, during which task sessions were performed using standard parameters.
AMPH did not produce a consistent effect on NP overall (see Figure 7a), because effects varied dependent upon the initial NP rate of the rats.
Figure 7.
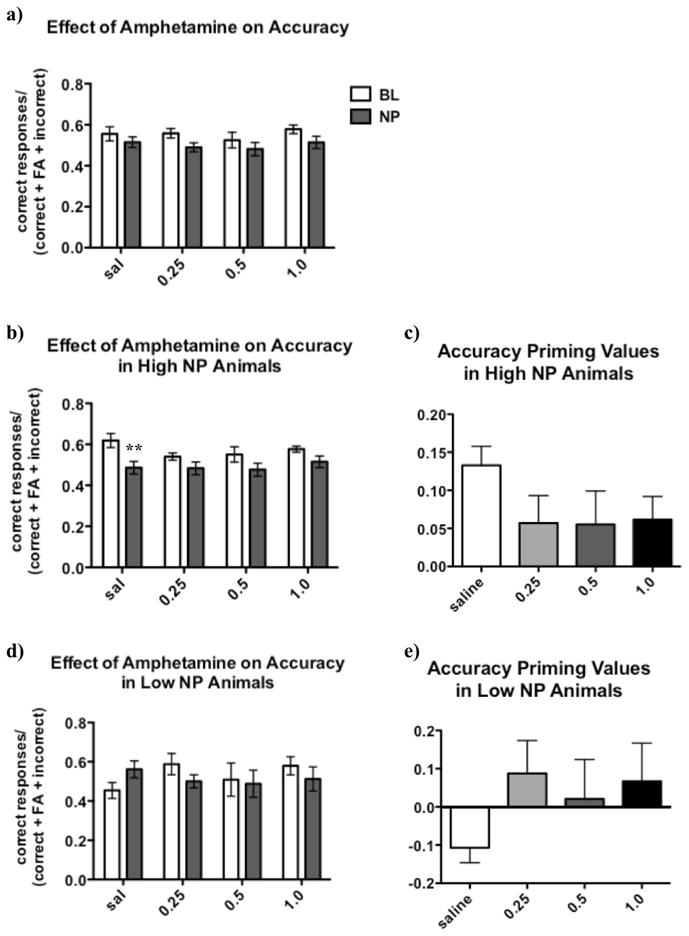
Amphetamine (AMPH) exhibited rate-dependent effects on negative priming (NP). Two-way ANOVA detected a significant effect of Trial Type on accuracy [F(1, 72) = 5.22, p < 0.05]; however, post hoc testing did not reveal any significant differences in accuracy between baseline and NP trials overall at any amphetamine dose (A). No main effects of Amphetamine Dose or Trial Type x Amphetamine Dose interaction were observed overall. However, when animals were examined separately based on their initial NP rate in the drug-free state, significant patterns emerged.
A three-way ANOVA of Median Split Group, Trial Type, and Amphetamine Dose revealed a very strong trend towards a significant interaction [F(3, 33) = 2.90, p = 0.05] between these factors. When accuracy priming values were calculated and compared for the two groups, a significant Median Split Group x Amphetamine Dose interaction [F(3, 33) = 2.98, p < 0.05] was observed. Post hoc testing indicated that groups differed in priming values only after saline administration (p < 0.05), while this difference was lost after AMPH administration at any dose.
In high NP rats, two-way ANOVA revealed a significant effect of Trial Type on accuracy [F(1, 42) = 13.47, p < 0.01]. Post hoc testing revealed that animals exhibited significantly lower accuracy in NP trials compared to baseline trials after saline treatment (p < 0.01). This NP effect was absent after amphetamine administration at any dose (B). Examination of accuracy priming values shows that high NP rats exhibited a robustly positive accuracy priming value after saline treatment that was attenuated by amphetamine treatment at every dose (C), although this effect did not reach statistical significance [F<1.7, ns].
In low NP rats, no significant effect of Trial Type was detected [F<1, ns]. However, a tendency toward lower accuracy in baseline trials compared to NP trials after saline administration was observed. This pattern was reversed after the 0.25 mg/kg dose of amphetamine; here, rats exhibited lower accuracy in NP trials compared to baseline trials, suggesting a strengthened NP effect. Accuracy differences between baseline and NP trials were absent after the higher amphetamine doses (D). While no significant differences in accuracy priming values for low NP were found [F<1.3, ns], inspection of the data suggests that rats did not exhibit an NP effect after saline administration, whereas after administration of the 0.25 mg/kg amphetamine dose, the accuracy difference became positive, indicating a stronger NP effect. At higher amphetamine doses, no NP effect was observed (E).
