Abstract
The current study examines participants’ attributions of change in a double-blind, randomized controlled trial of problem drinkers wanting to moderate their alcohol consumption. Participants were assigned to 12-weeks of naltrexone or placebo, which was paired with either combined motivational interviewing and cognitive behavioral therapy (MBSCT) along with an enhanced medication management intervention or enhanced medication management only. Upon treatment completion, a questionnaire assessed participants’ attributions of change along with their self-efficacy in their ability to maintain treatment gains. Participants differed in strength of attributions of change and self-efficacy according to both their therapy condition and their hypothesized medication condition. Specifically, those in the MBSCT condition who hypothesized that they received placebo displayed greater confidence in continuing changes without medication compared with the other groups. How treatment condition and attributions of change relate to self-efficacy for long-term maintenance of treatment gains are discussed.
Keywords: Naltrexone, cognitive behavioral therapy, attributions, self-efficacy
Self-efficacy represents an individual’s confidence in his ability to behave in such a way that he will achieve a desired goal. Various factors may affect the amount of self-efficacy one possesses in a given situation, including in vivo experience, known as enactive mastery, along with one’s physiological and affective states (Bandura, 1997). In relation to health behaviors, including recovery from addictive behaviors, self-efficacy consistently predicts change during treatment and maintenance of treatment gains (DiClemente, Fairhurst, & Piotrowski, 1995; Goldbeck, Myatt, Aitchison, 1997; Ilgen, McKellar, & Tiet, 2005; Schwarzer & Fuchs, 1996; Vielva & Iraugi, 2001; Hartzler, Witkiewitz, Villarroel, & Donovan, 2011; Witkiewitz, Donovan, & Hartzler, 2012). According to social cognitive theory, early mastery experiences in psychotherapy should enhance self-efficacy and motivation, which, in turn, increase use of coping skills and promote future treatment success (Bandura, 1997). Evidence supports this hypothesis as relevant for recovery of alcohol dependence, with a recent study suggesting that individuals who were able to achieve abstinence early in treatment were more likely to have maintained abstinence twelve weeks into treatment (Charney, Zikos, & Gill, 2010).
Conversely, early failures can undermine self-efficacy, motivation, and commitment to further behavior change. One way that practitioners and researchers attempt to increase success along with self-efficacy in early treatment is to add an effective medication regimen to psychotherapy. It is expected that the combination of medication and psychotherapy should work synergistically to improve treatment outcomes in a variety of populations, as medication should increase the occurrence of mastery experiences early in treatment and bolster self-efficacy. Evidence supports the efficacy of both psychotherapy (e.g. motivational interviewing, MI, and cognitive-behavior therapy, CBT) and medications (e.g. naltrexone, NTX) for the treatment of alcohol problems (e.g. Anton et al., 2006; Morgenstern & McKay 2007; O’Malley, et al., 1992; Volpicelli, Alterman, Hayashisa, & O’Brien, 1992), and the practice of combining medication and psychotherapy in alcohol treatment is increasingly common in both clinical and research settings (e.g., Ledgerwood, McCaul, & Petry, 2005; Zweben, 2001).
Despite the hypothesis that combination treatment will improve outcomes, efforts to maximize treatment efficacy by combining these treatment approaches have produced only modest long-term success (Anton et al., 2006; O’Malley et al., 1996). Results from Project COMBINE, a study evaluating the effectiveness of combined CBT and medication for problem drinking, suggest that the combination of NTX and CBT did not outperform either NTX or CBT alone during treatment (Anton, et al., 2006). In addition, no combination of treatment proved superior to any other at 1-year follow-up. One possible explanation for these results is that, while early mastery experiences fostered by medication may increase self-efficacy during active treatment, the addition of a medication component may also undermine self-efficacy via the under-development of internal attributions of change during therapy.
A basis for this hypothesis arises from studies of combined treatments in a variety of populations. For instance, some studies demonstrate that individuals taking medication while attending psychotherapy for anxiety disorders are prone to greater relapse rates post-treatment (Barlow, Gorman, Shear, & Woods, 2000; Foa, Liebowitz, Kozak, Davies, Campeas, & Franklin, 2005; Marks, Swinson, Basoglu, Kuck, Noshirvani, & Sullivan, 1993). This phenomenon has also occurred in insomnia and depression trials (Antonuccio, Danton, & DeNelsky, 1995; Morin, Colecchi, Stone, Sood, R., & Brink, 1999). In addition, studies suggest that those participants who attribute treatment improvements to active medication during a combined medication and psychotherapy trial for anxiety relapse at greater rates as compared to those who attribute changes to internal factors (Basoglu, Marks, Killic, Brewin, & Swinson, 1994; Biondi & Picardi, 2003). Furthermore, in a recent study of individuals with compulsive sexual behavior who completed a 12-week double-blind citalopram trial, participants in the placebo group had significantly higher future self-efficacy attributions without the use of medication as compared to the active medication group, and there was an inverse relationship between attributing change to the medication vs. internal factors (Muench, Blain, Morgenstern & Irwin, 2011). Taken together, these findings suggest that internal attributions of change promote maintenance of treatment success, and that simply taking a medication may reduce the likelihood that an individual will attribute changes to internal factors.
