Abstract
Objective
Endothelial cell (EC) inflammatory status is critical to many vascular diseases. Emerging data demonstrate that mutations of Krüppel like factor-11 (KLF11), a gene coding maturity-onset diabetes of the young type 7 (MODY7), contribute to the development of neonatal diabetes. However, the function of KLF11 in the cardiovascular system still remains to be uncovered. In this study, we aimed to investigate the role of KLF11 in vascular endothelial inflammation.
Methods and results
KLF11 is highly expressed in vascular endothelial cells (ECs) and induced by pro-inflammatory stimuli. Adenovirus-mediated KLF11 overexpression inhibits expression of tumor necrosis factors (TNF)-α-induced adhesion molecules. Moreover, siRNA-mediated KLF11 knockdown augments the pro-inflammatory status in ECs. KLF11 inhibits promoter activity of adhesion molecules induced by TNF-α and NF-κB p65 overexpression. Mechanistically, KLF11 potently inhibits NF-κB signaling pathway via physical interaction with p65. Furthermore, KLF11 knockdown results in increased binding of p65 to VCAM-1 and E-selectin promoters. At the whole organism level, KLF11-/- mice exhibit a significant increase in leukocyte recruitment to ECs after lipopolysaccharide (LPS) administration.
Conclusions
Taken together, our data demonstrate for the first time that KLF11 is a suppressor of EC inflammatory activation suggesting that KLF11constitutes a novel potential molecular target for inhibition of vascular inflammatory diseases.
Keywords: KLF11, endothelial cell, inflammation, atherosclerosis, NF-κB and adhesion molecules
Introduction
Endothelial cells are critical to maintain vascular wall structure and function, but sustained inflammatory status of endothelial cells leads to the onset of many inflammatory vascular diseases such as atherosclerosis and thrombosis. Pro-inflammatory factors such as tumor necrosis factor (TNF)-α, interleukin-1β (IL-1β) and oxidized low density lipoprotein (ox-LDL)1-3 trigger endothelial activation, which is characterized by abrupt increase in adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1) and E-selectin. Robust increase in these adhesion molecules and the ensuing recruitment of leukocytes to endothelial cells represent a hallmark of the early stage of atherosclerosis.4
KLFs, Sp1-like zinc finger transcription factors, are highly conserved in organisms ranging from flies to human, and are characterized by the presence of a DNA binding domain with three highly conserved zinc finger motifs as well as a variant carboxyl-terminal end.5 The KLFs are involved in the regulation of cell growth and differentiation in several tissues including those from the cardiovascular system. In particular, KLF2, KLF4, KLF5, and KLF6 have been implicated in developmental as well as pathological vascular processes.6 KLF11, one of the best-studied members of KLF family, is highly expressed in the pancreas and muscle.7 Upon induction, KLF11 binds to Sp1-like DNA sequences on target promoters to which, in turn, it recruits distinct chromatin remodeling and epigenetic factors including HATs, HDACs, and HMTs.7-9 For instance, mutation at the -331 site of the insulin gene promoter, which is causal of neonatal diabetes, disrupts the ability of KLF11 to bind and transcriptionally activate insulin biosynthesis.10 In addition, the KLF11-p300 pathway trans-regulates pancreatic-duodenal homeobox-1 gene (Pdx-1), a gene causing maturity onset diabetes of the young (MODY IV), by binding to consensus binding sites within its promoter.11 Furthermore, alterations in the coupling of KLF11 to HDACs due to variations in the sequence of the KLF11 promoter and protein have been found in selected human populations affected by type 2 diabetes mellitus (T2DM).12-15 Noteworthy, in spite of its strong-association with diabetes, the role of KLF11 in inflammatory responses that affect the cardiovascular system remains to be addressed. Consequently, the current study sought to define whether KLF11 influences the EC response to pro-inflammatory stimuli. We identified that KLF11 functions as a novel transcription factor in vascular endothelial cells (ECs) that potently inhibit pro-inflammatory adhesion molecules, which are crucial for the initiation and maintenance of vascular inflammation, a hallmark in many human diseases including atherosclerosis and diabetes. KLF11 interacts with NF-κB p65 to attenuate the effect of TNF-α on VCAM-1 and E-selectin expression. Analysis in vivo using genetically engineered KLF11-/- mice revealed an inhibitory role of KLF11 in leukocyte recruitment to ECs after lipopolysaccharide (LPS) administration. Thus, this study describes for the first time the role of KLF11 in modulating vascular inflammation and characterizes new molecular mechanisms underlying this function.
Methods and Materials
A detailed description is provided in the online-only Data Supplement (available online at http://atvb.ahajournals.org).
siRNA-mediated Gene Knockdown
HUVECs were transfected with siRNA-KLF11, siRNA-KLF2 or siRNA-KLF4 (Ambion Inc.) at about 50-60% confluence using Lipofectamine™ RNAiMAX Reagent (Invitrogen).16
Statistical Analysis
Statistical analysis between two groups was performed by two-tailed unpaired Student's t test and among three groups or more was performed by one-way ANOVA followed by Newman-Keuls test. A P value of <0.05 was considered statistically significant. Data are presented as mean ± SEM.
