Abstract
Objective
To examine the effect of a single bout of moderate-intensity aerobic exercise on preadolescent children with attention-deficit/hyperactivity disorder (ADHD) using objective measures of attention, brain neurophysiology, and academic performance.
Study design
Using a within-participants design, task performance and event-related brain potentials were assessed while participants performed an attentional-control task following a bout of exercise or seated reading during two separate, counterbalanced sessions.
Results
Following a single 20-minute bout of exercise, both children with ADHD and healthy match-control children exhibited greater response accuracy and stimulus-related processing – with children with ADHD also exhibiting selective enhancements in regulatory processes – compared with after a similar duration of seated reading. In addition, greater performance in the areas of reading and arithmetic were observed following exercise in both groups.
Conclusion
These findings indicate that single bouts of moderately-intense aerobic exercise may have positive implications for aspects of neurocognitive function and inhibitory control in children with ADHD.
Keywords: Aerobic Exercise, Academic Achievement, Cognitive Control, Preadolescent Children, Attention-Deficit/Hyperactivity Disorder
Attention-deficit/hyperactivity disorder (ADHD) affects over 2.5 million school-aged children in the US (1–3). This disorder is characterized by developmentally inappropriate levels of inattention, overactivity, distractibility, and impulsiveness, which manifest during childhood (1,4,5). Research suggests that failures in inhibitory control, and the neural processes subserving inhibitory control, may represent the core cognitive deficit underlying the manifestation of ADHD (6). Specifically, a growing body of research has suggested that ADHD-related deficits in inhibitory control are associated with failures in the cascade of processes underlying the stimulus-response relationship; including reductions in the allocation of attentional resources, delays in the speed in which stimuli are processed, and failures to appropriately implement action monitoring processes as assessed using neuroelectric measures (7–16). Although pharmacological treatments have largely been found effective in the management of ADHD symptoms (17), potential adverse effects, cost, and incomplete response argue other treatments for children with ADHD (18,19).
Reports from parents, teachers, and scholars have suggested that one such option may be single bouts of short-duration, moderate-intensity aerobic exercise (20–22). Despite some recent findings by Medina et al (23) suggesting that single bouts of exercise may facilitate reaction time-based measures of attentional vigilance, a paucity of empirically sound evidence exists in children with ADHD to support such claims. The vast majority of support for these assertions is drawn from previous research in healthy children, suggesting that participation in a single bout of structured physical activities lasting at least 20 minutes is beneficial for a variety of cognitive functions including aspects of concentration (24–26), brief tests of reading and mathematics achievement (27,28), and inhibitory control (28). The effects of single bouts of exercise also appear to mirror the neurocognitive deficits associated with ADHD; such that a single bout of moderate-intensity aerobic exercise serves to increase the allocation of attentional resources, and facilitates stimulus classification and processing speed, with a disproportionately larger effect for task conditions with the greatest inhibitory control demands (29–32). Accordingly, given the striking similarity between the aspects of cognition, which are influenced by acute exercise and those that exhibit ADHD-related deficits; the purpose of this study was to examine the effect of a single bout of aerobic exercise on the modulation of inhibitory control deficits in children with ADHD using objective measures of behavioral inhibition, neurocognitive function, and scholastic performance. It was hypothesized that children with ADHD would experience similar benefits from acute exercise as those experienced by children without ADHD (28), with greater response execution, attentional allocation, and scholastic achievement being observed after a bout of moderate-intensity exercise.
Methods
The ADHD group was comprised of 20 children (6 female) between the ages of 8 and 10 years recruited from the East-Central Illinois area based on suspected or diagnosed ADHD free of any comorbid conditions (Table I). The term suspected ADHD refers to children whose parents, school staff, or primary care provider expressed suspicion of ADHD; but no diagnostic assessment had been sought from a developmental specialist (82). Clinical status was verified through the ADHD supplement of the Kiddie-Sads-PL (K-SADS) semi-structured diagnostic interview using DSM-IV-TR criteria for any subtype of ADHD, including evidence for impairment in two or more settings and onset of symptoms before 7 years of age (1). Children with ADHD were screened to ensure that they were currently exhibiting ongoing ADHD symptoms using the ADHD Rating Scale-IV (33). Healthy match-control children were yoked by sex, age, pubertal status, and SES; with no significant differences observed between groups (t’s (38) ≤ 1.6, p’s ≥ 0.12). All participants had normal or corrected-to- normal vision, and were free of any central nervous system active drug therapy for at least 1 month before testing. All participants were screened for comorbid conditions, including autism spectrum disorders, using the Social Communication Questionnaire (34), and anxiety, conduct, somatic, and affective disorders (including depressive and bipolar disorders) using the DSM-oriented scores of the Child Behavioral Checklist (35). All participants provided written assent and their legal guardians provided written informed consent in accordance with the Institutional Review Board of the University of Illinois at Urbana-Champaign and Carle Foundation Hospital.
Table I.
Participant demographic and clinical characteristics (±1 SE).