New evidence from an experimental trial suggests that medication attributions may have a strong influence on maintenance of treatment gains. Recently, Powers, Smits, Whitley, Bystritsky, & Telch (2008) examined how treatment attributions may influence relapse rates for claustrophobia by giving three groups of participants a placebo and manipulating only end-treatment explanations of the placebo’s effect. Investigators told one group of participants that the pill they had ingested should have had a sedating effect, making the exposure easier. The second group was told the pill would have had no effect on the ease of treatment, and the third group was told the pill had stimulating effects that would have made treatment more difficult. Those who were told that they had taken a sedating pill experienced higher rates of relapse in the same exposure situation without medication at a one-week follow-up, which was mediated by a decrease in self-efficacy.
It is possible that the addition of a medication component to alcohol treatment may hinder maintenance of treatment gains in a similar manner by interfering with the development of clients’ internal attributions of behavior change along with their self-efficacy for maintenance of change after treatment discontinuation. Bandura (1997) posits that self-efficacy relates to a specific situation; for example, a client who is trying to moderate his drinking may have greater self-efficacy for moderating drinking on a Sunday morning than on a Friday night or when his mood is neutral as opposed to extremely positive or negative. In the context of combined medication and psychotherapy trials, if clients believe they are taking a medication that will place them in a physiological state in which drink refusal is made easier, they may attribute changes to the external influence of this medication. Clients’ self-efficacy for maintaining moderated levels of drinking may decrease when the situational influence of ingesting a pill that proposes to ease drink refusal is removed at treatment discontinuation. These changes in self-efficacy may then impact an individual’s level of success in achieving alcohol treatment goals. Interestingly, recent investigations indicate that self-efficacy mediated the relationship between both skills developed in therapy and the therapeutic bond and subsequent maintenance of treatment gains in the psychotherapy-only arm of the COMBINE study (Hartzler, Witkiewitz, Villarroel, Donovan, 2011; Witkiewitz, Donovan, & Hartzler, 2012), providing initial evidence for the importance of self-efficacy for post-treatment drinking outcomes. Notably, these investigations examined the relationship between self-efficacy and drinking abstinence, not moderation, and the therapy goal may influence the relationship between cognitive variables and treatment outcome.
The purpose of the current study was to explore participants’ attributions of change at treatment discontinuation based on their hypothesized medication condition in the context of alcohol moderation. In addition, we sought to examine participants’ self-efficacy related to maintenance of treatment gains based on their hypothesized medication condition. It was expected that participants who hypothesized that they were taking active medication would report fewer internal attributions for change and less confidence to maintain treatment gains without medication.
This investigation was a secondary analysis of data collected for Project SMART (Selecting Moderate Alcohol-Related Targets), a randomized controlled, single site trial of combined medication (naltrexone, NTX) and psychotherapy for moderation of problem drinking in a sample of 200 men who have sex with men (MSM). A complete description of Project SMART can be found elsewhere (Morgenstern, Kuerbis, Kahler, Bux, & Kranzler, In press), but the results are reviewed here briefly. In our main effects analysis (Morgenstern et al., In press), we found that a modified version of behavioral self control therapy (MBSCT, described further below) significantly reduced weekly sum of standard drinks, proportion of heavy drinking days and increased the likelihood of drinking a safe levels as defined by the National Institute for Alcohol Abuse and Alcoholism (NIAAA), and that MBSCT was superior to an enhanced medication management intervention. There were no main effects for NTX on any of the drinking outcomes. Interestingly, an interaction effect for NTX and therapy condition was found, such that those who received medication management only with NTX were significantly more likely to achieve safe levels of drinking than those in the medication management condition who received placebo (PBO). There was no such effect for the MSCBT condition.
Method
Participants
Advertising was targeted towards men who wished to reduce their drinking and did not want to quit drinking altogether. Participants (N = 200) were recruited through direct engagement with community outreach teams at gay bars and events, advertisements on social networking internet sites, flyers posted in gay community centers, and print media advertisements.