Results
KLF11 is an inducible transcription factor in response to pro-inflammatory stimuli in ECs
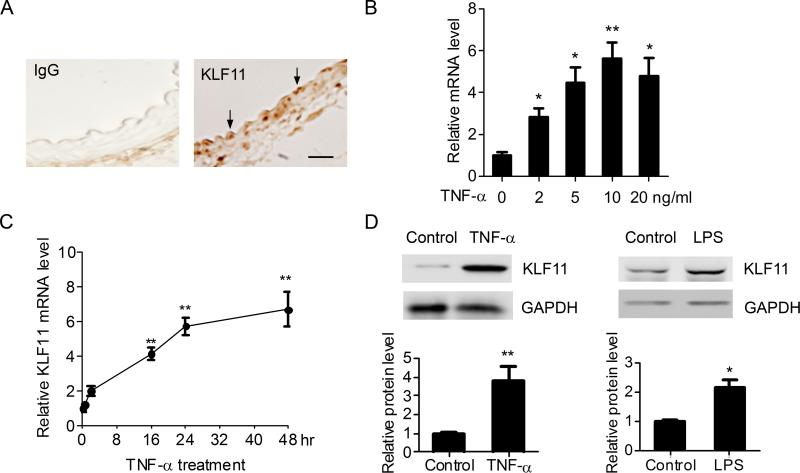
To assess expression of KLF11 in the vascular system, we first performed immunostaining on mouse aortas. The result of this analysis, as shown in Figure 1A, reveals that KLF11 is expressed in the normal artery with a particular enrichment in vascular ECs. The levels of KLF11 in human umbilical vein endothelial cells (HUVECs) were comparable to those for KLF2 and KLF4, which are well-characterized regulators of vascular ECs function (Supplemental Figure I).5, 17 This information led us to subsequently assess whether the expression of KLF11 is changed by pro-inflammatory stimuli in vascular ECs. Indeed, TNF-α upregulates the expression of KLF11 in a dose-dependent manner in HUVECs. TNF-α could significantly upregulate KLF11 mRNA at a dosage of 2ng/ml, and TNF-α (10ng/ml) increases KLF11 expression up to 6.05 ± 0.77 fold (versus control, p < 0.01, Figure 1B). Time course experiments show that the effect of TNF-α on KLF11 mRNA is maintained for at least 48 hours of exposure (Fig.1C). In agreement with these results, the KLF11 protein level is increased by 4.5 ± 0.61 fold in the TNF-α (10ng/ml)-stimulated HUVECs (Figure 1D). Moreover, in addition to TNF-α other pro-inflammatory stimuli such as LPS (10ng/ml) can activate KLF11 expression in HUVECs by 2.14 ± 0.25 fold (Figure 1D). Together, these experiments demonstrate that KLF11 is expressed in vascular ECs and is stimulated in response to well-characterized pro-inflammatory stimuli suggesting that KLF11 may modulate the inflammatory response in ECs.
Figure 1. The expression of KLF11 is increased in response to pro-inflammatory stimuli.
A, The expression of KLF11 in aortas from C57BL/6 mice was determined by immunostaining, and IgG served as negative control. Scale bar represents 20μm. Arrows indicate the endothelial cell layer. B-C, HUVECs (about 95% confluent) were treated with TNF-α at the indicated doses for 24 hours (B) or stimulated with TNF-α (10ng/ml) for the indicated time points (C). The expression of KLF11 was determined by RT-qPCR. D, Representative western blots from HUVECs treated with TNF-α (10ng/ml) (left panel) or LPS (10ng/ml) for 24 hours (right panel) showing the relative KLF11 protein levels. The band density was quantitatively analyzed and normalized against the internal control, GAPDH. Data are from three independent experiments and presented as mean ± SEM. * p < 0.05; ** p < 0.01.
Overexpression of KLF11 inhibits endothelial activation
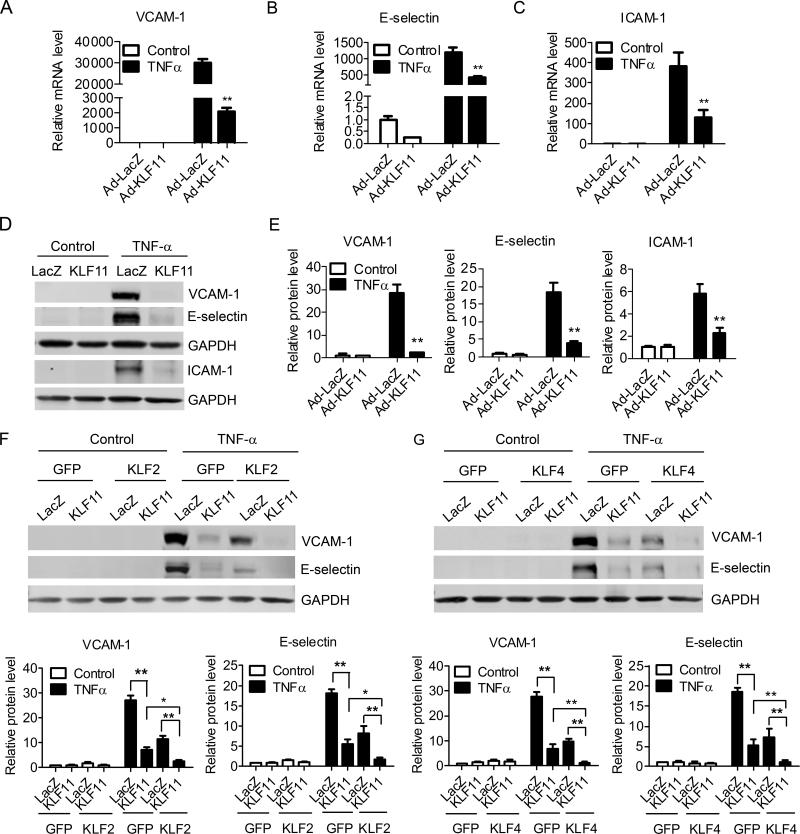
An important surrogate for EC activation by inflammatory chemokines is the expression of pro-inflammatory adhesion molecules. Thus, we analyzed the effect of KLF11 on the expression of adhesion molecules using HUVECs treated with TNF-α. Our data suggests that adenovirus-mediated overexpression of KLF11 dose-dependently inhibits the TNF-α-induced expression of adhesion molecules VCAM-1 , E-selectin and ICAM-1. KLF11 significantly inhibits EC inflammation at an expression level as low as about 2.5 fold (5 MOI) compared to basal level (Supplemental Figure II). At a 20 MOI, Ad-KLF11 inhibits the TNF-α-induced expression of the adhesion molecules VCAM-1, E-selectin, ICAM-1 by 92 ± 0.82%, 65 ± 3.3% and 70.6 ± 5.4%, respectively (Figure 2A-2C). KLF11 mediates a concomitant inhibition of other inflammatory mediators such as MCP-1 and IL-8 (Supplemental Figure IIIA). Consistent with these results, Figures 2D-2E and Supplemental Figure II show that in the presence of TNF-α (2ng/ml), KLF11 downregulates the protein levels of VCAM-1, E-selectin and ICAM-1 by 92 ± 0.35%, 78.5 ± 2.3% and 62% ± 6.2, respectively. Similar significant inhibitory effects of KLF11 on VCAM-1 and E-selectin were observed upon the treatment of EC with other inflammatory stimuli such as ox-LDL (40μg/ml) (Supplemental