| Measure | ADHD Subtype | Health Match-Control | p* | ||
|---|---|---|---|---|---|
| ADHD-C | ADHD-I | ADHD-H | |||
| N | 6 (2 females) | 11 (3 females) | 3 (1 female) | 20 (6 females) | |
| Age (years) | 9.3 ± 0.3 | 9.5 ± 0.3 | 9.6 ± 0.9 | 9.8 ± 0.1 | .13 |
| Tanner stage | 1.5 ± 0.1 | 1.5 ± 0.2 | 1.0 ± 0.0 | 1.4 ± 0.1 | .86 |
| K-BIT composite (IQ) | 111.7 ± 4.6 | 110.2 ± 4.0 | 121.3 ± 2.7 | 118.7 ± 2.9 | .12 |
| Socioeconomic status (SES) | 2.5 ± 0.3 | 2.0 ± 0.3 | 3.0 ± 0.0 | 2.3 ± 0.2 | .99 |
| Body mass index (kg/m2) | 16.7 ± 0.6 | 18.5 ± 0.9 | 14.1 ± 0.3 | 20.0 ± 1.2 | .06 |
| K-SADS inattentive symptoms | 7.3 ± 0.3† | 7.0 ± 0.3† | 3.7 ± 1.3 | --- | --- |
| K-SADS impulsive/hyperactive symptoms | 6.5 ± 0.2† | 3.3 ± 0.4 | 8.0 ± 1.0† | --- | --- |
| ADHD-IV composite percentile | 98.2 ± 1.3† | 98.2 ± 0.7† | 90.0 ± 5.5† | 31.3 ± 4.2 | < .001 |
| ADHD-IV inattentive percentile | 92.7 ± 4.1† | 92.0 ± 2.2† | 62.3 ± 12.3 | 36.8 ± 5.6 | <.001 |
| ADHD-IV impulsive/hyperactive percentile | 94.5 ± 2.1† | 80.5 ± 6.1 | 91.3 ± 0.9† | 37.5 ± 5.1 | <.001 |
| DBRS distractible subscale T-score | 66.2 ± 6.5† | 64.7 ± 3.5† | 49.3 ± 2.9 | 44.8 ± 1.2 | <.001 |
| DBRS impulsive-hyperactive subscale T-score | 65.5 ± 4.5† | 59.8 ± 3.2 | 56.0 ± 6.4 | 44.9 ± 1.3 | <.001 |
| DBRS oppositional defiance disorder subscale T-score | 57.0 ± 3.5 | 51.0 ± 3.3 | 47.7 ± 4.3 | 43.7 ± 1.0 | .001 |
| Autism spectrum disorder score | 7.0 ± 1.1 | 4.6 ± 1.0 | 7.3 ± 0.9 | 3.5 ± 0.8 | .04 |
Note: ADHD subtype was based upon K-SADS diagnostic interview classification.
Denotes clinically significant values for each of the severity scales. Percentiles greater than or equal to 90 on the ADHD-IV rating scale indicate high likelihood for the presence of ADHD. T-scores below 60 on the DBRS are considered to be normal behavioral ratings. Children with ADHD scoring high on the oppositional defiance disorder (ODD) subscale of the DBRS were retained given the high comorbidity between ADHD and ODD (83). Autism spectrum disorder scores below 15 indicate the absence of autism spectrum disorders.
Analysis was conducted between the participants with ADHD and the Healthy Match-Control group
Inhibitory Control Task
Participants completed a modified version of the Eriksen flanker task (9,36) to assess inhibitory aspects of cognitive control. This paradigm is conceptually simplistic in that it requires the discrimination of a centrally presented target stimulus amid lateral flanking stimuli. In this task, participants were required to make a left hand thumb press on a Neuroscan STIM system switch response pad (Compumedics, Charlotte, NC) when the target stimulus pointed left and a right hand thumb press when the target stimulus point right. Thus, participants were instructed to respond as accurately as possible to the direction of a centrally presented target fish amid either congruous (facing the same direction) or incongruous (the target faces the opposite direction) flanking goldfish. The task also manipulated stimulus-response compatibility to vary cognitive control requirements by instructing participants first to complete a compatible condition (described above) and then complete an incompatible condition whereby participants were instructed to respond in the direction opposite that of the centrally presented target arrow (ie, when the target fish pointed left the correct response was to the right, and vice-versa; 37). For each compatibility condition, two blocks of 100 trials were presented with equiprobable congruency and directionality. The stimuli were 3 cm tall yellow fish, which were presented focally for 200 ms on a blue background with a fixed inter-stimulus interval of 1700 ms. This task allows for the assessment of a number of variables including median reaction time (to better represent the central response tendency of children with ADHD; 38–40) and response accuracy. Further, the trial-by-trial nature of the task allows for the assessment of median RT for correct trials immediately following an error (n+1, termed post-error median RT) – which provide a behavioral indicator of the increased recruitment and implementation of top-down control (41,42) – as well as for correct trials following a match-correct trial (a subset of correct trials matched to specific error trials based on RT (43).
Neuroelectric Assessment
A Neuroscan Synamps 2 amplifier (Compumedics, Inc, Charlotte, NC) was used to acquire event-related brain potentials (ERPs) in response to the modified flanker task using established protocols for data acquisition and processing (37). ERPs refer to a class of electroencephalographic activity that occurs in response to, or in preparation for, an event (44). Accordingly, the evaluation of ERPs provides additional insight into the subset of processes that occur between stimulus encoding and response production. Of interest to the present investigation are two prominent ERP components known as the P3 (also known as the P300 or P3b) and the error-related negativity (ERN; also known as the Ne). The P3, which occurs in response to a stimulus, is a positive-going deflection in the ERP waveform with the size of the component reflecting the allocation of attentional resources towards stimulus engagement (45). The latency of P3, which refers to the time point corresponding to the maximum peak amplitude, is generally considered as a measure of stimulus classification and processing speed (46). The P3 component was evaluated as the mean amplitude within a 50 ms interval surrounding the largest positive going peak within a 300 – 700 ms latency window. The ERN, in contrast, occurs in response to conflicting actions, such as erroneous behavior, and is thought to reflect activation of action monitoring processes to initiate the upregulation of top-down compensatory processes (47). The ERN component was evaluated as the mean amplitude within a 50 ms interval surrounding the largest negative going peak within a 0 – 150 ms window relative to the response.