To be eligible for this study, men had to: 1) be between ages 18 to 65; 2) have an average weekly consumption of greater than twenty-four standard drinks per week over the last 90 days; 3) self identify as being sexually active with other men; and 4) read English at an eighth grade level or higher. Participants with a lifetime diagnosis of bipolar disorder, schizophrenia, or other psychotic disorder; untreated current major depressive disorder; or current physiological dependence on alcohol or other drugs (with the exception of cannabis) were excluded. Participants assessed by the study psychiatrist as at risk for serious medication side effects from NTX, such as those taking contraindicated medications or with severe liver abnormalities, were also excluded. Participants were not enrolled in concurrent drug or alcohol related treatment during the treatment phase of Project SMART. Men in this trial reported an average baseline drinking consumption of 43.1 standard drinks per week (SD = 25.5), with a mean of over 8 drinks per drinking day. Over 90% of men met DSM-IV criteria for alcohol dependence at baseline, indicating that medication and psychotherapy were both viable treatment options for this population.
Procedure
The Institutional Review Board at the participating institution approved this study. Potential participants called for a brief phone interview to receive information about study procedures. Those who were interested were asked to provide verbal consent and screened for initial study eligibility. Eligible participants were then scheduled for an in-person interview during which they participated in full consenting and screening procedures. Participants who met full eligibility criteria were enrolled in the study and randomly assigned to one of two medication conditions, NTX or placebo (PBO), and one of two counseling conditions, Brief Behavioral Compliance Enhancement Therapy (BBCET) or BBCET in combination with MBSCT.
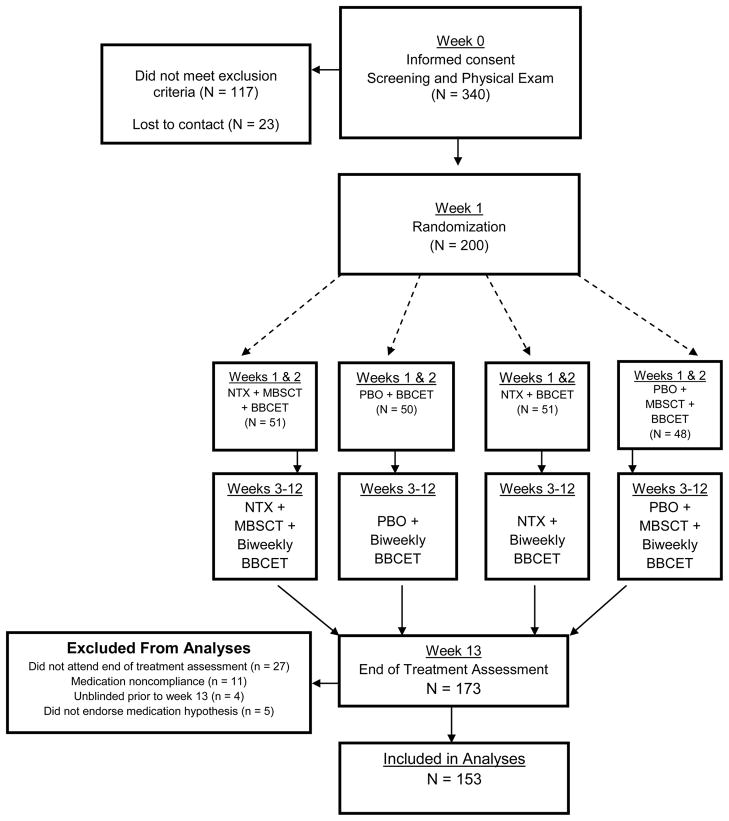
The treatment phase of the trial lasted twelve weeks, with a follow-up assessments at one-week post treatment termination. After completing the end of treatment assessment, participants were provided with a sealed envelope revealing their medication condition. Staff remained blind to participants’ medication condition through the study’s follow-up phase. (See figure 1 for detailed flow of study procedures).
Figure 1.
Flow of Participants through the Study.