Figure IIIB) and in the presence of LPS (10ng/ml), a key microbial component involved in the initiation of the sepsis syndrome and EC activation18 (Supplemental Figure IIIC). Altogether, our data indicate that KLF11 behaves as an inhibitor of EC activation in response to a variety of the best characterized pro-inflammatory stimuli. KLF2 and KLF4 are well known to inhibit inflammation in ECs.19, 20 We sought to determine whether KLF11 inhibits pro-inflammatory adhesion molecules in a KLF2 or KLF4-dependent manner. First, we performed siRNA-mediated downregulation of KLF2 and KLF4 (Supplemental Figure IV) in combination with KLF11 overexpression. The expression of VCAM-1 is significantly upregulated by knockdown of KLF2 (1.9 ± 0.24 fold) or KLF4 (1.82 ± 0.04 fold), consistent with prior reports.20, 21 However, KLF11 overexpression results in an inhibitory effect on VCAM-1 expression in the absence of either KLF2 or KLF4 that is not significantly different to that in control ECs (Supplemental Figure IVB). Most interestingly, although KLF11 overexpression does not affect endogenous levels of either KLF2 or KLF4 (Supplemental Figure VA), we found a synergistic inhibitory effect between KLF11 and KLF2 or KLF4 on EC inflammation. Combination of KLF11 and KLF2 or KLF4 adenoviral-mediated overexpression inhibits EC pro-inflammatory adhesion molecules to an extreme low level at both mRNA (Supplemental Figure VI) and protein levels (Figures 2F and 2G). Taken together, these data indicate that the significant KLF11 inhibition of EC inflammation does not operate indirectly via KLF2 or KLF4.
Figure 2. KLF11 potently inhibits EC inflammatory response.
About 95% confluent HUVECs were infected with Ad-LacZ or Ad-KLF11 (20 MOI). Forty-eight hours post-infection, cells were stimulated with TNF-α (2ng/ml) for 4 hours. The expression of VCAM-1, E-selectin, and ICAM-1 was determined by RT-qPCR (A-C) and Western blot (D). Band density of Western blots was quantitatively analyzed and normalized against GAPDH (E). F-G, HUVECs were infected with Ad-LacZ or Ad-KLF11 plus Ad-KLF2 or Ad-KLF4 (10 MOI/adenovirus). Forty-eight hours post-infection, cells were stimulated with TNF-α (2ng/ml) for 4 hours. The expression of VCAM-1 and E-selectin was determined by Western blot. Band density of Western blots was quantitatively analyzed and normalized against GAPDH. Data are from three independent experiments and presented as mean ± SEM. * p < 0.05; ** p < 0.01.
KLF11 knockdown exacerbates the inflammatory response in ECs
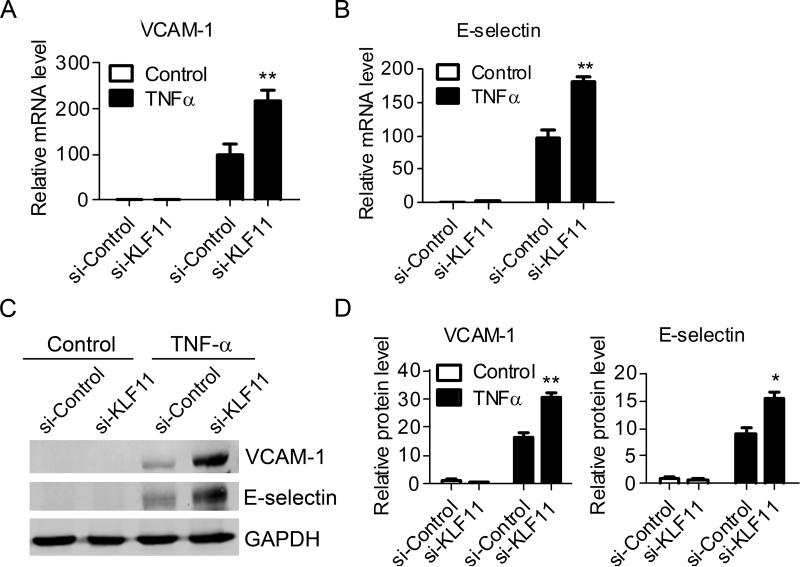
To further characterize the effect of KLF11 on EC inflammation, we also applied siRNA technology to knock down the expression of KLF11. The specific siRNA against KLF11 results in about 80% knockdown in the expression of KLF11 at both mRNA and protein levels (Supplemental Figure VII) without any significant effects on the expression of endogenous KLF2 and KLF4 (Supplemental Figure VB). The knockdown of KLF11 increases the TNF-α-induced expression of VCAM-1 and E-selectin by 2.2 ± 0.21 fold, 1.88 ± 0.11 fold, respectively at the mRNA level (Figures 3A and 3B) and 1.86 ± 0.1 fold and 1.68 ± 0.15 fold at the protein level (Figures 3C and 3D). These results suggest the possibility that endogenous KLF11 is required to prevent excessive up-regulation of adhesion molecules, a hallmark of endothelial cell activation, in response to the pro-inflammatory stimuli.
Figure 3. KLF11 knockdown aggravates EC inflammation.
HUVECs were transfected with siRNA-control or siRNA-KLF11 (40nM) for 72 hours and then stimulated with TNF-α (2ng/ml) for 4 hours. The expression of VCAM-1 and E-selectin was determined by RT-qPCR (A-B) and Western blot (C). Band density of Western blots was quantitatively analyzed and normalized against GAPDH (D). Data are from three independent experiments and presented as mean ± SEM. * p < 0.05; ** p < 0.01.