No main effect of Amphetamine Dose and no interaction were found in either high NP or low NP rats. No significant effect of Trial Type or Amphetamine Dose and no Trial Type x Amphetamine Dose interaction on correct response latency were observed (data not shown).
Data were analyzed using two-way ANOVA, with the two factors Trial Type (baseline or NP) and Amphetamine Dose. Post hoc comparisons of significant effects were conducted using Bonferroni tests. Values are expressed as mean ± SEM. Asterisks (**p < 0.01) denote significant differences compared with baseline trials. BL, baseline; NP, negative priming.
In order to assess the effect of amphetamine on animals that exhibited strong vs. weak negative priming in the drug-free state, we divided rats into groups, identifying “high NP” rats (accuracy priming values above 0 on the day of saline treatment) and “low NP” rats (accuracy priming values below 0 on the day of saline treatment). Accuracy in the task exhibited significant interactions between trial type, AMPH dose, and negative priming levels in the drug-free state. Accuracy priming values likewise exhibited significant interactions between AMPH dose and priming levels in the drug-free state, with high NP and low NP rats differing in their priming values only after saline administration. The robustness of these findings is underlined by the fact that this pattern of significant interactions was still observed when rats were grouped into high NP and low NP groups based on a range of different cutoff criteria, such as drug-free NP values > or < 0.5.
Significant NP was observed in high NP rats after saline administration, but was abolished in these rats after AMPH administration at all doses. This finding was reflected by the fact that high NP rats exhibited higher accuracy in baseline vs. NP trials only after saline administration, but not after AMPH (see Figure 7b), and that accuracy priming values tended to be reduced after all AMPH doses (see Figure 7c). In contrast, in rats with low NP values in the drug-free state, the 0.25 mg/kg AMPH dose appeared to enhance negative priming, with accuracy tending to be higher in baseline vs. NP trials after this dose, a pattern that was absent after saline administration in these animals (see Figure 7d). This pattern was lost at higher AMPH doses. Confirming this finding, the accuracy priming values in this group were highest after the 0.25 mg/kg AMPH dose (see Figure 7e).
The total number of trials completed were unaffected by AMPH. While the total number of correct responses exhibited a trend towards decrease after the highest AMPH dose, rats still completed significant numbers of correct responses even after this dose, demonstrating that task performance was not grossly disrupted by the doses of AMPH used. Premature responses were increased significantly by all doses of AMPH (see Table 1).
Table 1.
Effects of amphetamine (AMPH) on task performance. Total trials were unaffected by any amphetamine dose. There was a trend towards a main effect of Amphetamine Dose to reduce total correct responses [F(3, 36) = 2.58, p = 0.07]. A main effect of Amphetamine Dose on premature responses before the prime was observed [F(3, 36) = 6.96, p < 0.001]; post hoc testing confirmed that premature responses were increased after each amphetamine dose compared to saline (p < 0.01 for 0.25 mg/kg, p < 0.001 for 0.5 mg/kg, p < 0.05 for 1 mg/kg).
Data were analyzed using one-way repeated measures ANOVA. Post hoc comparisons of significant effects were conducted using Newman-Keuls tests. Values are expressed as mean ± SEM.
| saline | 0.25 mg/kg | 0.5 mg/kg | 1 mg/kg | |
|---|---|---|---|---|
| trials | 111.69 ± 7.01 | 116.85 ± 8.11 | 95.08 ± 12.72 | 98.00 ± 11.22 |
| total correct responses | 40.23 ± 4.40 | 38.62 ± 3.47 | 31.38 ± 5.61 | 27.54 ± 4.52† |
| premature responses | 8.00 ± 1.51 | 35.08 ± 6.93** | 40.69 ± 9.95*** | 26.23 ± 6.56* |
Asterisks (*p < 0.05, **p < 0.01, ***p < 0.001) denote significant differences and dagger symbol (†) denotes a trend toward difference compared with performance after saline administration.
