Abstract
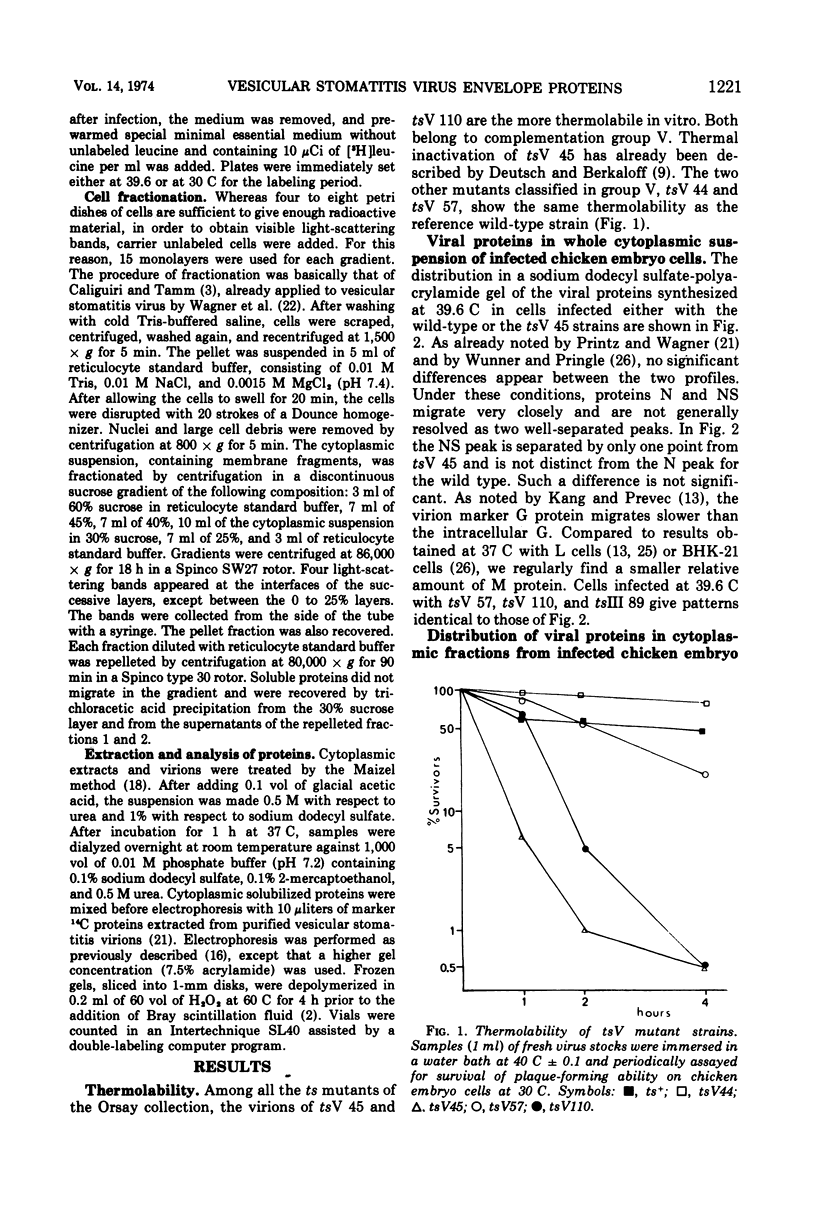
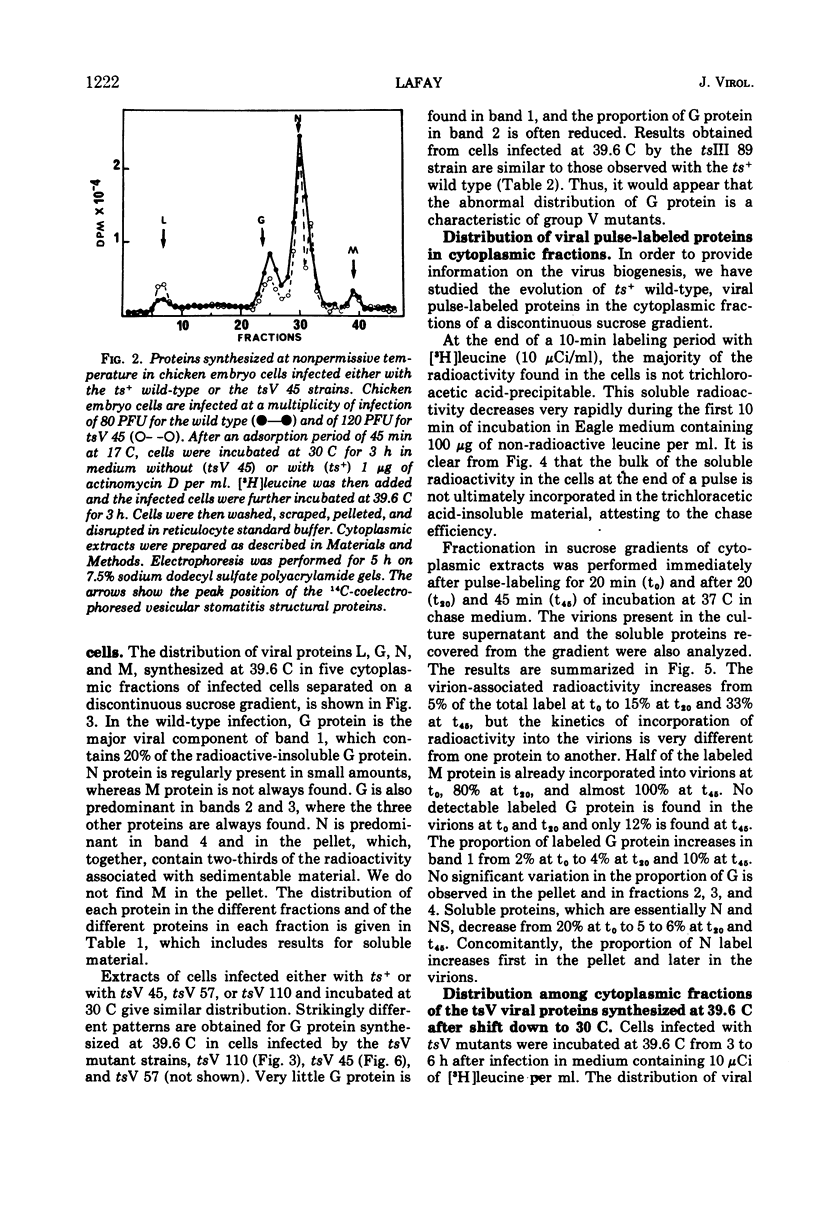
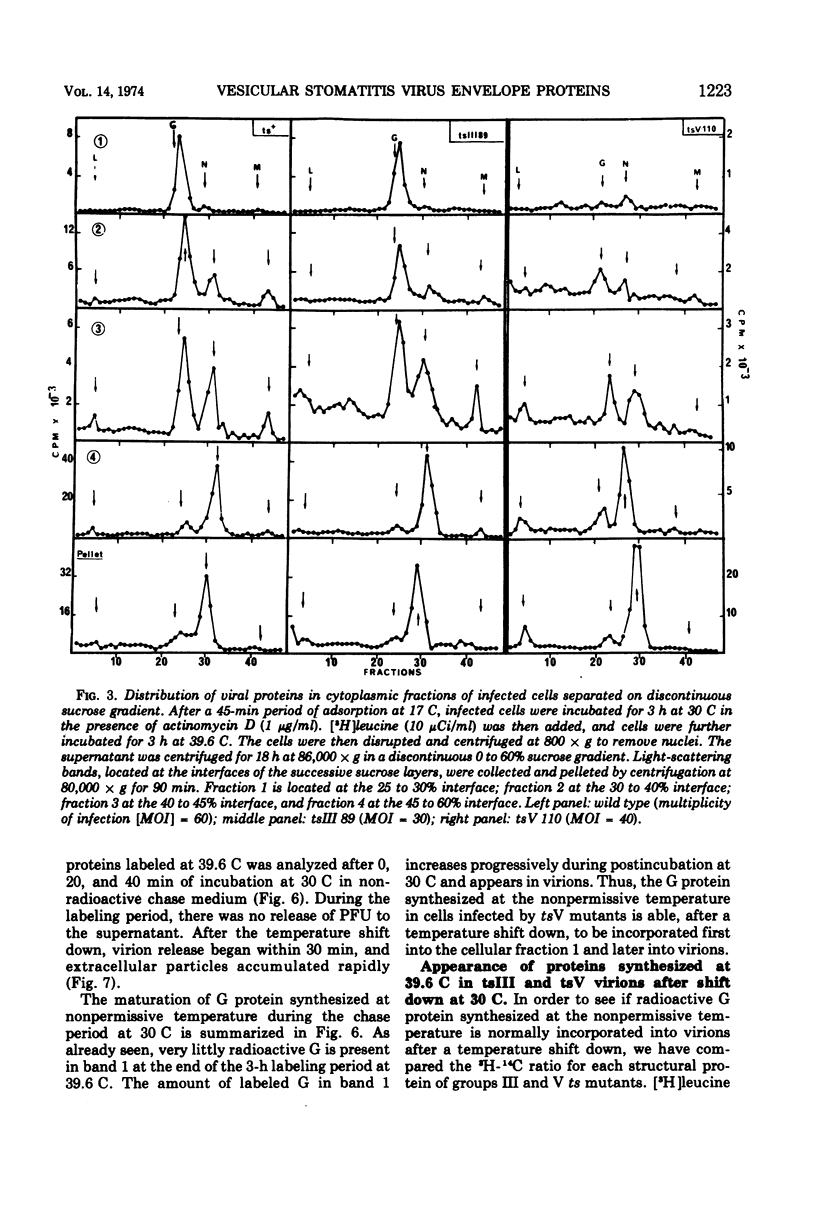
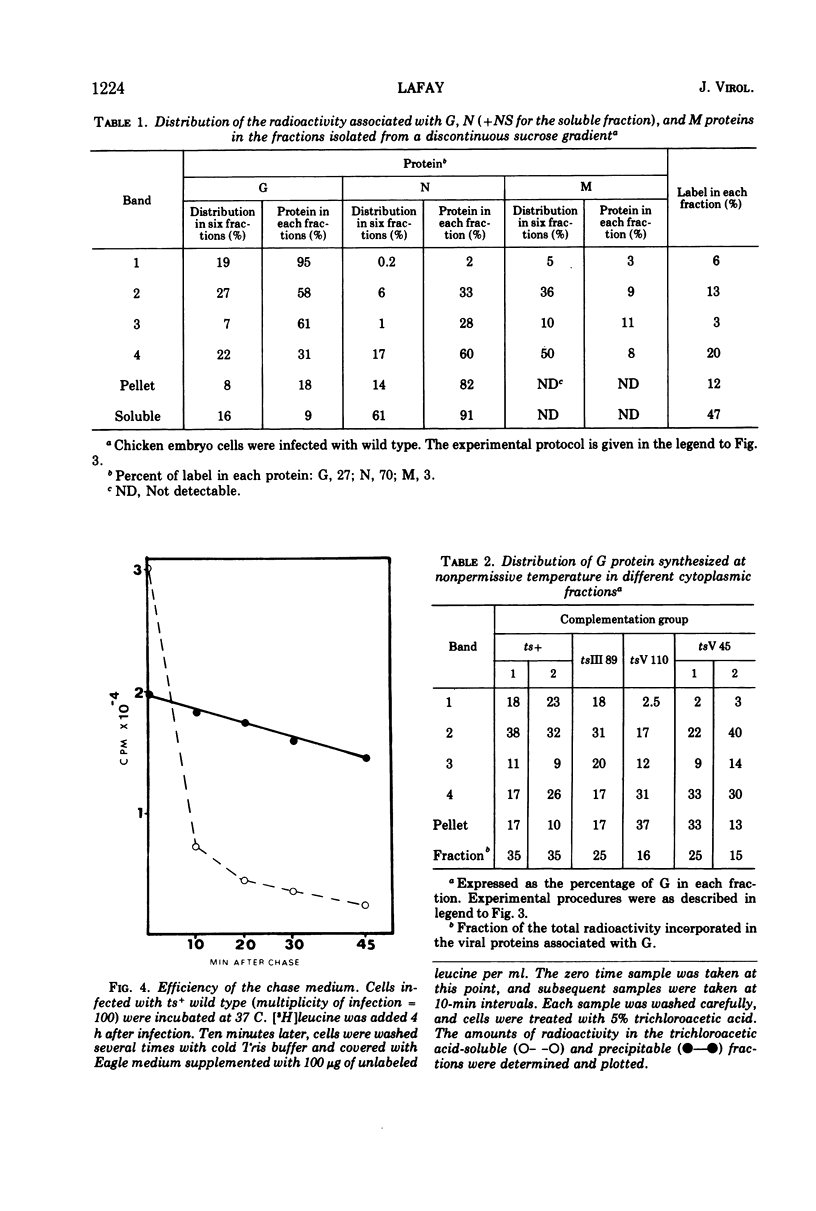
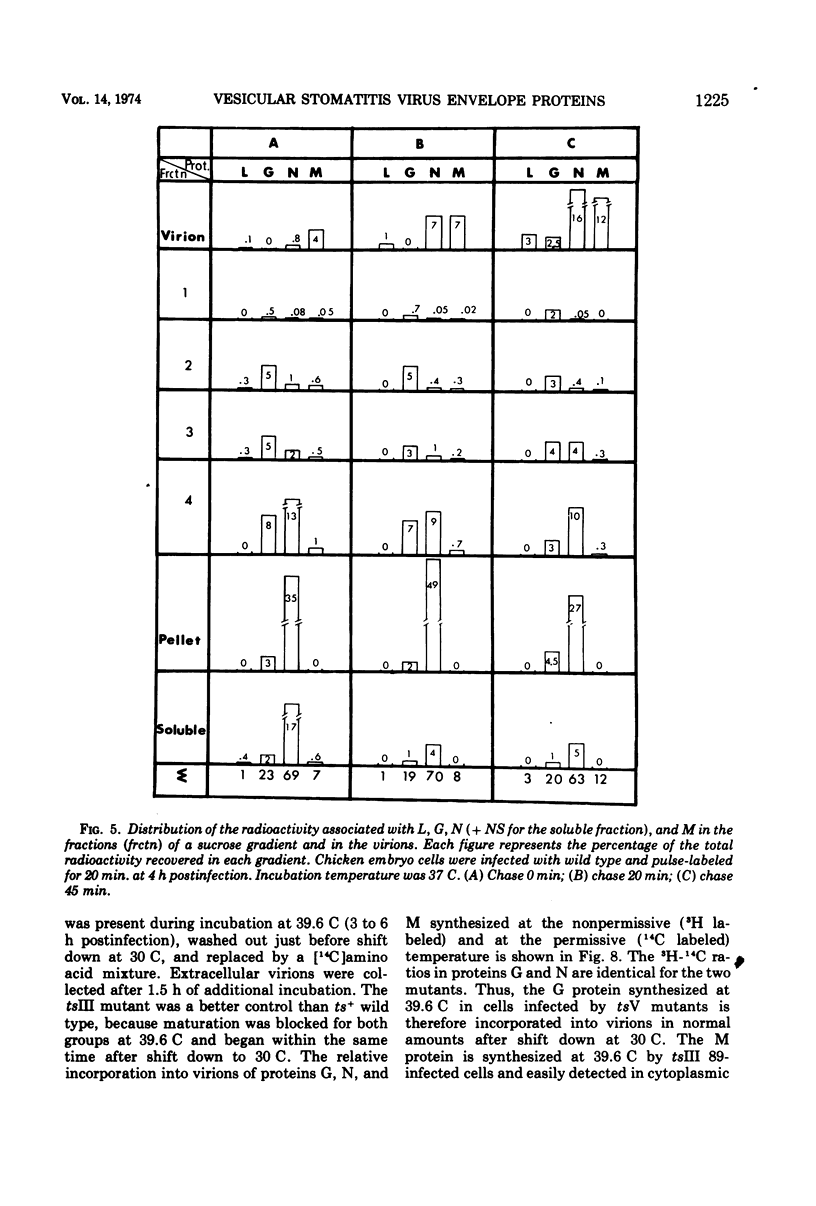
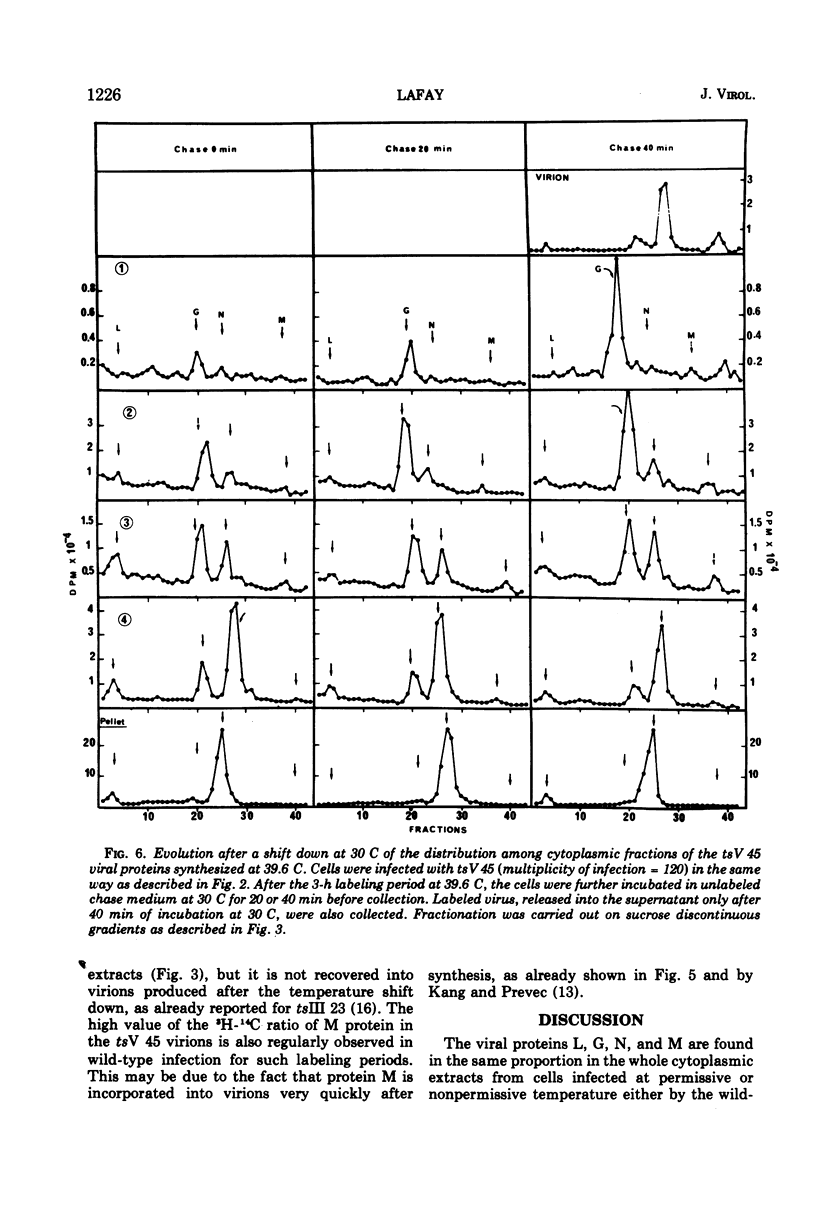
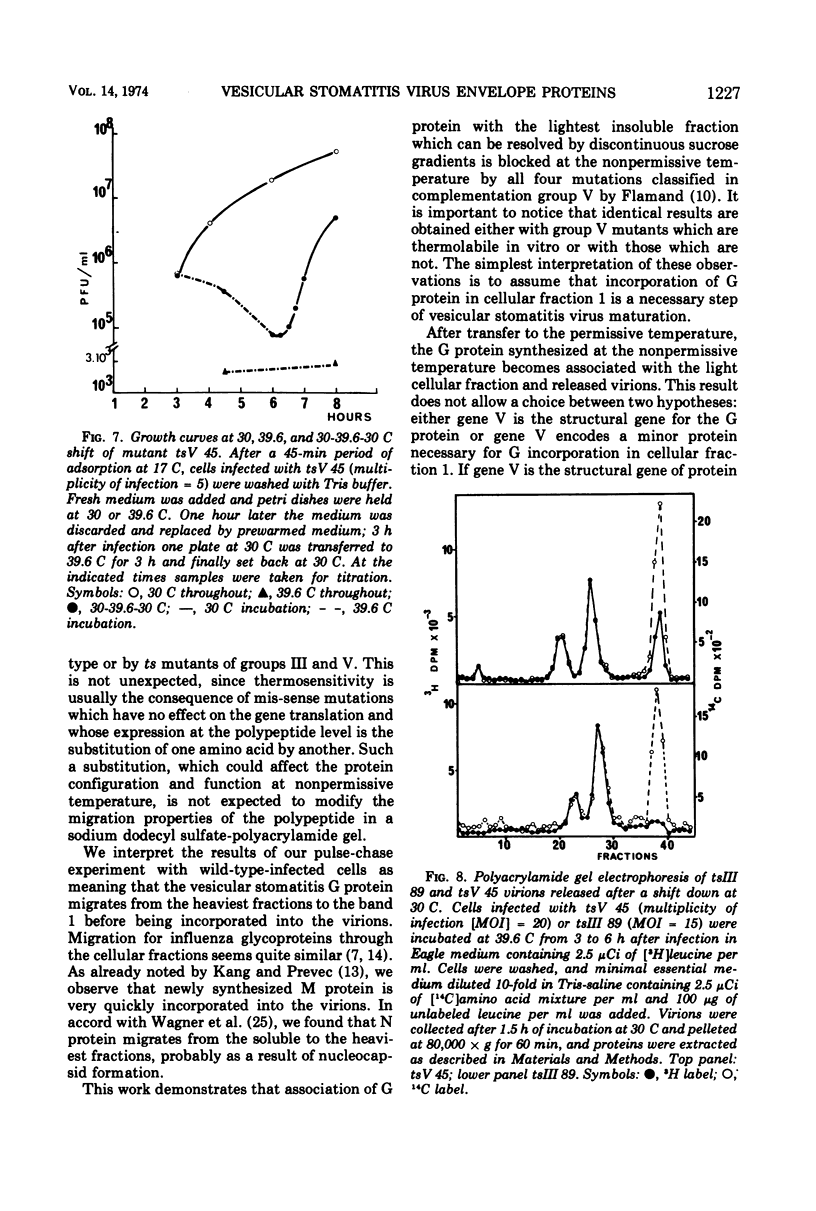
All five major viral proteins were synthesized in chicken embryo cells infected with vesicular stomatitis virus temperature-sensitive (ts) mutants of complementation groups III and V and maintained at the nonpermissive temperature. The distribution of these proteins among cytoplasmic cellular fractions separated on discontinuous sucrose gradients was identical for wild-type and tsIII-infected cells. Strikingly different patterns were observed for the G protein in gradients