Increase in KLF11 inhibits the TNF-α-induced promoter activity of adhesion molecules
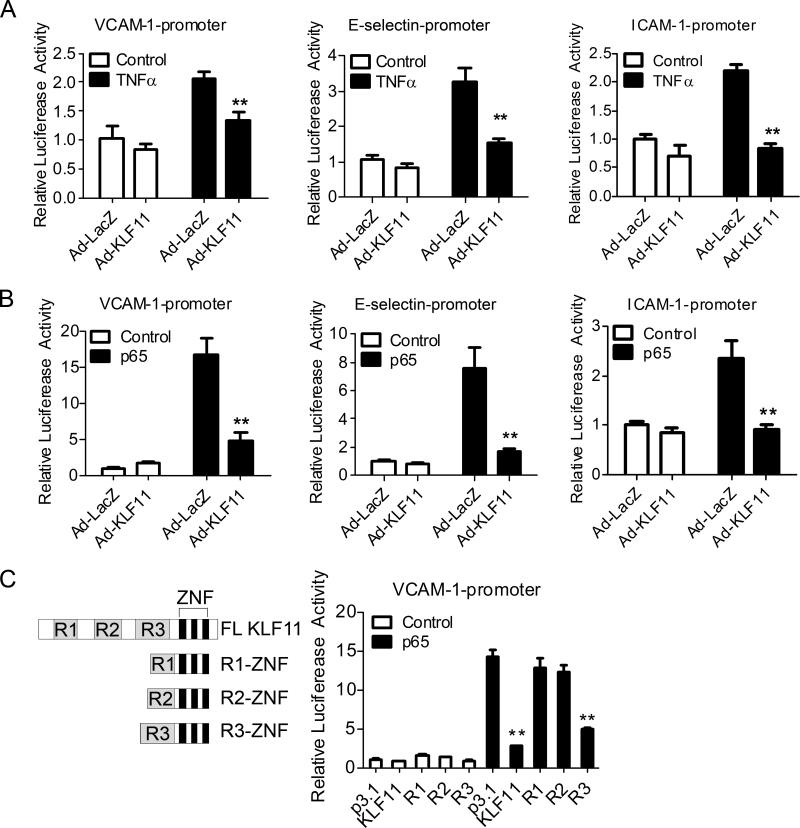
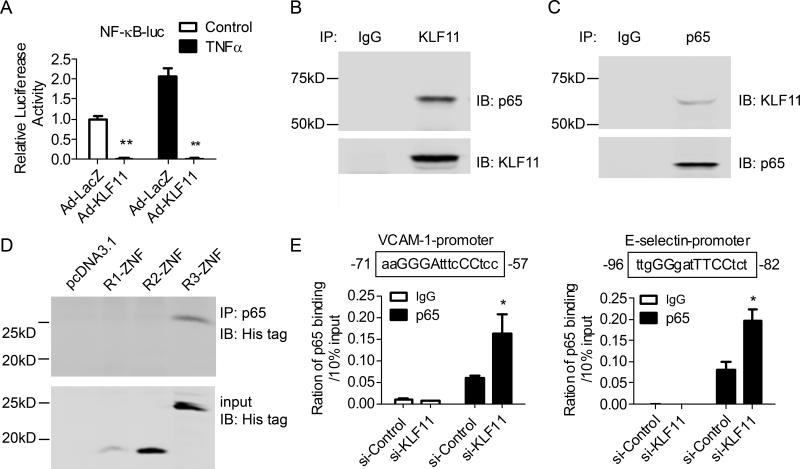
To further investigate the effect of KLF11 on the transcriptional regulation of adhesion molecules by inflammatory chemokines, we used adenoviral-mediated delivery of KLF11 in ECs to modulate the response of the VCAM-1, E-selectin and ICAM-1 reporter constructs to TNF-α. While TNF-α induces the activity of these reporters in bovine aortic endothelial cells (BAECs), KLF11 acts as a robust inhibitor of this response (Figure 4A). These results suggest that KLF11 negatively regulates VCAM-1, E-selectin and ICAM-1, at least in part, at the transcription level. Interestingly, the ability of TNF-α to stimulate these promoters is known to proceed mainly via the p65 subunit of NF-κB. In experiments depicted in Figure 4B, we demonstrate that NF-κB p65 indeed activates VCAM-1, E-selectin and ICAM-1 promoters while KLF11 antagonizes this effect. KLFs regulate gene expression by concomitantly binding to DNA and deploying its gene silencing effect through three well-characterized transcriptional repression domains (R1, R2, and R3).22, 23 We defined their relative contribution to the repression of the reporters by cotransfection of constructs encoding each of these domains with the VCAM-1 reporter in AD-293 cells. Our results show that whereas KLF11 R3-ZNF (R3) inhibits p65-induced VCAM-1 promoter activity as effective as full length (FL) KLF11, neither the R1-ZNF (R1) nor the R2-ZNF (R2) is sufficient to achieve this suppressive function (Figure 4C). Overall, this analysis reveals the ability of KLF11 to suppress the transcriptional activation of the VCAM-1 promoter in ECs via the p65 NF-κB subunit, thereby extending the mechanistic understanding of this phenomenon.
Figure 4. KLF11 inhibits the expression of pro-inflammatory adhesion molecules at the transcription level.
A, BAECs were transfected with VCAM-1-Luciferase (VCAM-1-Luc), E-selectin-Luc or ICAM-1-Luc for 8 hours, and then infected with Ad-LacZ or Ad-KLF11 (20 MOI) for 24 hours before TNF-α (2ng/ml) stimulation for 16 hours. B, BAECs were cotransfected with p65 and VCAM-1-Luc, E-selectin-Luc or ICAM-1-Luc for 8 hours, and then infected with Ad-LacZ or Ad-KLF11 (20 MOI) for 24 hours. C, AD-293 cells were cotransfected with VCAM-1-Luc, p65 and different KLF11 fragment constructs (left panel) for 24 hours. Promoter activity was detected by Firefly luciferase levels and normalized against Renilla luciferase activity. Data are from three independent experiments and presented as mean ± SEM. ** p < 0.01.