4. Discussion
Animal models of TS have been proposed, with different degrees of face, predictive and construct validity (Swerdlow and Sutherland, 2005). Many of these models rely on the appearance (e.g. (Campbell et al., 2000)) or suppression (e.g. (Tizabi et al., 2001)) of a “tic-like” repetitive, stereotyped movement in a rodent as a behavioral end-point. The limitations and potential anthropomorphic pitfalls of such models have been discussed elsewhere (Swerdlow and Sutherland, 2005, 2006). An alternative strategy in developing TS-relevant models is to identify simple measures of information processing that detect deficits in TS patients, and which can be translated across species for studies in infra-humans. This approach was taken previously with one measure of automatic inhibition – prepulse inhibition of the startle reflex (PPI) – which is deficient in TS (Castellanos et al., 1996; Swerdlow et al., 2001) and has been used to study the anatomy (Baldan Ramsey et al., 2011b), pharmacology (Swerdlow et al., 2006) and genetics (Baldan Ramsey et al., 2011a) of this disorder. Negative priming is another simple measure of inhibitory deficits in TS, with implications for both pathophysiology (Wright et al., 2006; Wright et al., 2005) and therapeutics (Deckersbach et al., 2006), and the original studies reported here describe the usefulness of a novel animal model for further understanding the biology of NP deficits in TS.
In the studies reported here, rats exhibited robust negative visuospatial priming, reflected by degraded performance when the targets were presented in the location previously occupied by the non-target distractor during the preceding prime (NP trials), compared to unrelated target locations in prime and probe trial (baseline trials). This NP effect was replicable, and individual negative priming values for each rat were significantly positively correlated across repeated trials (see Figure 6), indicating that the degree of NP in this task appears to be a stable trait inherent to the animal. To our knowledge, this is the first documented rat task assessing visuospatial priming.
4.1 Species Differences
The negative priming effect reported here was most robustly observed in response accuracy: significantly lower accuracy was detected in NP trials compared to baseline trials (see Figures 3a, b and 4a, b). While a tendency towards longer correct response latencies in NP trials compared to baseline trials was observed, this effect was less reliable and never reached statistical significance (see Figures 3c, d and 4c, d). In the human visuospatial priming task, NP effects on response accuracy have been observed, but the most robust NP effect in humans is slowed response latency (Tipper, 2001). This differential sensitivity across species may reflect the fact that rats adopted a task strategy different from that used by humans performing an analogous task.
Sensory-based cognitive tasks typically feature a speed-accuracy trade-off: longer processing of a stimulus before making a response increases response accuracy, while faster responding generally leads to reduced accuracy. Test subjects can therefore adopt strategies that either optimize response speed at the cost of accuracy or vice versa, depending on aspects of the task, such as reward probability, reward size, error cost, etc. (Kay et al., 2006), and characteristics of the test subjects. A strategy of sacrificing response speed for the maintenance of accuracy is commonly observed in humans (Busemeyer and Townsend, 1993). Interestingly, while both speed-maximizing and accuracy-maximizing strategies have been observed in rats and mice (Abraham et al., 2004; Kaneko et al., 2006; Rinberg et al., 2006; Uchida and Mainen, 2003; Young et al., 2010), rats frequently favor strategies that maintain high response speeds while sacrificing accuracy. For example, rats exhibit lower accuracy, but do not slow their reaction times, in higher-difficulty trials of both an odor-discrimination task (Uchida and Mainen, 2003) and an attentional set-shifting task (Barense et al., 2002). In contrast, mice maintain high accuracy, while performing with longer response latencies, in similar tasks when challenged with more difficult odor-discrimination trials (Abraham et al., 2004) or set-shifting tested at older ages (Young et al., 2010). The tendency of rats to maintain speed of responding at the cost of accuracy may therefore explain why the NP effect in our task primarily impacted response accuracy rather than response latencies.
Species differences in task demands might also contribute to the predominant NP effects on accuracy in the rat paradigm vs. latency in the human paradigm. Thus, in the human NP paradigm, subjects perform a minimal finger movement to press a keyboard, and NP effects on response latency can be as small as several tens of milliseconds (Hartston and Swerdlow, 1999; Swerdlow et al., 1996). Changes in response latency on that scale would be difficult to detect in this rat NP task, which requires the rat to make a whole-body movement across the experimental chamber to respond to the stimulus.