Academic Performance Assessment
Participants also completed the Wide Range Achievement Test - 3rd edition (WRAT3; Wide Range, Inc., Wilmington, DE) to assess performance in reading comprehension, spelling, and arithmetic. The WRAT3 is a brief (~15 minutes) assessment, which allows for repeated administration through the use of two equivalent forms (48). Performance on the WRAT3 has been found to strongly correlate with the California Achievement Test – Form E and the Stanford Achievement Test (48). Administration order of the WRAT3 subtests was counterbalanced across participants, yet fixed between experimental conditions.
Procedure
A within-participants design had participants visit the laboratory on three separate days (M = 6.45 ± 7.3 days apart, M = 2.0 ± 2.2 hours time of day difference) on which they had not previously engaged in physical education. Following completion of the informed consent/assent, participants completed the Kaufman Brief Intelligence Test (K-BIT; 49); participants’ legal guardians completed the Physical Activity Readiness Questionnaire (50), the modified Tanner Staging System questionnaire (51), the Social Communication Questionnaire (34), the Child Behavioral Checklist (35), the ADHD Rating Scale IV (33), the Disruptive Behavior Rating Scale (DBRS; 52), and a health history and demographics questionnaire. Participants were then counterbalanced into two different session orders (day 2: Reading, day 3: Exercise vs. day 2: Exercise, day 3: Reading) to ensure that the observed effects were not due to the specific order in which participants received the exercise and rest conditions. No significant differences for any of the dependent variables (p ≥ 0.08) were observed between session orders. The experimental conditions consisted of 20 minutes of either seated reading or aerobic exercise on a motor-driven treadmill at an intensity between 65% and 75% of their maximum heart rate (HRduring exercise = 132.1 ± 10.3% bpm) recorded in response to a maximal exercise test using a Polar heart rate monitor (Model A1, Polar Electro, Finland; maximal exercise tests were conducted using the methodology reported in 28). Following the completion of the experimental conditions, participants were outfitted with an electrode cap and provided task instructions and practice trials. Once HR returned to within 10% of pre-experimental condition levels (28), the two conditions of the flanker task were performed (Compatible: 16.0 ± 0.6 minutes post-exercise; Incompatible: 27.4 ± 0.8 minutes post-exercise) followed by administration of the WRAT3 (38.1 ± 1.4 minutes post exercise).
Statistical Analyses
All statistical analyses were conducted using a significance level of p = .05, and analyses with three or more within-subjects levels used the Greenhouse-Geisser statistic with subsidiary univariate ANOVAs and Bonferroni corrected t tests for post hoc comparisons. Analysis were conducted using a 2 (Group: ADHD, Healthy Match-Control) × 2 (Session: Post Exercise, Post Reading) multivariate repeated measures ANOVA with additional variables nested within the primary analytical procedure based on the specific analysis. Specifically, analysis of task performance measures (median RT and response accuracy) were conducted separately within 2 (Compatibility: Compatible, Incompatible) × 2 (Congruency: Congruent, Incongruent). Post-trial median RT was also assessed within 2 (Compatibility: Compatible, Incompatible) × 2 (Accuracy: Post Error, Post Match Correct). The P3 ERP component was assessed separately for amplitude and latency within 2 (Compatibility: Compatible, Incompatible) × 2 (Congruency: Congruent, Incongruent) × 7 (Site: Fz, FCz, Cz, CPz, Pz, POz, Oz) (37). The ERN component was assessed at the FCz electrode site (53–55) within 2 (Accuracy: Error, Match Correct). Finally, analysis of academic performance was conducted separately for each academic subject. The data analysis was performed in PASW Statistics, 19.0. A statistical summary table for all variables of interest is provided in Table II.
Table II.
Statistical summary table for task performance, neuroelectric measures, and academic performance.
| Measure | Effect | df | F | p | η2 | Observed Power |
|---|---|---|---|---|---|---|
| Median Reaction Time | ||||||
| Compatibility | 1, 38 | 6.9 | .01 | .15 | .72 | |
| Congruency | 1, 38 | 198.3 | < .001 | .84 | 1.0 | |
| Response Accuracy | ||||||
| Group | 1, 38 | 5.4 | .026 | .12 | .62 | |
| Session | 1, 38 | 7.1 | .011 | .16 | .74 | |
| Compatibility | 1, 38 | 16.1 | < .001 | .30 | .97 | |
| Congruency | 1, 38 | 58.8 | < .001 | .60 | 1.0 | |
| Post-Trial Median Reaction Time | ||||||
| Accuracy | 1, 38 | 20.3 | < .001 | .35 | .99 | |
| Group × Session × Accuracy | 1, 38 | 6.2 | .017 | .14 | .68 | |
| Compatibility | 1, 38 | 13.8 | .001 | .27 | .95 | |
| P3 ERP Amplitude | ||||||
| Session | 1, 38 | 25.9 | < .001 | .41 | 1.0 | |
| Site | 6, 38 | 7.8 | < .001 | .17 | .91 | |
| Group | 1, 38 | 4.3 | .044 | .10 | .53 | |
| Group × Congruency | 1, 38 | 5.9 | .02 | .14 | .66 | |
| P3 ERP Latency | ||||||
| Session | 1, 38 | 13.0 | .001 | .25 | .94 | |
| Site | 1, 38 | 12.5 | < .001 | .25 | 1.0 | |
| Session × Site | 6, 38 | 3.1 | .025 | .08 | .75 | |
| Compatibility | 1, 38 | 9.2 | .004 | .20 | .84 | |
| Congruency | 1, 38 | 14.8 | < .001 | .28 | .96 | |
| ERN ERP Amplitude | ||||||
| Accuracy | 1, 34 | 115.9 | < .001 | .77 | 1.0 | |
| Session × Accuracy | 1, 34 | 7.5 | .01 | .18 | .76 | |
| Group × Session × Accuracy | 1, 34 | 5.4 | .026 | .14 | .62 | |
| WRAT3 Reading Comprehension | ||||||
| Session | 1, 38 | 20.1 | < .001 | .35 | .99 | |
| WRAT3 Arithmetic | ||||||
| Session | 1, 38 | 5.0 | .032 | .12 | .58 | |
Note: Only significant (p < .05) effects are reported.