Increase in KLF11 suppresses NF-κB signaling pathway via interaction with p65
The results described above support a key role of KLF11 in the regulation of NF-κB signaling pathway in EC. Consequently, this data stimulated us to further investigate whether transcriptional regulation of adhesion molecule genes by KLF11 in response to TNF-α is limited to its repressive effect on the p65. It has been long known that pro-inflammatory cytokines such as TNF-α induce IκB-α phosphorylation and its ensuing degradation, allowing NF-κB p65/p50 translocation into the nucleus to activate the expression of adhesion molecules and pro-inflammatory cytokines.24 Our results shown in Figure 5A reveal that KLF11 inhibits the binding of NF-κB to consensus NF-κB binding sites assayed by reporter-gene activation. More remarkably, endogenous KLF11 interacts with p65 as determined by co-IP assays (Figure 5B-5C), suggesting that the association of these two transcriptional regulators in a complex contribute to the inhibition of NF-κB activity. To gain a better mechanistic understanding of this phenomenon, we next dissected which of the transcriptional regulatory domains of KLF11 binds to p65. Using co-IP assays, we demonstrate that R3-ZNF readily binds to p65 (Figure 5D). Moreover, the siRNA-mediated knockdown of KLF11 augments the binding of p65 to a distinct NF-κB binding site located at (-57) – (-71) bps from the transcription initiation site within the VCAM-1 promoter. A similar result was observed on the NF-κB binding site located at (-82) – (-96) bps within E-selectin promoter (Figure 5E). Thus, these results reveal for the first time an interaction between KLF11 and p65 mediating the inhibition of the inflammatory response in ECs.
Figure 5. KLF11 potently inhibits NF-κB activity.
A, BAECs were transfected with NF-κB-Luc for 8 hours, and then infected with Ad-LacZ or Ad-KLF11 for 24 hours. Promoter activity was detected after TNF-α (2ng/ml) stimulation for 16 hours by dual-luciferase assay and normalized against Renilla activity. B-C, Co-IP assays were performed to determine the interaction between endogenous KLF11 and p65 with an antibody against KLF11 (B) or p65 (C) in HUVECs, respectively. D, AD-293 cells were co-transfected with p65 and the His-tagged KLF11 fragments for 24 hours, and then Co-IP assays were performed with an antibody against p65 and Western blot was performed with anti-His antibody. E, HUVECs were transfected with siRNA-control or siRNA-KLF11 (40nM) for 72 hours and then stimulated with TNF-α (2ng/ml) for 1 hour. CHIP assays were performed using an antibody against p65 and normal rabbit IgG. The binding of p65 to the VCAM-1 and E-selectin promoters was determined with qPCR. Data shown are from three independent experiments and presented as mean ± SEM. * p < 0.05; ** p < 0.01.
KLF11 functions as an inhibitor of leukocytes-EC adhesion in vitro and in vivo
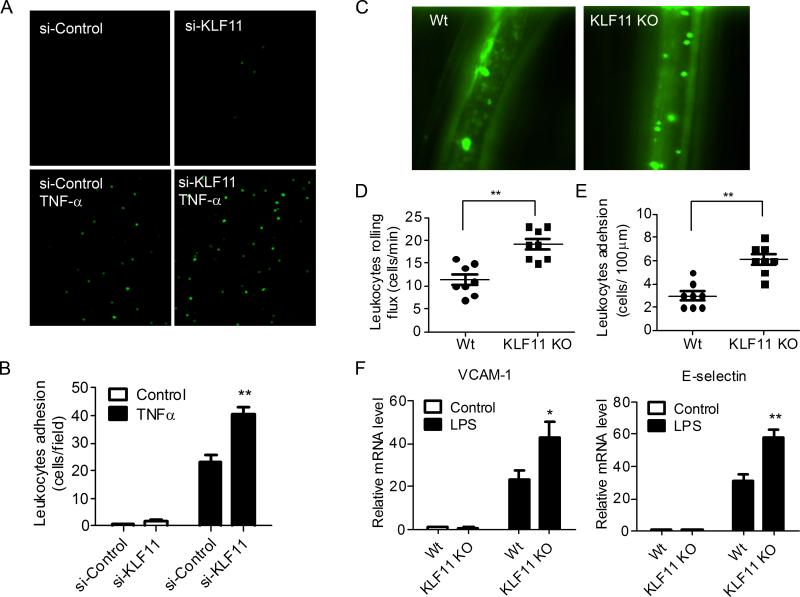
To further examine the biological impact of the KLF11-mediated inhibition of the inflammatory response in ECs, we performed leukocyte-EC adhesion assays in vitro and in vivo.25 The siRNA-mediated KLF11 knockdown significantly increases the TNF-α-induced leukocyte (GFP-labeled THP-1 cells) adhesion to ECs by 1.77 ± 0.13 fold (p < 0.05) in vitro (Figure 6A and 6B). In addition, we determined the effect of KLF11 deficiency on rolling and adhesion of leukocytes on ECs in vivo using a genetically engineered KLF11-/- mouse model.26-28 The lack of expression of KLF11 in aortas from KLF11-/- mice in comparison with littermates was determined by Western blot analyses (Supplemental Figure VIII) and, additionally, it was determined that KLF11 knockout had no effect on the expression of KLF2 and KLF4 in these animals (Supplemental Figure VC). We demonstrate that administration of LPS (30μg/kg) by tail vein injection to the KLF11-/- mice increases leukocytes rolling and adhesion of leukocytes on ECs by 1.7 ± 0.1 fold and 2.04 ± 0.15 fold (p < 0.01), respectively (Figures 6C-6E). In addition, experiments depicted in Figure 6F reveal that the expression of VCAM-1 and E-selectin is significantly increased in aortas from KLF11-/- mice administered LPS. Collectively, our data identify an important homeostatic inhibitory role of KLF11 in mediating the recruitment of leukocytes to ECs in vivo by modulating the expression of adhesion molecules in the endothelium.
Figure 6. KLF11 inhibits leukocyte-EC adhesion in vitro and in vivo.