from cells infected by tsV mutants; very little, if any, G protein was found in the lightest fraction. Pulse and chase experiments with wild-type, virus-infected cells showed that protein G moves from the heaviest to the lightest fraction before being incorporated into the virion. After shift down to the permissive temperature (30 C), G protein synthesized at 39.6 C in tsV-infected cells became associated with the lightest cellular fraction and later with the released virions. In contrast, M protein, synthesized at 39.6 C in tsIII-infected cells, was not incorporated into the virions after shift down. These data strongly suggest, first, that M protein is encoded by the vesicular stomatitis gene III, and second, that incorporation of G protein in the lightest cellular fraction is a necessary step of vesicular stomatitis maturation. This step is impaired by tsV mutations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. doi: 10.1128/jvi.10.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Cartwright B. The distribution of virus proteins in BHK 21 cells infected with vesicular stomatitis virus (Indiana C). J Gen Virol. 1973 Nov;21(2):407–411. doi: 10.1099/0022-1317-21-2-407. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Atkinson P. H., Summers D. F. Interactions of vesicular stomatitis virus structural proteins with HeLa plasma membranes. Nat New Biol. 1971 May 26;231(21):121–123. doi: 10.1038/newbio231121a0. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Summers D. F. In vitro association of vesicular stomatitis virus proteins with purified HeLa and erythrocyte plasma membranes. Virology. 