Results
Task Performance
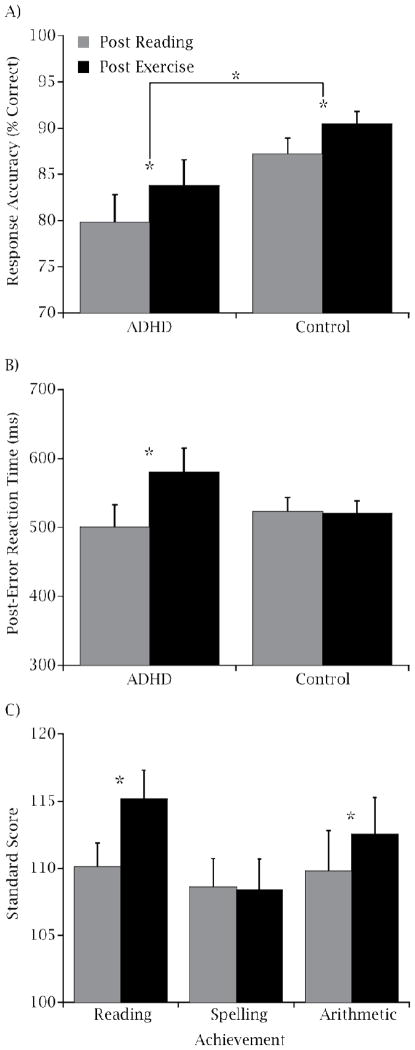
Figure 1 illustrates the effects of Group and Session for response accuracy and post-error slowing. Analysis revealed that children with ADHD (81.8 ± 2.7 %) exhibited decreased overall response accuracy relative to the Healthy Match-Control group (88.8 ± 1.3 %; p = .026, Cohen d = 1.7). However, following the single bout of exercise (87.1 ± 1.7 %), both groups exhibited greater response accuracy relative to following reading (83.5 ± 1.8 %; p = .011, Cohen d = .94). No significant main effects or interactions involving Group or Session were observed for median RT (p ≥ 0.1). Analysis of median RT for trials immediately following an error revealed greater post-error slowing following the exercise condition (579.4 ± 35.1 ms) relative to following the reading condition (500.3 ± 32.4 ms) only for children with ADHD, t (19) = 3.0, p = 0.008, Cohen d = 1.36.
Figure 1.
Mean (± SE) response accuracy (A) and median (± SE) post error reaction time (B) collapsed across compatibility and congruency conditions for each session by group. Mean (± SE) standard score for each session on each of the three WRAT3 academic performance tests collapsed across ADHD and Healthy Match-Control groups (C).
Neuroelectric Measures
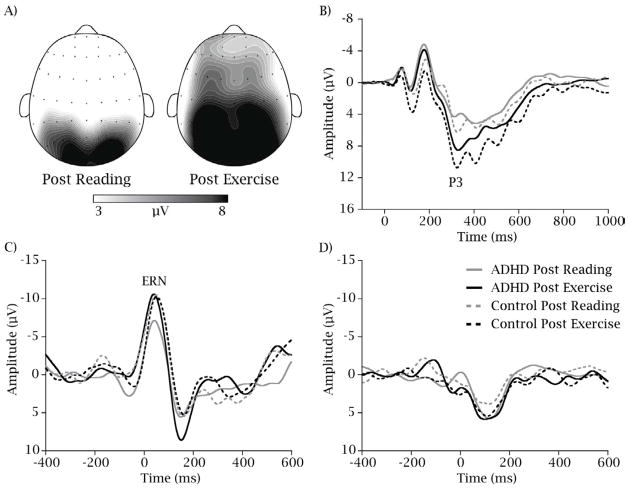
Figure 2 illustrates the topographic distribution of P3 amplitude across the scalp and provides grand-average stimulus and response-locked ERP waveforms for each group and session.
Figure 2.
Topographic-plots of P3 amplitude collapsed across group (A), the stimulus-locked grand-average waveform from the PZ electrode site (B), the response-locked grand average waveform for error (C), and match-correct trials (D) for each group (solid lines indicate ADHD group) and session (black lines indicate Post Exercise) with all graphs collapsed across compatibility and congruency conditions.
P3
Analysis of the P3 component revealed that children with ADHD exhibited smaller P3 amplitude (7.8 ± 0.6 μV) relative to the Healthy Match-Control group (10.1 ± 0.6 μV), only for the incongruent trials of the flanker task, t (38) = 2.8, p = 0.009, Cohen’s d = .91. However, both children with ADHD and the Healthy Match-Control children exhibited larger P3 amplitude following the bout of exercise (10.9 ± 0.6 μV) relative to following reading (7.9 ± 0.5 μV; p = < .001, Cohen’s d = .8). Further, shorter P3 latency was also observed following exercise, relative to following reading, at the FCz, Cz, and CPz electrode sites, t’s (39) ≥ 3.1, p ≤ 0.004, Cohen d = .99.