Interventions
Medication (NTX or PBO)
Pill assignment was double-blind, and psychiatrists administering BBCET were also blind to participants’ therapy conditions. Participants titrated up from a daily 25 mg to 100 mg of NTX or PBO during the first three weeks of treatment and then remained at this dose through the remainder of treatment, and 95% of participants titrated up to a full 100 mg. Participants were considered compliant with medication if they reported taking at least 80% of the medication during the 12 week treatment period. Medication adherence was assessed and recorded by the treating physician at each BBCET visit, and 89.2% of participants were medication compliant throughout the trial. Participants who, when asked by the treating physician at medication management visits, reported noncompliance with a therapeutic dose of NTX or PBO (at least 50 mg or more) for seven days or more were discontinued from the treatment phase of the trial but continued in follow-up. We withdrew eight participants for being without medication for more than seven days. These participants were evenly dispersed across condition, with no significant differences. Sixty-two percent of participants reported experiencing some side effects. The most common side effects were nausea, insomnia, diarrhea, fatigue, and loss of libido. Most of these side effects dissipated once participants had titrated to the full 100 mg dose. Those with extreme side effects were titrated down to a lower dosage or ceased taking medication altogether under physician supervision. Six participants withdrew themselves from the study altogether due to uncomfortable side effects. While there were slightly more individuals in the NTX condition who dropped out or removed themselves from treatment due to side effects, this difference was not statistically significant.
BBCET
All participants, regardless of condition, received BBCET, a series of 20-minute sessions with a psychiatrist at weeks one through three, and then every other week thereafter for twelve weeks. BBCET integrates medication management with compliance enhancement techniques. Each session addresses patient issues related to the personal barriers of compliance, focuses on how medication can assist the patient in achieving goals related to the control of drinking, and, if necessary, addresses management of side effects. The treatment emphasizes the role of medication and a problem-solving approach to resolving any issues related to compliance and modification of drinking. Advice from the physician was limited to qualitative suggestions for medically safe levels of drinking, and physicians were directed to avoid implementing any MI or CBT techniques. BBCET sessions were designed to replicate care that one would likely receive in a primary care setting. Thirty audiotapes of BBCET sessions were randomly selected an evaluated by coders to evaluate treatment fidelity, including the degree to which physicians refrained from utilizing MBSCT techniques. All of these sessions demonstrated high adherence to the BBCET protocol.
MBSCT
Based on existing behavioral self-control therapy (Sanchez-Craig, Annis, Bornet, & MacDonald, 1984), MBSCT is a manual-based amalgam of Motivational Interviewing (MI) and Cognitive Behavioral Therapy (CBT). MBSCT is targeted towards moderation of problem drinking, and it was initially designed for problem drinking MSM (Morgenstern et al., 2007). Both MI (Hettema, Steele, & Miller, 2005; Morgenstern et al., 2007) and CBT (Morgenstern & McKay, 2007) have received significant empirical support for treatment of problem drinking. In the current study, treatment was comprised of 12 one-hour sessions that focused on moderation of problem drinking. The first two weeks of counseling utilized MI and the development of a change plan, and the last 10 sessions focused on the development of skills to modify behavior patterns of excessive drinking based on an initial functional analysis. Ten master’s and doctoral level therapists delivered this intervention. All therapists had extensive training in implementing both MI and CBT and attended weekly supervision with the study’s principal investigator, and all but one therapist had more than five years of experience implementing these interventions prior to the current study.
Treatment fidelity was monitored in weekly supervision, which reviewed videotaped therapy sessions, and through the Yale Adherence Competence Scale (YACS, Carroll et al., 2000; Madson & Campbell, 2006), a measure that is frequently used to evaluate therapist skill and utilization of CBT and MI techniques. Two independent raters coded a randomly selected 10% of all videotaped therapy sessions according to YACS coding, and therapists demonstrated a high level of adherence and skill for MI and CBT components, with a mean YACS score of 6.43 (SD = .27) out of a possible 7 (Morgenstern et al., In press). There were no significant differences among therapists with respect to treatment fidelity nor were there significant therapist effects on study outcome.
Measures
Demographics
Age, sexual orientation, education, income, and ethnicity were obtained through structured interview procedures.
Substance use and other psychiatric disorders
The Composite International Diagnostic Instrument, Substance Abuse Module (CIDI-SAM; Cottler, Robins, & Helzer, 1989) was used to evaluate substance dependence exclusion criteria. It is a well-established diagnostic interview that has demonstrated excellent reliability for individual symptoms of substance abuse and dependence (Cottler, et al., 1989). Participants were screened for psychosis and other thought disorders using the Structured Clinical Interview for DSM-IV, psychotic screening and bipolar disorder sections (SCID; First, Spitzer, Gibbon, & Williams, 1996) and the Mini-Mental Status Examination (MMSE; Folstein, Folstein, & McHugh, 1975), respectively, for exclusion criteria.