1974 Feb;57(2):566–569. doi: 10.1016/0042-6822(74)90195-0. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Influenza virus proteins. II. Association with components of the cytoplasm. Virology. 1973 Jan;51(1):56–70. doi: 10.1016/0042-6822(73)90365-6. [DOI] [PubMed] [Google Scholar]
- David A. E. Assembly of the vesicular stomatitis virus envelope: incorporation of viral polypeptides into the host plasma membrane. J Mol Biol. 1973 May 5;76(1):135–148. doi: 10.1016/0022-2836(73)90085-5. [DOI] [PubMed] [Google Scholar]
- Deutsch V., Berkaloff A. Analyse d'un mutant thermolabile du virus de la stomatite vésiculaire (VSV. Ann Inst Pasteur (Paris) 1971 Jul;121(1):101–106. [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Flamand A., Pringle C. R. The homologies of spontaneous and induced temperature-sensitive mutants of vesicular stomatitis virus isolated in chick embryo and BHK 21 cells. J Gen Virol. 1971 May;11(2):81–85. doi: 10.1099/0022-1317-11-2-81. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Wöllert W., Rott R., Scholtissek C. Association of influenza virus proteins with cytoplasmic fractions. Virology. 1974 Jan;57(1):28–41. doi: 10.1016/0042-6822(74)90105-6. [DOI] [PubMed] [Google Scholar]
- Lafay F., Berkaloff A. Etude des mutants thermosensibles du virus de la stomatite vésiculaire (VSV). Mutants de maturation. C R Acad Sci Hebd Seances Acad Sci D. 1969 Sep 15;269(11):1031–1034. [PubMed] [Google Scholar]