4.2 Role of Prime Duration
It is notable that robust negative priming effects in our task were observed only when rats were challenged by reducing the duration of the prime stimuli from 0.5 to 0.25 s (see Figures 3 and 4). This pattern – that the priming effect became stronger when the prime duration was reduced – was consistent with the observation that lengthening the prime duration from 0.5 to 1 s weakened the priming effect (see Figure 3). Conceivably, shorter duration primes may favor processing by preconscious/automatic mechanisms, which may be required for the negative priming effect. Indeed, stimuli presented below the level of conscious awareness can have a greater impact on response characteristics than stimuli that are consciously perceived (Bargh, 1992; Bornstein, 1989). In one potentially relevant paradigm, prepulse inhibition (PPI) of the startle response is maximal when the interval between the weak lead stimulus and the startling stimulus is 60–120 ms; longer intervals result in less inhibition of startle (Braff et al., 1978; Graham, 1975). The shorter prepulse-pulse intervals associated with maximal PPI have been linked to more automatic, preconscious inhibition, whereas longer intervals are linked to more volitional, consciously controlled inhibition (Swerdlow et al., 2008).
4.3 Effects of Modulation of Dopamine Levels
Administration of low doses of D-amphetamine (AMPH) before testing under conditions that would normally produce negative priming (i.e., decreased duration of prime stimuli) altered performance in a manner that was dependent on the rats’ amount of drug-free negative priming (i.e., while challenged with shorter prime stimuli but administered only saline). In rats that exhibited high levels of drug-free negative priming, all doses of AMPH disrupted negative priming (see Figure 7b, d). This abolition of negative priming was a selective behavioral effect: AMPH did not alter the number of trials completed by the rats, nor did it significantly reduce the number of correct responses performed (see Table 1). In contrast, in rats that exhibited low levels of drug-free negative priming, the 0.25 mg/kg dose of AMPH increased negative priming. It was only at this dose – and not after saline administration – that these rats exhibited lower accuracy in the NP trials compared to baseline trials (see Figure 7c, e).
This “rate-dependent” effect of AMPH recalls the widely observed pattern described in the Yerkes-Dodson law, which states that performance, especially in difficult tasks, initially increases with physiological or mental arousal, but when levels of arousal become too high, performance is degraded (see Figure 8). The finding that AMPH improves NP in animals with normally low levels of NP, while reducing NP in animals with high levels of NP, suggests that an optimal level of dopamine transmission might produce maximal amount of negative visuospatial priming, and that levels either above or below that optimal point result in reduced or no negative priming. Such a role for “optimal dopamine levels” has been suggested in other features of information processing and cognition, as evidenced by rate-dependent effects of AMPH in humans (Mattay et al., 2003; Talledo et al., 2009), including tasks with specific visuospatial processing demands (Hamidovic et al., 2010). Parallel findings have been reported with systemic AMPH administration in rats (Lyon and Robbins, 1975; Talledo et al., 2009) and mice (Papaleo et al., 2008), and after infusion of dopamine D1 agonists and antagonists into the prefrontal cortex (PFC) of rats performing an attentional task (Granon et al., 2000). The PFC has also been implicated in the control of visuospatial priming in humans (Wright et al., 2006; Wright et al., 2005), and AMPH significantly increases PFC dopamine release in rats (Moghaddam and Bunney, 1989). Optimal dopamine transmission levels in the PFC may therefore be required for maximum levels of PFC-controlled functions, including visuospatial priming.
Figure 8.