ERN
Previous research has established that a minimum of six error-of-commission trials are necessary to obtain a stable ERN component (56). Thus, compatible and incompatible trials were collapsed after matching error and correct trials within each compatibility in order to account for potential artifacts that may exist due to differences in response latency between correct and incorrect trials (43). Remaining participants with fewer than six errors of commission were discarded from analysis of the ERN component (N = 4; 2 ADHD), leaving a total of 36 participants. No significant differences between Groups were observed in this subset of participants for demographic variables, t’s (34) ≤ 1.8, p ≥ 0.09. Accordingly, analysis of the ERN component (i.e., the error’s trials in the Group × Session × Accuracy interaction, depicted in Figure 2c) revealed that children with ADHD exhibited smaller ERN amplitude (−7.3 ± 1.1 μV) relative to the Healthy Match-Control group (−11.2 ± 1.1 μV) following the reading session, t (34) = 2.5, p = 0.017, Cohen’s d = .86. However, after the single bout of exercise no differences between the ADHD (−10.8 ± 1.0 μV) and Healthy Match-Control (−10.8 ± 1.1 μV) groups were observed, t (34) = 0.03, p = 0.98, Cohen’s d = 0.01. No main effects or interactions involving Group or Session were observed for match correct response-locked trials (i.e., the match correct trials in the Group × Session × Accuracy interaction; p ≥ 0.21, depicted in Figure 2d).
Academic Performance
Analysis revealed that both children with ADHD and Healthy Match-Control children exhibited enhanced performance following exercise on tests of reading comprehension (115.2 ± 2.2) and arithmetic (112.5 ± 2.7) relative to following the seated reading condition (reading comprehension: 110.1 ± 1.8, p < .001, Cohen’s d = 1.58; arithmetic: 110.0 ± 3.1, p = .03, Cohen’s d = 1.25; see Figure 1). No main effects or interactions involving Group or Session were observed for spelling (p ≥ 0.15).
Discussion
This investigation provides initial evidence suggesting that single bouts of moderate-intensity aerobic exercise may be a tool in the non-pharmaceutical treatment of children with ADHD. That is, utilizing objective measures to assess the effect of exercise on aspects of cognition, these findings suggest that both children with ADHD and healthy match-control children exhibit overall enhancements in inhibitory control and the allocation of attentional resources, coupled with a selective enhancement in stimulus classification and processing speed, following a single 20 minute bout of moderate-intensity aerobic exercise. Further, acute exercise appears to have added benefit to children with ADHD who exhibited exercise-induced facilitations in action monitoring processes (i.e., ERN amplitude) and regulatory adjustments in behavior (i.e., post-error slowing). These acute exercise-induced enhancements in response production and neurocognitive function may also have relevance for maximizing scholastic performance in all children, as evidenced by the exercise-induced improvements in the areas of reading comprehension and arithmetic – subjects which have been found to depend heavily upon the successful inhibition of unrelated information (57,58).
Interestingly, these findings provide partial support for the hypoarousal model of ADHD (59), which suggests that cognitive and attentional deficits related to ADHD may arise as a result of underarousal of the central nervous system (CNS). However, more recent research has suggested that these deficits are not necessarily a result of deficient arousal, but instead occur due to insufficient task-related activation (60). Consistent with this assertion, findings from the present investigation revealed that children with ADHD exhibited smaller P3 amplitude following reading, relative to healthy match-control children, only in response to the incongruent trials – replicating previous research, which has observed ADHD-related deficits in the allocation of attentional resources for task conditions requiring the greatest amount of inhibitory control (9). Accordingly, the finding that a single bout of physical activity served to generally enhance the allocation of attentional resources towards stimulus engagement suggests that such an intervention may act to reduce the core deficit associated with ADHD proposed by the hypoarousal model of ADHD. Although pharmacological treatments have largely been found effective in the short-term management of ADHD (17), an estimated 30 to 50% of all clinically diagnosed cases persist into adulthood (61). Given that ADHD represents one of the most prevalent childhood disorders in the United States (1–3); a growing push has been towards non-pharmacological treatment strategies to reduce the potential effects of, and costs associated with, long-term psycho-stimulant use (18). These novel findings suggesting that single bouts of exercise may be an effective aid in the treatment of ADHD, are both relevant, and timely to this growing movement and are consistent with the aims of recently released guidelines for the treatment of ADHD (19).
Further, although speculative, given that changes in cognition associated with chronic physical activity participation may be progressively accrued through repeated bouts of acute exercise; such a treatment tool may also serve to create more long-term changes in inhibitory control. That is, individuals with greater chronic physical activity participation and aerobic fitness levels have been found to exhibit increased tissue volume in the basal ganglia (62) and hippocampus (63) as well as a greater ability to recruit neural resources in the frontal and parietal regions (64); in addition to functional enhancements in neural processes related to the allocation of attentional resources (37,65), and greater integrity of action monitoring processes (37). Given that these neural structures and processes mirror those which exhibit deficits in children with ADHD (8,10–13,15,16,66–75), over the course of repeated bouts of acute aerobic exercise, these neuronal structures and functions may be sufficiently enhanced to more enduringly alter, in part, the underlying etiology of ADHD (20). Consonant with this assertion, an initial investigation into the effects of a chronic 10-week physical activity program in 10 children with ADHD observed enhancements in information processing, visual search, and sustained attention relative to a similarly-sized control group (76). However, further research is necessary to better understand how chronic physical activity participation may serve to influence inhibitory control processes in children with ADHD.
Research is still necessary to understand the specific components of exercise that optimize its influence on cognition and how other factors (i.e., age, personality, nutrition) may relate to changes in cognition associated with acute exercise. Further, given that the ADHD group used within the present investigation represents a subpopulation of children experiencing less severe symptoms of ADHD, the extent to which the effects observed within the current investigation generalize to children with more severe cases of ADHD, those with comorbid conditions, or children undergoing pharmacological treatment is still unknown. Thus, future research will need to investigate these factors further to better understand the utility of acute exercise in enhancing inhibition in these populations and how acute bouts of exercise may combine and compare with other more traditional ADHD treatment strategies. However, given that approximately 44% of US children with ADHD do not undergo drug treatments (77), these findings may have clinical utility in enhancing cognitive function in children with ADHD.