Timeline Followback Interview (TLFB)
The TLFB is an interviewer-assisted, calendar based method that utilizes specific recall techniques (e.g., memory cues) for participants to recall daily drinking (in standard drink equivalents) and types and frequency of other drug use. The TLFB has demonstrated reliability and validity for the collection of alcohol and other drug use data for recall periods of up to one year (Fals-Stewart, O’Farrell, Freitas, McFarlin, & Rutigliano, 2000). The TLFB was administered at the initial screen, baseline, and end of treatment assessments. Daily data was then converted into summary level variables for two three-month time periods: baseline drinking and drinking during treatment (referred to by its assessment--end of treatment drinking). We used TLFB data to compute mean drinks per day (MDD) along with proportion of heavy drinking days (PHD) in each time period. Heavy drinking days were defined as days in which participants consumed at least six standard drinks.
Medication Attributions and Self-Efficacy Questionnaire (MASE)
Participants completed the MASE at their end of treatment assessment prior to unblinding. This computerized questionnaire asked participants to subjectively rate how their drinking had changed since beginning treatment. Participants also rated how much of change they attributed to a variety of factors, including therapist behaviors, internal factors, external factors (e.g., life events such as the death of a loved one, loss of a job, or the breakup of a relationship), medication, and physician behaviors, and each attribution was measured by a single item. Internal factors were defined for participants as things that “you did consciously or deliberately to moderate your drinking (like resisting urges to drink even though you wanted to, or changing things in your life to make it easier to moderate drinking). It does NOT include the things outside of your control or things that just that happened to you by coincidence.” All of these items were rated on a 5-point Likert scale (0 = Not at all and 4 = Completely). Finally, participants were asked to rate how confident they were that they could continue their changes, along with how confident they were that they could continue changes without medication, on a 10-point Likert scale (0 = Not at all confident and 9 = Completely confident).
Medication Questionnaire (MED-Q)
The Medication Questionnaire (MED-Q) was a computerized self report questionnaire created to ask participants about their perspective of their experience in the study including their hypothesized medication condition. Participants completed the MED-Q at the end of treatment assessment, immediately after the MASE, and prior to unblinding.
Analytic Plan
Participants in this study were randomized to one of four treatment conditions, MBSCT or BBCET only combined with NTX or placebo (see figure 1). Such a design was used in order to evaluate the relative utility of psychotherapy and NTX for moderation of problem drinking. For the primary analyses in this study, we focused on participants’ hypothesized medication condition rather then their actual medication condition, as client’s attributions of change prior to unblinding should have related to this hypothesis. To be eligible for these analyses, men must have completed an end of treatment assessment, attended at least 5 of their BBCET visits during the trial, and remained blind to their medication condition throughout treatment. Of 200 men who were enrolled in the trial, 27 did not complete an end of treatment assessment. An additional 11 men attended fewer than five BBCET visits while receiving treatment, indicating inadequate medication compliance. Four additional participants were unblinded to their medication condition prior to the end of treatment, and five participants did not endorse a hypothesis regarding their medication condition. The 47 men who were excluded based on these criteria did not show any baseline differences from the other men on age, MDD, or PHD. Subsequent analyses proceeded with the sample of 153 men.
Our preliminary analyses included outlining demographic information for our sample and examining baseline equivalence of drinking levels among the four treatment conditions. Because drinking data were skewed, a logarithmic transformation of data was preformed prior to analyses. We also examined whether participants in each condition differed in terms of how they subjectively viewed their change in drinking behavior at end of treatment by using a between subjects ANOVA. The subjective change data was slightly skewed and normalized through a square root transformation prior to analyses. In addition, we briefly examined changes in MDD and PHD from baseline to end of treatment based on hypothesized and actual medication condition, along with interactions of these variables and therapy condition.
Primary data analyses included between subjects ANCOVAs that examined the predictive association between attribution variables, hypothesized medication condition, therapy condition, and confidence to maintain treatment gains. Attribution variables included single items from the MASE that assessed the extent to which individuals attributed treatment gains to “internal factors” and “the medication”. Therapy condition and hypothesized medication were treated as independent variables, with attribution variables and confidence to maintain treatment gains as dependent variables. We utilized hypothesized medication condition rather than actual medication condition as the predictor of attributions and self-efficacy because participants were not certain of their medication condition during the end-treatment assessment. Their attributions of change, then, would be based on their hypothesis rather than their actual medication condition in cases where participants were incorrect about this condition. Prior to the primary analysis of each outcome variable, the relationship between actual medication condition and these variables was assessed. In cases where this relationship is significant, actual medication condition is entered as a covariate in subsequent analyses.