A-B, HUVECs were transfected with siRNA-control or siRNA-KLF11 (40nM) for 72 hours and then stimulated with TNF-α (2ng/ml) for 16 hours. Activated HUVECs were incubated with GFP-expressing THP-1 cells for 30 min. The binding of THP-1 cells to ECs was visualized on fluorescence microscopy (A). The number of bound THP-1 cells was quantified by counting four microscopic fields per well in triplicates (B). C-F, KLF11-/- and littermate mice were administered LPS (30μg/Kg) by tail vein injection. Four hours later, the leukocyte recruitment was analyzed with intravital microscopy (C). The rolling (D) and adhesion of leukocytes (E) on vascular walls were quantitatively analyzed, n=8. F, The expression of VCAM-1 and E-selectin in aortas from KLF11-/- and littermate mice was determined by RT-qPCR at 4 hours after LPS administration, n = 4. Data shown are presented as mean ± SEM. * p < 0.05; ** p < 0.01.
Discussion
Cardiovascular disease (CVD) is the leading cause of illness and death in the United States and the major cause of mortality and disability in patients with diabetes mellitus.29, 30 It has been documented that KLF11 is a diabetes relevant gene.11, 31-33 Indeed, recent research reveals that alterations in the KLF11 pathway impact the development of neonatal and juvenile diabetes.10 Here, the observation that KLF11 is highly expressed in ECs and that its expression could be induced by TNF-α prompted us to address the relationship between KLF11 and EC activation.
The recruitment of leukocytes to ECs, an early stage of inflammatory diseases such as atherosclerosis, is mediated by the endothelial expression of adhesion molecules such as ICAM-1, VCAM-1 and E-selectin.4 Through in vitro gain- and loss-of-function approaches, we demonstrate that KLF11 overexpression potently inhibits TNF-α-induced expression of adhesion molecules in endothelial cells while, in response to inflammatory stimuli, THP-1 cells show increased adhesion to si-KLF11-treated HUVECs concomitant with increased levels in VCAM-1 and E-selectin in those cells. Furthermore, a more fundamental contribution to our understanding KLF11 function in the present study is provided by the finding that KLF11 deficiency in vivo exacerbates the rolling and adhesion of leukocytes on endothelial cells in an LPS-induced animal model of endothelial dysfunction. The conventional KLF11-/- mice used here also lack KLF11 in the leukocytes. Our observation of increased adhesion of THP-1 (wild type for endogenous KLF11) to si-KLF11-treated HUVECs in vitro argues that this effect is mostly driven by the exacerbated activation of the endothelium in the KLF11-/- mice resulting in enhanced expression of adhesion molecules. Taken together, our study provides evidence that KLF11 has a potent anti-inflammatory effect on vascular ECs and is critical for modulation of leukocytes-EC interaction.
KLF11, a nuclear-located transcription factor, regulates target genes through binding to a consensus sequence present in their promoters.5 We investigated the transcriptional mechanism underlying the anti-inflammatory effect of KLF11. We found that although KLF11 does not significantly regulate the pro-inflammatory adhesion molecules and cytokines under basal conditions, its effects are evident in response to TNF-α and other stimuli. NF-κB plays a critical mediator role in response to most inflammatory stimuli in various cell lines and in ECs in particular.34, 35 In the present study, we demonstrate that KLF11 inhibits NF-κB signaling via a physical interaction with NF-κB p65. CHIP assay data further showed that depletion of KLF11 by RNAi exacerbates the binding of p65 to the VCAM-1 and E-selectin promoters in ECs, thereby confirming that KLF11 regulation of adhesion molecules at the level of transcription involves negative regulation of the NF-κB pro-inflammatory response. The repressor domains R1, R2, R3 and the zinc fingers of KLF11 bind independently to multiple chromatin remodelers to fulfill its functions.22, 23, 33, 36 Noteworthy, studies looking at genetic variations in the KLF11 gene in distinct human populations revealed a variant (Ala347Ser) within R3 domain that segregates with diabetes in families with early-onset type 2 diabetes.31 Here, we demonstrate that KLF11 R3-ZNF could inhibit the p65-induced transcription of the VCAM-1 promoter (Figure 4C) and the R3-ZNF physically binds to p65 (Figure 5D). In fact, our data suggest that this specific repression domain of KLF11 is sufficient to regulate pro-inflammatory adhesion molecules expression in response to TNF-α in EC and demonstrate that NF-κB, a good example of regulatory pathways of EC inflammation, is at least one of the most important signaling molecules mediating this effect. Actually, many inflammatory mediators such as activator protein-1 (AP-1), nuclear factor of activated T-cells (NFAT), and Ets137, 38 are involved in the transduction of extracellular pro-inflammatory stimuli to intracellular signaling pathways. Based on the data from the present study, we cannot completely rule out that KLF11 might inhibit EC inflammation via multiple signaling pathways under conditions of pro-inflammatory stimulation. Nevertheless, the data here reported provides biochemical and cell biological evidence demonstrating that KLF11 is upregulated in response to inflammatory stimuli and inhibits EC inflammation via inhibition of p65. KLF11 possibly acts in a negative-feedback manner to ensure the optimal p65 activation levels thus maintaining the pro- and anti-inflammatory homeostatic balance, which is required for proper “physiological” inflammatory responses.
It has been documented that KLFs 2, 4 and 6 are expressed in ECs and have important roles in EC biology. KLF6 is induced after vascular injury and stimulates endogenous endoglin expression in vascular repair.39 KLF2 and KLF4 can be induced by laminar shear stress in ECs.40, 41 However, KLF2 is downregulated while KLF4 is increased in TNF-α-stimulated ECs.20 Both KLF2 and KLF4 suppress TNF-α-induced EC activation evidenced by decreased expression of pro-inflammatory adhesion molecules.20,19, 42 Our data indicates that the anti-inflammatory effects of KLF11 on EC cannot be explained by KLF11 interfering with KLF2 or KLF4 since we found that changes in KLF11 expression do not translate in changes in the expression of KLF2 or KLF4 either in vitro or in vivo and KLF11 inhibitory effect on the expression of EC adhesion molecules is not affected by KLF2 or KLF4 knockdown in ECs. The synergistic effects observed between KLF11 and KLF2 or KLF4 on the VCAM-1 and E-selectin expression may be a result of a concomitant inhibition of the NF-κB transcriptional activity resulting from sequestration of its co-transcriptional activator CBP/p30043 or the concurrent inhibition of NF-κB pathway by KLF11, possibilities that remain to be addressed. More interestingly, we demonstrate that KLF11 may have broader negative effects on adhesion molecules, since KLF11 potently inhibits ICAM-1 expression while KLF2 does not.19 A number of studies have demonstrated that the ICAM-1 promoter contains transcription factor binding sites such as NF-κB, AP-1, ets-1, SP1, STAT, and PKC-zeta.44, 45 KLF2 may activate some of these transcription factors thus resulting in a distinct, overall non-inhibitory effect for KLF2 overexpression on the ICAM-1 promoter.