It is also important to note that we do not yet have a clear understanding regarding the half-life of a single bout of exercise as limited research in this area has investigated multiple time points following an acute bout of physical activity to examine how long exercise-induced modulations persist. Within the small body of existent research, conflicting findings have emerged with some findings suggesting that acute exercise may only exhibit a short duration influence over aspects of cognitive processing speed (78), and others have observed exercise-induced enhancements in inhibition and working memory persisting for at least 60 minutes following the cessation of exercise (28,79). Clearly, one area of future research that is much needed is to better characterize the duration of the potential benefits for cognition incurred by an acute bout of physical activity, and such matters are likely complicated by the mode, intensity, and duration of the exercise bout, as well as individual difference factors, and the sensitivity of the measure of cognitive function utilized.
Given that previous research has observed that children with ADHD are less likely to participate in vigorous physical activity and organized sports relative to children without ADHD (80), the current findings suggest that motivating children with ADHD to be physically active may have positive implications for aspects of neurocognitive function and inhibitory control. These findings, which observed positive effects in children with and without ADHD, provides additional support for recommendations by the National Association for Sport and Physical Education that short bouts of exercise be incorporated during the school day, as part of a comprehensive school-based physical activity program (81).
Acknowledgments
Supported by the National Institute of Child Health and Human Development (NICHD; RO1 HD055352 to C.H.) and the NICHD Developmental Psychobiology and Neurobiology Training Grant at the University of Illinois (2 T32 HD007333 to M.P.).
Abbreviations
- ADHD
attention-deficit/hyperactivity disorder
- BMI
body mass index
- WRAT3
Wide Range Achievement Test 3rd edition
- IQ
intelligence quotient, SES, socioeconomic status
- ERPs
event-related brain potentials
- ERN
error-related negativity
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Biederman J. Attention-deficit/hyperactivity disorder: A life-span perspective. J Clin Psychiatry. 1998;59:4–16. [PubMed] [Google Scholar]
- 3.Wolraich ML, Hannah JN, Baumgaertel A, Feurer ID. Examination of DSM-IV criteria for attention deficit hyperactivity disorder in a county-wide sample. J Dev Behav Pediatr. 1998;19:162–168. doi: 10.1097/00004703-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Banaschewski T, Ruppert S, Tannock R, Albrecht B, Becker A, Uebel H, et al. Colour perception in ADHD. J Child Psychol Psychiatry. 2006;47:568–572. doi: 10.1111/j.1469-7610.2005.01540.x. [DOI] [PubMed] [Google Scholar]
- 5.Schachar R, Mota VL, Logan GD, Tannock R, Klim P. Confirmation of an inhibitory control deficit in attention-deficit/hyperactivity disorder. J Abnorm Child Psychol. 2000;28:227–235. doi: 10.1023/a:1005140103162. [DOI] [PubMed] [Google Scholar]
- 6.Barkley RA. Behavioral inhibition, sustained attention, and executive functions. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 7.Albrecht B, Brandeis D, Uebel H, Heinrich H, Mueller UC, Hasselhorn M, et al. Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: Evidence for an endophenotype. Biol Psychiatry. 2008;64:615–625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry RJ, Johnstone SJ, Clarke AR. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neuropsychol. 2003;114:184–198. doi: 10.1016/s1388-2457(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 9.Jonkman LM, Kemner C, Verbaten MN, Van Engeland H, Kenemans JL, Camfferman G, et al. Perceptual and response interference in children with attention-deficit hyperactivity disorder, and the effects of methylphenidate. Psychophys. 1999;36:419–429. [PubMed] [Google Scholar]
- 10.Jonkman LM, Kemner C, Verbaten MN, Van Engeland H, Camfferman G, Buitelaar JK, et al. Attentional capacity, a probe ERP study: Differences between children with attentional-deficit hyperactivity disorder and normal control children and effects of methylphenidate. Psychophys. 2000;37:334–346. [PubMed] [Google Scholar]
- 11.Kemner C, Verbaten MN, Koelega HS, Buitelaar JK, van der Gaag R, Camfferman G, et al. Event-related brain potentials in children with attention-deficit and hyperactivity disorder: Effects of stimulus deviancy and task relevance in the visual and auditory modality. Biol Psychiatry. 1996;40:522–534. doi: 10.1016/0006-3223(95)00429-7. [DOI] [PubMed] [Google Scholar]
- 12.Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- 13.Liotti M, Pliszka SR, Perez R, Luus B, Glahn D, Semrud-Clikeman M. Electrophysiological correlates of response inhibition in children and adolescents with ADHD: Influence of gender, age, and previous treatment history. Psychophys. 2007;44:936–948. doi: 10.1111/j.1469-8986.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 14.Loiselle DL, Stamm JS, Maitinsky S, Whipple SC. Evoked potential and behavioral signs of attentive dysfunctions in hyperactive boys. Psychophys. 1980;17:193–201. doi: 10.1111/j.1469-8986.1980.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 15.van Meel CS, Heslenfeld DJ, Oosterlan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): The role of error processing. Psychiatry Res. 2007;151:211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Wiersema R, van der Meere J, Roeyers H, Coster RV, Baeyens D. Event rate and event-related potentials in ADHD. J Child Psychol Psychiatry. 2006;47:560–567. doi: 10.1111/j.1469-7610.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- 17.Solanto MV, Arnsten AT, Castellanos FX. Stimulant drugs and ADHD: Basic and clinical neuroscience. New York, NY: Oxford University Press; 2001. [Google Scholar]
- 18.Wilson LJ, Jennings JN. Parents’ acceptability of alternative treatments for attention-deficit hyperactivity disorder. J Atten Disord. 1996;1:114–121. [Google Scholar]