Significant interaction terms that were found in the primary analyses are followed-up with t-tests to examine hypothesized medication condition differences within each therapy condition. In addition, we calculated correlations between these variables (See Table 1). MDD at baseline was included as a covariate in all analyses. Cohen’s d effect sizes for differences among these groups are reported in addition to significance. Cohen’s d sizes ranging from .2–.5 represent small effects, .5–.8 represent moderate effects, and .8 or larger represent large effects (Cohen, 1988).
Table 1.
Intercorrelations between drinking outcomes, attributions, and self-efficacy.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. MDD (BL) | -- | .36 | −.03 | .04 | −.06 | −.09 | .02 |
| 2. MDD (w13) | -- | .43 | −.26 | −.43 | −.47 | −.20 | |
| 3. Subjective Δ | -- | .34 | .60 | .59 | .36 | ||
| 4. NTX att. | -- | .08 | .29 | −.16 | |||
| 5. Internal att. | -- | .59 | .53 | ||||
| 6. Confident | -- | .52 | |||||
| 7. Confident - NTX | -- |
Note. N = 92. MDD = mean drinks per day; BL = baseline assessment; w13 = week 13 (end of treatment) assessment. Subjective Δ = subjective change in drinking over the 13-week trial. NTX att = attribution of change to medication (naltrexone) factors. Internal att = attribution of change to internal factors. Confident = self-rated confidence in ability to continue changes over the upcoming three-month period, Confident –NTX = self-rated confidence in ability to continue changes in the upcoming three-month period without medication. All correlations with an absolute value greater than .15 are significant, p < .05
Results
Preliminary Analyses
Sample characteristics
Among the sample of 153 men, ages ranged from nineteen to sixty-five at the time of study enrollment (M = 41.52, SD = 11.15), and 91.5% of these men self-identified as gay, while 6.5% identified as bisexual. Seventy-five percent of these men were Caucasian, 7.2% were of mixed race, 7.8% were Black, 3.3% were Asian or Pacific Islander, and 6.5% were of another race. Additionally, 13.1% of these men identified as Hispanic or Latino. A sizable minority (16.3%) of the sample reported that they were HIV-positive at baseline.
Of the 153 men, 79 participated in the MBSCT condition, while 74 received BBCET only. Within the MBSCT condition, roughly half of the sample (n = 39) believed they were taking PBO at the end of treatment assessment, while 40 men believed they had been taking NTX. Forty-one men in MBSCT were actually taking PBO, with 38 taking NTX. Within the BBCET condition, 52 men believed they took PBO, and the minority of men (n = 22) believed they took NTX. In reality, 40 received PBO and 34 men received NTX. Overall, 60.7% of the sample correctly guessed their medication condition. An exact-binomial sign test indicated that this was significantly better than chance, p = .01. In addition, a slightly greater percentage of men in BBCET (64.9%) as compared to MBSCT (57.0%) correctly guessed their medication condition.
Baseline equivalence of groups
Those randomized to MBSCT and those randomized to BBCET displayed equivalent drinking patterns at baseline. Those in BBCET (n = 74) reported a mean of 6.57 (SD = 3.53) drinks per day in the ninety days prior to baseline, while those in MBSCT (n= 79) reported an average of 6.10 (SD = 4.21) drinks per day during this time period. Participants in both conditions also reported a similar PHD at baseline (BBCET, M = .64, SD = .32; MBSCT, M = .62, SD = .31). Comparing baseline characteristics of the sample by hypothesized medication condition, the NTX group (n = 62) did not differ from the PBO group (n = 91). Each group reported similar MDD (NTX, M = 5.99, SD = 4.66; PBO, M = 6.56, SD = 3.27) along with PHD (NTX, M = .61, SD = .31; PBO, M = .64, SD = .32).
End of treatment outcomes
In this sample, an ANCOVA revealed a significant effect of time on MDD (See Table 2 for means), F (1, 149) = 155.434, p < .05. The Time X Hypothesized Medication Condition effect for MDD was not significant, nor was a Time X Treatment Condition X Hypothesized Medication Condition interaction. These analyses were repeated with actual medication condition, and a similar pattern of results emerged.
Table 2.