In summary, here we demonstrate for the first time an important homeostatic role of KLF11 as an anti-inflammatory factor controlling leukocytes recruitment via modulation of physiological responses in the expression of pro-inflammatory adhesion molecules in ECs. This knowledge extends the current understanding of how KLF11 regulate key cellular functions in the vascular system thereby helping to further underscore the important role of this transcription pathway in maintaining EC homeostasis. KLF11 is thus a potential molecular target for treatment of EC inflammation-associated cardiovascular diseases such as atherosclerosis and diabetic vascular pathologies.
Supplementary Material
Acknowledgements
The authors thank Dr. Minerva Garcia-Barrio (Cardiovascular Research Institute, Morehouse School of Medicine, Atlanta) for helpful discussions and critical comments.
Sources of Funding
This work was partially supported by the NIH grants HL068878, HL105114, and HL089544 (Y.E.C.), American Heart Association 10POST3270008 (Y.F.), 0835237N (J.Z.). Y.E.C. is an Established Investigator of American Heart Association (0840025N). R.U. was funded by the Mayo Foundation and the National Institutes of Health (DK52913).
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest. 1995;95:1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 3.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.McConnell BB, Yang VW. Mammalian kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005;25:1135–1141. doi: 10.1161/01.ATV.0000165656.65359.23. [DOI] [PubMed] [Google Scholar]
- 7.Cook T, Gebelein B, Mesa K, Mladek A, Urrutia R. Molecular cloning and characterization of tieg2 reveals a new subfamily of transforming growth factor-beta-inducible sp1-like zinc finger-encoding genes involved in the regulation of cell growth. J Biol Chem. 1998;273:25929–25936. doi: 10.1074/jbc.273.40.25929. [DOI] [PubMed] [Google Scholar]
- 8.Spittau B, Wang Z, Boinska D, Krieglstein K. Functional domains of the tgf-beta-inducible transcription factor tieg3 and detection of two putative nuclear localization signals within the zinc finger DNA-binding domain. J Cell Biochem. 2007;101:712–722. doi: 10.1002/jcb.21228. [DOI] [PubMed] [Google Scholar]
- 9.Lomberk G, Mathison AJ, Grzenda A, Seo S, Demars CJ, Rizvi S, Bonilla-Velez J, Calvo E, Fernandez-Zapico ME, Iovanna J, Buttar NS, Urrutia R. Sequence-specific recruitment of heterochromatin protein 1 via interaction with kruppel-like factor 11, a human transcription factor involved in tumor suppression and metabolic diseases. J Biol Chem. 2012 doi: 10.1074/jbc.M112.342634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnefond A, Lomberk G, Buttar N, Busiah K, Vaillant E, Lobbens S, Yengo L, Dechaume A, Mignot B, Simon A, Scharfmann R, Neve B, Tanyolac S, Hodoglugil U, Pattou F, Cave H, Iovanna J, Stein R, Polak M, Vaxillaire M, Froguel P, Urrutia R. Disruption of a novel kruppel-like transcription factor p300-regulated pathway for insulin biosynthesis revealed by studies of the c.-331 ins mutation found in neonatal diabetes mellitus. J Biol Chem. 2011;286:28414–28424. doi: 10.1074/jbc.M110.215822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Zapico ME, van Velkinburgh JC, Gutierrez-Aguilar R, Neve B, Froguel P, Urrutia R, Stein R. Mody7 gene, klf11, is a novel p300-dependent regulator of pdx-1 (mody4) transcription in pancreatic islet beta cells. J Biol Chem. 2009;284:36482–36490. doi: 10.1074/jbc.M109.028852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florez JC, Saxena R, Winckler W, Burtt NP, Almgren P, Bengtsson Bostrom K, Tuomi T, Gaudet D, Ardlie KG, Daly MJ, Altshuler D, Hirschhorn JN, Groop L. The kruppel-like factor 11 (klf11) q62r polymorphism is not associated with type 2 diabetes in 8,676 people. Diabetes. 2006;55:3620–3624. doi: 10.2337/db06-0867. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Aguilar R, Froguel P, Hamid YH, Benmezroua Y, Jorgensen T, Borch-Johnsen K, Hansen T, Pedersen O, Neve B. Genetic analysis of kruppel-like zinc finger 11 variants in 5864 danish individuals: Potential effect on insulin resistance and modified signal transducer and activator of transcription-3 binding by promoter variant -1659g>c. J Clin Endocrinol Metab. 2008;93:3128–3135. doi: 10.1210/jc.2007-2504. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Hanson RL, Que LN, Mack JL, Franks PW, Infante AM, Kobes S, Bogardus C, Baier LJ. Association analysis of kruppel-like factor 11 variants with type 2 diabetes in pima indians. J Clin Endocrinol Metab. 2008;93:3644–3649. doi: 10.1210/jc.2008-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanahashi T, Shinohara K, Keshavarz P, Yamaguchi Y, Miyawaki K, Kunika K, Moritani M, Nakamura N, Yoshikawa T, Shiota H, Inoue H, Itakura M. The association of genetic variants in kruppel-like factor 11 and type 2 diabetes in the japanese population. Diabet Med. 2008;25:19–26. doi: 10.1111/j.1464-5491.2007.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan Y, Guo Y, Hamblin M, Chang L, Zhang J, Chen YE. Inhibition of gluconeogenic genes by calcium-regulated heat-stable protein 1 via repression of peroxisome proliferator-activated receptor alpha. J Biol Chem. 2011;286:40584–40594. doi: 10.1074/jbc.M111.232918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkins GB, Jain MK. Role of kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 18.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- 19.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr., Garcia-Cardena G, Jain MK. Klf2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 21.Fang Y, Davies PF. Site-specific microrna-92a regulation of kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook T, Gebelein B, Belal M, Mesa K, Urrutia R. Three conserved transcriptional repressor domains are a defining feature of the tieg subfamily of sp1-like zinc finger proteins. J Biol Chem. 1999;274:29500–29504. doi: 10.1074/jbc.274.41.29500. [DOI] [PubMed] [Google Scholar]