- 19.American Academy of Pediatrics. ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gapin JI, Labban JD, Etnier JL. The effects of physical activity on attention deficit hyperactivity disorder symptoms: The evidence. Prev Med. 2011;52:S70–S74. doi: 10.1016/j.ypmed.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Panksepp J. Can PLAY diminish ADHD and facilitate the construction of the social brain. J Am Acad Child Adolesc Psychiatry. 2007;16:57–66. [PMC free article] [PubMed] [Google Scholar]
- 22.Tantillo M, Kesick CM, Hynd GW, Dishman RK. The effects of exercise on children with attention-deficit hyperactivity disorder. Med Sci Sports Exer. 2002;34:203–212. doi: 10.1097/00005768-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Medina JA, Netto TLB, Muszkat M, Medina AC, Botter D, Orbetelli R, et al. Exercise impact on sustained attention of ADHD children, methylphenidate effects. ADHD Atten Deficit Hyper Dis. 2010;2:49–58. doi: 10.1007/s12402-009-0018-y. [DOI] [PubMed] [Google Scholar]
- 24.Caterino MC, Polack ED. Effects of two types of activity on the performance of second-, third-, and fourth-grade students on a test of concentration. Percept Mot Skills. 1999;89:245–248. doi: 10.2466/pms.1999.89.1.245. [DOI] [PubMed] [Google Scholar]
- 25.Mahar MT, Murphy SK, Rowe DA, Golden J, Shields AT, Raedeke TD. Effects of a classroom-based program on physical activity and on-task behavior. Med Sci Sports Exer. 2006;38:2086–2094. doi: 10.1249/01.mss.0000235359.16685.a3. [DOI] [PubMed] [Google Scholar]
- 26.McNaughten D, Gabbard C. Physical exertion and the immediate mental performance of sixth-grade children. Percept Mot Skills. 1993;77:1155–1159. doi: 10.2466/pms.1993.77.3f.1155. [DOI] [PubMed] [Google Scholar]
- 27.Gabbard C, Barton J. Effects of physical activity on mathematical computation among young children. J Psychol. 1979;103:287–288. [Google Scholar]
- 28.Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neurosci. 2009;159:1044–1054. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillman CH, Pontifex MB, Themanson JR. Acute aerobic exercise effects on event-related brain potentials. Exercise and Cognitive Function. 2009;8:161–178. [Google Scholar]
- 30.Kamijo K, Nishihira Y, Hatta A, Kaneda T, Wasaka T, Kida T, et al. Differential influences of exercise intensity on information processing in the central nervous system. Eur J Appl Physiol. 2004;92:305–311. doi: 10.1007/s00421-004-1097-2. [DOI] [PubMed] [Google Scholar]
- 31.Kamijo K, Nishihira Y, Higashiura T, Kuroiwa K. The interactive effect of exercise intensity and task difficulty on human cognitive processing. Int J Psychophys. 2007;65:114–121. doi: 10.1016/j.ijpsycho.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Kamijo K, Hayashi Y, Sakai T, Yahiro T, Tanaka K, Nishihira Y. Acute effects of aerobic exercise on cognitive function in older adults. J Geront, Series B: Psychol Sci. 2009;64:356–363. doi: 10.1093/geronb/gbp030. [DOI] [PubMed] [Google Scholar]
- 33.DuPaul GT, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale - IV: Checklists, norms, and clinical interpretation. New York, NY: The Guilford Press; 1998. [Google Scholar]
- 34.Rutter M, Bailey A, Lord C. The social communication questionnaire manual. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 35.Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 36.Eriksen CW, Eriksen BA. Effects of noise letters upon the identification of a target letter in a non-search task. Percept Psychophys. 1974;25:249–263. [Google Scholar]
- 37.Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, et al. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J Cogn Neurosci. 2011;23:1332–1345. doi: 10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- 38.Epstein JN, Brinkman WB, Froehlich T, Langberg JM, Narad ME, Antonini TN, et al. Effects of stimulant medication, incentives, and event rate on reaction time variability in children with ADHD. Neuropsychopharm. 2011;36:1060–1072. doi: 10.1038/npp.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, et al. Response variability in attention deficit hyperactivity disorder: Evidence for neuropsychological heterogeneity. Neuropsycholog. 2007;45:630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 40.Mezzacappa E. Alerting, orienting, and executive attention: Developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Dev. 2004;75:1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- 41.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psych Sci. 1993;4:385–390. [Google Scholar]
- 42.Kerns JG, Cohen JD, MacDonald AWI, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 43.Mathewson KJ, Dywan J, Segalowitz SJ. Brain bases of error-related ERPs as influenced by age and task. Biol Psychol. 2005;70:88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Coles MGH, Gratton G, Fabiani M. Event-related potentials. In: Cacioppo JT, Tassinary LG, editors. Principles of psychophysiology: Physical, social, and inferential elements. New York, NY: Cambridge University Press; 1990. pp. 413–455. [Google Scholar]
- 45.Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ilan AB, Polich J. P300 and resposne time from a manual Stroop task. Clin Neurophysiol. 1999;110:367–373. doi: 10.1016/s0168-5597(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 47.Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson GS. Wide range achievement test 3: Administration manual. Wilminton, DE: Jastak Associates; 1993. [Google Scholar]
- 49.Kaufman AS, Kaufman NL. Kaufman brief intelligence test manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- 50.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q) Canadian J Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- 51.Taylor SC, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Coock DG. Performance of a new pubertal self-assessment questinnaire: A preliminary study. Paediatr Perinat Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 52.Erford BT. Disruptive behavior rating scale parent/treacher manual. East Aurora, NY: Slosson Educational Publications, Inc; 1993. [Google Scholar]
- 53.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulated cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 54.Dehaene S, Posner MI, Tucker DM. Psychol Sci. 1994. Localization of a neural system for error detection and compensation; pp. 303–305. [Google Scholar]