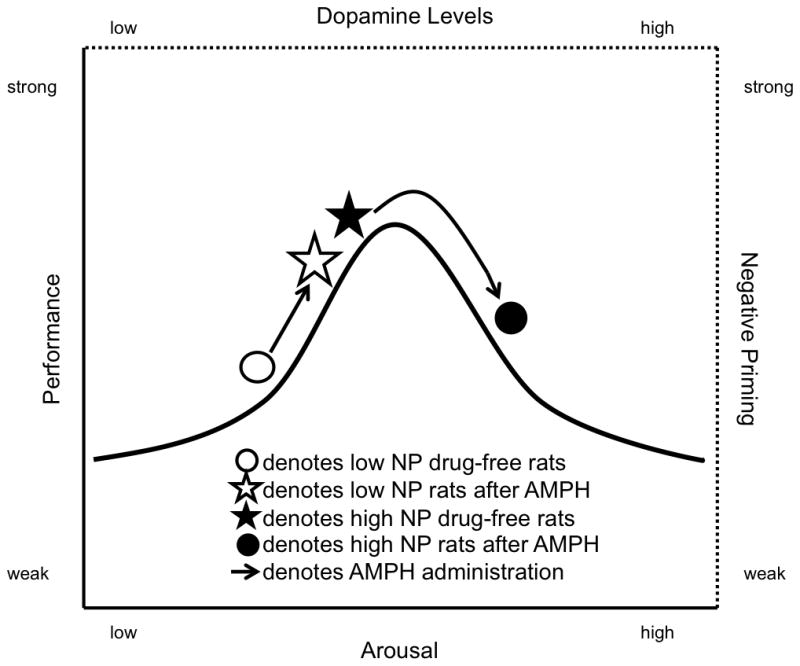
Schematic of the Yerkes-Dodson law in relation to the effects of amphetamine observed in this study. Variables of the original Yerkes-Dodson law are marked along the solid axes; variables relating to this study are marked along the dotted axes. As described in the original Yerkes-Dodson law, low arousal levels are associated with low performance. At higher arousal levels, performance improves up to a point; however, at very high arousal levels, performance deteriorates. Analogously, in the hypothetical pattern underlying the findings in our study, low levels of dopamine transmission are associated with low levels of negative priming (NP). As dopamine levels increase, NP at first increases also, e.g. in low NP (O) rats administered amphetamine (☆). Increasing dopamine levels past a presumed optimal point, e.g. in high NP rats (★) administered amphetamine (●), results in NP deteriorating.
4.4 Predictions and Therapeutic Implications
One “reverse translational” and testable prediction from the present findings would be that AMPH should increase NP in TS patients with lowest NP levels. Conceivably, such a drug effect might predict an AMPH-enhanced response to habit reversal therapy in these subjects, given that greater levels of NP predict a positive therapeutic response to this treatment (Deckersbach et al., 2006). This prediction is not terribly bold, as stimulants have been reported to enhance attentional mechanisms and, in some cases, reduce tic severity in children with co-morbid TS and attention deficit disorder (Tourette’s Syndrome Study Group, 2002). More generally, the fact that intact NP in TS patients predicts a positive response to behavioral therapy suggests that the neural substrates of that regulate NP may be engaged through the process of gaining volitional control over automatic responses. The ability of novel medications to enhance negative priming in the present model might therefore be reasonably expected to predict their utility in augmenting behavioral therapies for TS.
Alternatively, the abolition of NP after administration of higher doses of AMPH (0.5 – 1.0 mg/kg) may be analogous to the NP deficits seen in TS. This interpretation would suggest that the increased dopamine release induced by AMPH mimics the excessive dopamine function postulated in TS etiology (Albin et al., 2003; Leckman et al., 2010). In this case, AMPH would be expected to disrupt NP in healthy human subjects with high NP levels, and possibly in TS patients with relatively high NP, mirroring the disruption of PPI observed in humans with high baseline levels of PPI (Talledo et al., 2009).
TS is a complex clinical phenotype, and “pure” TS is much less common than forms that are comorbid with syndromes or diagnoses of attention deficit/hyperactivity disorder (ADHD) and/or obsessive-compulsive disorder (OCD). Whether these clinical phenotypes represent “mixtures” of different disorders rather than neurobiologically distinct entities is not known; perhaps more importantly, no animal model is likely to capture the full neurobiology relevant to all of the varied forms of TS. By modeling several symptoms of TS, however, any compounds developed that attenuate deficits in several of these models are more likely to be efficacious in patients, a concept referred to as convergent validity. Hence, modeling NP deficits is a step forward. Moreover, NP deficits in TS as first reported in a visuospatial priming task were independent of comorbid ADHD and OCD (Swerdlow et al. 1996), and comorbid conditions did not impact the predictive value of priming deficits on therapeutic response to habit reversal therapy (Deckersbach et al. 2006). Based on a different paradigm, some (Ozonoff et al. 1998) have suggested a role of symptom severity and/or comorbid OCD and/or ADHD in TS-related NP deficits, while others (Tomporowski et al. 1994) have reported that visuospatial attentional performance in a related visuospatial priming paradigm did not differ between healthy children and children with non-TS-related ADHD. Thus, the present available literature suggests that NP deficits in TS patients reflect pathology of relevance to some, if not all, forms of this disorder.