Group means and standard deviations for baseline drinking and dependent measures.
| Group | PHD-BL | PHD-w13 | MDD - BL | MDD - w13 | Subjective Δ | NTX att. | Internal att. | Confident | Confident - NTX |
|---|---|---|---|---|---|---|---|---|---|
| M(SD) | M(SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| BBCET (n = 74) | .63 (.32) | .44 (.37) | 6.55 (3.49) | 4.16 (2.39) | 4.47 (1.13) | 1.13 (1.19) | 2.24 (.94) | 6.23 (2.25) | 6.24 (2.38) |
| H-PBO (n = 52) | .64 (.32) | .50 (.27) | 6.67 (3.69) | 4.58 (2.58) | 4.24 (1.14) | .70 (.89) | 2.16 (.98) | 6.02 (2.45) | 6.28 (2.37) |
| H-NTX (n = 22) | .61 (.32) | .29 (.40) | 6.23 (3.01) | 3.10 (1.37) | 5.05 (.89) | 2.20 (1.20) | 2.45 (.83) | 6.75 (1.59) | 6.15 (2.46) |
| MBSCT (n = 79) | .61 (.31) | .26 (.25) | 6.00 (4.23) | 2.70 (1.68) | 5.32 (.88) | 1.33 (1.28) | 2.78 (.90) | 7.46 (1.45) | 6.89 (2.24) |
| H-PBO (n = 39) | .62 (.30) | .30 (.26) | 6.08 (2.33) | 2.79 (1.96) | 5.33 (.76) | .53 (.81) | 2.97 (.94) | 7.47 (1.70) | 7.64 (1.96) |
| H-NTX (n = 40) | .61 (.32) | .23 (.33) | 5.92 (5.43) | 2.61 (1.39) | 5.30 (.99) | 2.05 (1.20) | 2.60 (.86) | 7.45 (1.20) | 6.23 (2.28) |
Note: PHD = proportion of heavy drinking days MDD = mean drinks per day; BL = baseline assessment; w13 = week 13 (end of treatment) assessment. Subjective Δ = subjective change in drinking over the 13-week trial. NTX att = attribution of change to medication (naltrexone) factors. Internal att = attribution of change to internal factors. Confident = self-rated confidence in ability to continue changes over the upcoming three-month period. Confident –NTX = self-rated confidence in ability to continue changes in the upcoming three-month period without medication. H-PBO = Hypothesized placebo. H-NTX = Hypothesized Naltrexone
For PHD, a slightly different pattern of results emerged. A significant time effect appeared for PHD, F (1, 149) = 114.084, p < .05, such that individuals reduced their PHD during treatment. A significant Time X Hypothesized Medication Condition interaction also appeared. Those who believed they had been taking NTX showed greater decreases in their drinking than those who believed they were taking PBO, F (1, 149) = 4.03, p < .05; however, this interaction was not significant for actual medication condition. Thus, changes in heavy drinking behavior may have led individuals to believe that they were taking medication. This finding may relate to expectancies specific to NTX. NTX is purported to make drinking, and heavy drinking in particular, less pleasurable (Kranzler, Armeli, Feinn, & Tennen, 2004; McCaul, Wand, Stauffer, Lee, & Rohde, 2001). Clients received education about this medication effect, which may have then influenced their hypothesis about their medication condition. No 3-way Time X Treatment Condition X Medication Condition effect reached significance.
With regard to how participants subjectively viewed their change in drinking behavior at end of treatment, those in MBSCT reported more change in their drinking than those in BBCET, F (1, 148) = 14.70, p < .05. In addition, those who hypothesized they had taken NTX showed greater subjective change than those who believed they had taken PBO, F (1, 148) = 8.07, p = .05. Furthermore, an interaction emerged such that those who believed they had taken NTX showed greater levels of subjective change only in the BBCET condition, F (1, 148) = 5.40, p < .05. Again, when examining actual medication condition as opposed to hypothesized medication condition, no differences emerged between those taking NTX and PBO, and no Time X Treatment Condition X Medication Condition or Hypothesized Medication Condition interaction appeared. Overall, the discrepancy in results when examining actual as compared to hypothesized medication condition is interesting. This pattern of results suggests that a perceived change in drinking behavior may lead one to believe that he was taking an active medication. Furthermore, this result is most pronounced when individuals are not attending weekly psychotherapy. Notably, a power analysis indicated that our sample size (N = 153) permitted us to detect large and medium, but not small, three-way interaction effects.
Primary Analyses
Correlations among attribution and confidence items
The correlations between attribution variables, hypothesized medication condition, treatment condition, and confidence to maintain treatment gains are shown in Table 1. Internal attributions displayed a strong positive correlation with both confidence to continue changes, r = .59, p < .05, and confidence to continue changes without medication, r = .53, p < .05. Medication attributions appeared positively related to confidence to continue changes, r = .29, p < .05, and negatively related to confidence to continue changes without medication, r = −.16, p = .05.