- 23.Buttar NS, DeMars CJ, Lomberk G, Rizvi S, Bonilla-Velez J, Achra S, Rashtak S, Wang KK, Fernandez-Zapico ME, Urrutia R. Distinct role of kruppel-like factor 11 in the regulation of prostaglandin e2 biosynthesis. J Biol Chem. 2010;285:11433–11444. doi: 10.1074/jbc.M109.077065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tedgui A, Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 25.Fan Y, Wang Y, Tang Z, Zhang H, Qin X, Zhu Y, Guan Y, Wang X, Staels B, Chien S, Wang N. Suppression of pro-inflammatory adhesion molecules by ppar-delta in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:315–321. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- 26.Song CZ, Gavriilidis G, Asano H, Stamatoyannopoulos G. Functional study of transcription factor klf11 by targeted gene inactivation. Blood Cells Mol Dis. 2005;34:53–59. doi: 10.1016/j.bcmd.2004.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villar IC, Scotland RS, Khambata RS, Chan M, Duchene J, Sampaio AL, Perretti M, Hobbs AJ, Ahluwalia A. Suppression of endothelial p-selectin expression contributes to reduced cell trafficking in females: An effect independent of no and prostacyclin. Arterioscler Thromb Vasc Biol. 2011;31:1075–1083. doi: 10.1161/ATVBAHA.111.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walshe TE, Dole VS, Maharaj AS, Patten IS, Wagner DD, D'Amore PA. Inhibition of vegf or tgf-{beta} signaling activates endothelium and increases leukocyte rolling. Arterioscler Thromb Vasc Biol. 2009;29:1185–1192. doi: 10.1161/ATVBAHA.109.186742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabel EG. Cardiovascular disease. N Engl J Med. 2003;349:60–72. doi: 10.1056/NEJMra035098. [DOI] [PubMed] [Google Scholar]
- 30.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 31.Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, Dina C, Hamid YH, Joly E, Vaillant E, Benmezroua Y, Durand E, Bakaher N, Delannoy V, Vaxillaire M, Cook T, Dallinga-Thie GM, Jansen H, Charles MA, Clement K, Galan P, Hercberg S, Helbecque N, Charpentier G, Prentki M, Hansen T, Pedersen O, Urrutia R, Melloul D, Froguel P. Role of transcription factor klf11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005;102:4807–4812. doi: 10.1073/pnas.0409177102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu X, Perakakis N, Laubner K, Limbert C, Stahl T, Brendel MD, Bretzel RG, Seufert J, Path G. Human kruppel-like factor 11 inhibits human proinsulin promoter activity in pancreatic beta cells. Diabetologia. 2007;50:1433–1441. doi: 10.1007/s00125-007-0667-3. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Zapico ME, Mladek A, Ellenrieder V, Folch-Puy E, Miller L, Urrutia R. An msin3a interaction domain links the transcriptional activity of klf11 with its role in growth regulation. EMBO J. 2003;22:4748–4758. doi: 10.1093/emboj/cdg470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karin M, Greten FR. Nf-kappab: Linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 35.Tak PP, Firestein GS. Nf-kappab: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JS, Moncrieffe MC, Kaczynski J, Ellenrieder V, Prendergast FG, Urrutia R. A conserved alpha-helical motif mediates the interaction of sp1-like transcriptional repressors with the corepressor msin3a. Mol Cell Biol. 2001;21:5041–5049. doi: 10.1128/MCB.21.15.5041-5049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oettgen P. Regulation of vascular inflammation and remodeling by ets factors. Circ Res. 2006;99:1159–1166. doi: 10.1161/01.RES.0000251056.85990.db. [DOI] [PubMed] [Google Scholar]
- 38.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 39.Botella LM, Sanchez-Elsner T, Sanz-Rodriguez F, Kojima S, Shimada J, Guerrero-Esteo M, Cooreman MP, Ratziu V, Langa C, Vary CP, Ramirez JR, Friedman S, Bernabeu C. Transcriptional activation of endoglin and transforming growth factor-beta signaling components by cooperative interaction between sp1 and klf6: Their potential role in the response to vascular injury. Blood. 2002;100:4001–4010. doi: 10.1182/blood.V100.12.4001. [DOI] [PubMed] [Google Scholar]
- 40.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung kruppel-like factor (klf2). Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 41.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr., Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-dependent regulation of kruppel-like factor 2 is mediated by microrna-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fledderus JO, van Thienen JV, Boon RA, Dekker RJ, Rohlena J, Volger OL, Bijnens AP, Daemen MJ, Kuiper J, van Berkel TJ, Pannekoek H, Horrevoets AJ. Prolonged shear stress and klf2 suppress constitutive proinflammatory transcription through inhibition of atf2. Blood. 2007;109:4249–4257. doi: 10.1182/blood-2006-07-036020. [DOI] [PubMed] [Google Scholar]
- 43.Allen KL, Hamik A, Jain MK, McCrae KR. Endothelial cell activation by antiphospholipid antibodies is modulated by kruppel-like transcription factors. Blood. 2011;117:6383–6391. doi: 10.1182/blood-2010-10-313072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: Nf-kappa b and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 45.Rahman A, Anwar KN, Malik AB. Protein kinase c-zeta mediates tnf-alpha-induced icam-1 gene transcription in endothelial cells. Am J Physiol Cell Physiol. 2000;279:C906–914. doi: 10.1152/ajpcell.2000.279.4.C906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.