4.5 Summary
In summary, we describe a novel visuospatial priming task that detects negative priming in rats. Rodent negative priming is sensitive to modification by pharmacological intervention with relevance to TS pathology. This novel task may facilitate cross-species studies of inhibitory information processing, its dysfunction in brain disorders including TS, and the discovery of novel therapeutic approaches to these disorders.
Highlights.
We developed a novel rat task to assess visuospatial priming, a measure of competitive information processing with relevance to Tourette syndrome.
Rats exhibited robust negative priming (NP) in our task.
Amphetamine had rate-dependent effects on NP, disrupting NP in high NP rats and enhancing NP in low NP rats.
Our task may constitute a valuable novel tool for investigating the neural bases of NP deficits in TS and other neuropsychiatric disorders.
Acknowledgments
Supported by NIH grants R21 MH091571 and R01 MH059803, as well as the Tourette’s Syndrome Association. The funding sources had no role in the design of this study; in the collection, analysis and interpretation of data presented here; in the writing of this report; or in the decision to submit this article for publication. The authors would like to thank Dr. Mark Geyer for his advice and support, and Ms. Mahalah Buell, Ms. Shereen Cohen, and Ms. Maria Bongiovanni for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Albin RL, Koeppe RA, Bohnen NI, Nichols TE, Meyer P, Wernette K, Minoshima S, Kilbourn MR, Frey KA. Increased ventral striatal monoaminergic innervation in Tourette syndrome. Neurology. 2003;61:310–315. doi: 10.1212/01.wnl.0000076181.39162.fc. [DOI] [PubMed] [Google Scholar]
- Azrin NH, Peterson AL. Habit reversal for the treatment of Tourette syndrome. Behav Res Ther. 1988;26:347–351. doi: 10.1016/0005-7967(88)90089-7. [DOI] [PubMed] [Google Scholar]
- Baldan Ramsey LC, Williams K, Gallezot J, Crowley M, Anderson G, Leventhal BL, Ohtsu H, Krystal JH, Mayes L, de Araujo I, Ding Y, State MW, Pittenger C. Histidine decarboxylase deficiency produces tourette syndrome phenomenology and dopamine dysregulation in humans and mice. Neuropsychopharmacology. 2011a;36:S198. [Google Scholar]
- Baldan Ramsey LC, Xu M, Wood N, Pittenger C. Lesions of the dorsomedial striatum disrupt prepulse inhibition. Neuroscience. 2011b;180:222–228. doi: 10.1016/j.neuroscience.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargh JA. The ecology of automaticity: toward establishing the conditions needed to produce automatic processing effects. Am J Psychol. 1992;105:181–199. [PubMed] [Google Scholar]
- Bornstein R. Exposure and affect: Overview and meta-analysis of research, 1968–1987. Psychological Bulletin. 1989;106:265–289. [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Busemeyer JR, Townsend JT. Decision field theory: a dynamic-cognitive approach to decision making in an uncertain environment. Psychol Rev. 1993;100:432–459. doi: 10.1037/0033-295x.100.3.432. [DOI] [PubMed] [Google Scholar]
- Campbell KM, Veldman MB, McGrath MJ, Burton FH. TS+OCD-like neuropotentiated mice are supersensitive to seizure induction. Neuroreport. 2000;11:2335–2338. doi: 10.1097/00001756-200007140-00053. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biol Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Rauch S, Buhlmann U, Wilhelm S. Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44:1079–1090. doi: 10.1016/j.brat.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Dénes J, Keedwell AD. Latin squares and their applications. Academic Press; New York-London: 1974. [Google Scholar]
- Elkins IJ, Cromwell RL. Priming effects in schizophrenia: associative interference and facilitation as a function of visual context. J Abnorm Psychol. 1994;103:791–800. doi: 10.1037//0021-843x.103.4.791. [DOI] [PubMed] [Google Scholar]
- Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilly DM, Loveland A. What is a “low dose” of d-amphetamine for inducing behavioral effects in laboratory rats? Psychopharmacology (Berl) 2001;153:155–169. doi: 10.1007/s002130000580. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Palmer AA, de Wit H. Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine. Psychiatr Genet. 2010;20:85–92. doi: 10.1097/YPG.0b013e32833a1f3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartston HJ, Swerdlow NR. Visuospatial priming and stroop performance in patients with obsessive compulsive disorder. Neuropsychology. 1999;13:447–457. doi: 10.1037//0894-4105.13.3.447. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Tamura H, Kawashima T, Suzuki SS. A choice reaction-time task in the rat: a new model using air-puff stimuli and lever-release responses. Behav Brain Res. 2006;174:151–159. doi: 10.1016/j.bbr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Martin C. When good enough is best. Neuron. 