- Lafay F. Etude des fonctions du virus de la stomatite vésiculaire altérées par une mutation thermosensible: mise en evidence dela protéine structurale affectée par la mutation ts 23. J Gen Virol. 1971 Dec;13(3):449–453. doi: 10.1099/0022-1317-13-3-449. [DOI] [PubMed] [Google Scholar]
- Lafay F. Etude des mutants thermosensibles du Virus de la Stomatite Vésiculaire (VSV). Classification de quelques mutants d'après des critères de fonctionnement. C R Acad Sci Hebd Seances Acad Sci D. 1969 May 12;268(19):2385–2388. [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Simpson R. W. Conditional lethal mutants of vesicular stomatitis virus. II. Synthesis of virus-specific polypeptides in nonpermissive cells infected with "RNA-" host-restricted mutants. Virology. 1974 Feb;57(2):369–377. doi: 10.1016/0042-6822(74)90176-7. [DOI] [PubMed] [Google Scholar]
- Printz P., Wagner R. R. Temperature-sensitive mutants of vesicular stomatitis virus: synthesis of virus-specific proteins. J Virol. 1971 May;7(5):651–662. doi: 10.1128/jvi.7.5.651-662.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Kiley M. P., Snyder R. M., Schnaitman C. A. Cytoplasmic compartmentalization of the protein and ribonucleic acid species of vesicular stomatitis virus. J Virol. 1972 Apr;9(4):672–683. doi: 10.1128/jvi.9.4.672-683.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Holloway A. F., Cormack D. V. Characterization of three complementation groups of vesicular stomatitis virus. Virology. 1972 Dec;50(3):829–840. doi: 10.1016/0042-6822(72)90437-0. [DOI] [PubMed] [Google Scholar]