- 55.Miltner WR, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MGH. Implementation of error-processing in the human anterior cingulated cortex: A source analysis of the magnetic equivalent of the error-related negativity. Biol Psychol. 2003;64:157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 56.Pontifex MB, Scudder MR, Brown M, O’Leary KC, Wu C, Themanson JR, et al. On the number of trials necessary for stabilization of error-related brain activity across the lifespan. Psychophys. 2010;47:767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- 57.Bull R, Scerif G. Executive functioning as a predictor of children’s mathematics ability: Inhibition, switching, and working memory. Dev Neuropsychol. 2001;19:273–293. doi: 10.1207/S15326942DN1903_3. [DOI] [PubMed] [Google Scholar]
- 58.St Clair-Thompson HL, Gathercole SE. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Q J Exp Psychol (Hove) 2006;59:745–759. doi: 10.1080/17470210500162854. [DOI] [PubMed] [Google Scholar]
- 59.Satterfield JH, Cantwell DP. Proceedings: CNS function and response to methylphenidate in hyperactive children. Psychopharm Bull. 1974;10:36–38. [PubMed] [Google Scholar]
- 60.Barry RJ, Clarke AR, Johnstone SJ, McCarthy R, Selikowitz M. Electroencephalogram Theta/Beta ratio and arousal in attention-deficit/hyperactivity disorder: Evidence of independent processes. Biol Psychiatry. 2009;66:398–401. doi: 10.1016/j.biopsych.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 61.Bracken BA, Boatwright BS. Clinical assessment of attention deficit-child/adult professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2005. [Google Scholar]
- 62.Chaddock L, Erickson KI, Prakash RS, VanPatter M, Voss MW, Pontifex MB, et al. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev Neuro. 2010;32:249–256. doi: 10.1159/000316648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, VanPatter M, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaddock L, Erickson KI, Prakash RS, Voss MW, VanPatter M, Pontifex MB, et al. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biol Psychol. 2012;89:260–268. doi: 10.1016/j.biopsycho.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 65.Hillman CH, Buck SM, Themanson JR, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Dev Psychol. 2009;45:114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- 66.Aylward EH, Reiss AL, Reader MJ, Singer HS, Brown JE, Denckla MB. Basal ganglia volumes in children with attention-deficit hyperactivity disorder. J Child Neurol. 1996;11:112–115. doi: 10.1177/088307389601100210. [DOI] [PubMed] [Google Scholar]
- 67.Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, et al. Quantitative brain magnetic resonance imaging in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 68.Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, et al. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 69.Dimoska A, Johnstone SJ, Barry RJ, Clarke AR. Inhibitory motor control in children with attention-deficit/hyperactivity disorder: Event-related potentials in the stop-signal paradigm. Biol Psychiatry. 2003;54:1345–1354. doi: 10.1016/s0006-3223(03)00703-0. [DOI] [PubMed] [Google Scholar]
- 70.Giedd JN, Castellanos FX, Casey BJ, Kozuch P, King AC, Hamberger SD, et al. Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:665–669. doi: 10.1176/ajp.151.5.665. [DOI] [PubMed] [Google Scholar]
- 71.Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D, Lyytinen H. Corpus callosum morphology in attention deficit-hyperactivity disorder: Morphometric analysis of MRI. J Learn Disabil. 1991;24:141–146. doi: 10.1177/002221949102400302. [DOI] [PubMed] [Google Scholar]
- 72.Johnstone SJ, Barry RJ. Auditory event-related potentials to a two-tone discrimination paradigm in attention deficit hyperactivity disorder. Psychiatry Res. 1996;64:179–192. doi: 10.1016/s0165-1781(96)02893-4. [DOI] [PubMed] [Google Scholar]
- 73.Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: Event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biol Psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- 74.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higer-motor control: A study with functional mri. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 75.Yeo RA, Hill DE, Campbell RA, Vigil J, Petropoulos H, Hart B, et al. Proton magnetic resonance spectroscopy investigation of the right frontal lobe in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42:303–310. doi: 10.1097/00004583-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 76.Verret C, Guay MC, Berthiaume C, Gardiner P, Béliveau L. A physical activity program improves behavior and cognitive functions in children with ADHD: An exploratory study. J Atten Disord. 2012;16:71–80. doi: 10.1177/1087054710379735. [DOI] [PubMed] [Google Scholar]
- 77.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 78.Barella LA, Etnier JL, Chang YK. The immediate and delayed effects of an acute bout of exercise on cognitive performance in healthy older adults. J Aging Phys Activity. 2010;18:87–98. doi: 10.1123/japa.18.1.87. [DOI] [PubMed] [Google Scholar]
- 79.Pontifex MB, Hillman CH, Fernhall B, Thompson KM, Valentini TA. The effect of acute aerobic and resistance exercise on working memory. Med Sci Sport Exer. 2009;41:927–934. doi: 10.1249/MSS.0b013e3181907d69. [DOI] [PubMed] [Google Scholar]
- 80.Kim J, Mutyala B, Agiovlasitis S, Fernhall B. Health behaviors and obesity among US children with attention deficit hyperactivity disorder by gender and medication use. Prev Med. 2011;52:218–222. doi: 10.1016/j.ypmed.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 81.National Association for Sport and Physical Education. Comprehensive school physical activity programs [Position statement] Reston, VA: 2008. [Google Scholar]
- 82.Bussing R, Zima BT, Gary FA, Garvan CW. Barriers to detection, help-seeking, and service use for children with ADHD symptoms. J Behav Health Serv Res. 2003;30:176–189. doi: 10.1007/BF02289806. [DOI] [PubMed] [Google Scholar]
- 83.Jenson PS, Martin D, Cantwell DP. Comorbidity in ADHD: Implications for research, practice, and DSM-V. J Am Acad Child Adolesc Psychiatry. 1997;36:1065–1079. doi: 10.1097/00004583-199708000-00014. [DOI] [PubMed] [Google Scholar]