2006;51:277–278. doi: 10.1016/j.neuron.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Ko T, Evenden J. The effects of psychotomimetic and putative cognitive-enhancing drugs on the performance of a n-back working memory task in rats. Psychopharmacology (Berl) 2009;202:67–78. doi: 10.1007/s00213-008-1314-5. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol. 2010;20:237–247. doi: 10.1089/cap.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M, Robbins TW. The action of central nervous system stimulant drugs: a general theory concerning amphetamine effects. In: Essman W, Valzeli C, editors. Current developments in psychopharmacology 2. Spectrum; New York: 1975. pp. 79–163. [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Differential effect of cocaine on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens: comparison to amphetamine. Synapse. 1989;4:156–161. doi: 10.1002/syn.890040209. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Ricciardi J, Wetzler C, Hanania T. Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and d-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neurosci Res. 2011;69:41–50. doi: 10.1016/j.neures.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, Ginsburg GS, Deckersbach T, Dziura J, Levi-Pearl S, Walkup JT. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA. 2010;303:1929–1937. doi: 10.1001/jama.2010.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006;51:351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bongiovanni MJ, Tochen L, Shoemaker JM. Separable noradrenergic and dopaminergic regulation of prepulse inhibition in rats: implications for predictive validity and Tourette Syndrome. Psychopharmacology (Berl) 2006;186:246–254. doi: 10.1007/s00213-006-0374-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry. 1997;41:452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette’s syndrome: in search of an “fMRI-friendly” startle paradigm. Biol Psychiatry. 2001;50:578–585. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Magulac M, Filion D, Zinner S. Visuospatial priming and latent inhibition in children and adults with Tourette’s disorder. Neuropsychology. 1996;10:485–494. [Google Scholar]
- Swerdlow NR, Sutherland AN. Using animal models to develop therapeutics for Tourette Syndrome. Pharmacol Ther. 2005;108:281–293. doi: 10.1016/j.pharmthera.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Sutherland AN. Preclinical models relevant to Tourette syndrome. Adv Neurol. 2006;99:69–88. [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talledo JA, Sutherland Owens AN, Schortinghuis T, Swerdlow NR. Amphetamine effects on startle gating in normal women and female rats. Psychopharmacology (Berl) 2009;204:165–175. doi: 10.1007/s00213-008-1446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper SP. The negative priming effect: inhibitory priming by ignored objects. Q J Exp Psychol A. 1985;37:571–590. doi: 10.1080/14640748508400920. [DOI] [PubMed] [Google Scholar]
- Tipper SP. Does negative priming reflect inhibitory mechanisms? A review and integration of conflicting views. Q J Exp Psychol A. 2001;54:321–343. doi: 10.1080/713755969. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Russell LT, Johnson M, Darmani NA. Nicotine attenuates DOI-induced head-twitch response in mice: implications for Tourette syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1445–1457. doi: 10.1016/s0278-5846(01)00194-4. [DOI] [PubMed] [Google Scholar]
- Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58:527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Wright CI, Keuthen NJ, Savage CR, Martis B, Williams D, Wedig M, McMullin K, Rauch SL. Brain correlates of negative and positive visuospatial priming in adults. Neuroimage. 2006;30:983–991. doi: 10.1016/j.neuroimage.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Wright CI, McMullin K, Martis B, Fischer H, Rauch SL. Brain correlates of negative visuospatial priming in healthy children. Psychiatry Res. 2005;139:41–52. doi: 10.1016/j.pscychresns.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wylie SA, Stout JC. Enhanced negative priming in Parkinson’s disease. Neuropsychology. 2002;16:242–250. [PubMed] [Google Scholar]
- Yamaguchi S, Kobayashi S. Contributions of the dopaminergic system to voluntary and automatic orienting of visuospatial attention. J Neurosci. 1998;18:1869–1878. doi: 10.1523/JNEUROSCI.18-05-01869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA, Jeste DV, Risbrough VB. The mouse attentional-set-shifting task: a method for assaying successful cognitive aging? Cogn Affect Behav Neurosci. 2010;10:243–251. doi: 10.3758/CABN.10.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]