Attributions
With regards to the attribution variables, as expected, those who believed they took NTX reported a greater attribution to the pill than those who believed they were taking PBO, F (1, 145) = 68.61, p < .05, d = 1.46. No treatment condition effect or Treatment X Hypothesized Medication Condition interaction emerged on pill attributions. A different pattern appeared for internal attributions. No main effect for hypothesized medication condition appeared; however, those in the MBSCT condition reported greater internal attributions than those in BBCET, F (1, 147) = 7.08, p < .05, d = .28. The Hypothesized Medication Condition X Treatment Condition interaction also reached significance, F (1, 147) = 3.93, p < .05. An examination of the means of internal attributions shows that, in the BBCET condition, those who believed they were taking NTX showed higher internal attributions of change than those who believed they were taking PBO, d = .31. One possible explanation for this pattern of findings may be that individuals who experienced subjective changes in their drinking behavior in the BBCET condition were also more likely to believe that they were on NTX, as discussed above. Due to their success, these individuals, then, may have been more likely to attribute their change to internal factors than their less successful counterparts who believed they were taking PBO. In the MBSCT condition, the opposite relationship appeared, such that those taking PBO showed higher internal attributions of change compared to those taking NTX, d = .38.
Confidence to maintain changes in the future
Overall treatment group differences emerged with regards to self-efficacy to continue changes at post-treatment, as those in MBSCT reported greater confidence to continue changes than those in BBCET, F (1, 145) = 10.34, p < .05, d = .64. No main effect for hypothesized medication or Treatment Condition X Hypothesized Medication Condition interaction emerged.
Confidence to maintain changes in the future without medication
When examining participants’ confidence to continue changes without medication, the main effect of treatment condition remained significant, F (1, 145) = 4.08, p < .05, d = .32. In addition, hypothesized Medication Condition differences, F (1, 145) = 4.78, p < .05, d = .32, along with the Treatment Condition X Hypothesized Medication Condition interaction, F (1, 145) = 4.07, p < .05, both reached significance. In this context, those who were in the MBSCT condition reported greater confidence to continue changes overall than those in the BBCET condition. In addition, those who thought they took PBO indicated greater overall confidence to continue changes than those who believed they took NTX.
We followed up on this interaction by examining the simple main effect of hypothesized medication condition within each treatment condition. While no significant difference in confidence to continue changes without medication emerged between the NTX and PBO groups within the BBCET condition, those who believed they took PBO in the MBSCT group reported greater confidence to continue changes without medication than those in MBSCT who believed they took NTX, t (76) = 3.28, p < .05, d = .74 (see Figure 1). Furthermore, a post-hoc Tukey’s B test found that the MBSCT/PBO group reported greater confidence to continue changes without medication than the three other groups combined, p < .05, suggesting a clinically relevant difference between this group and others with regards to their confidence to continue changes without medication.
Discussion
Results from this study indicate that treatment approach (e.g., psychotherapy versus medication management only) represents an influential factor in clients’ attributions for change during treatment along with their self-efficacy for maintaining treatment gains. In addition, hypothesized medication condition bears some relation to one’s confidence to maintain changes after medication is discontinued in the context of alcohol moderation, such that those who believed that they received PBO reported higher self-efficacy in regards to maintaining their changes after ceasing the medication. While longer-term drinking outcomes have yet to be explored, these findings cast further doubt on the previously held hypothesis that adding a medication component to psychotherapy should increase one’s confidence in his ability to change.
Recently, researchers have examined factors that predict maintenance of treatment gains in abstinence-based alcohol treatment, and findings indicate that self-efficacy mediates the relationship between cognitive-behavioral interventions and one-year treatment outcomes (Hartzler, et al., 2011; Witkiewitz, et al., 2012). In particular, these researchers found that self-efficacy mediated the relationship between the therapeutic bond and treatment outcomes in the COMBINE study; however, this relationship only emerged when clients were not also ingesting a pill (Hartzler et al., 2011). Furthermore, the effects of drink refusal skills training appeared to be mediated by changes in self-efficacy (Witkiewitz, et al., 2012). Together with these findings, the current study promotes the importance of developing self-efficacy as a key factor in the success of individuals after treatment discontinuation.
Notably, participants in this study endorsed a moderated drinking goal and reported baseline drinking at levels for which professionals deemed moderation an appropriate goal. While some individuals in this study were drinking at very high levels at treatment entry, they were required to be sober when presenting to all outpatient treatment sessions, and individuals who were unable to safely maintain a blood alcohol content of 0.00 during treatment sessions were referred for more intensive treatment. Thus, medication effects on client progress may differ when clients have an abstinence goal or when clients initially present as more severely dependent on alcohol. In addition, our findings may not generalize to other populations, such as those who have less education, as the development of attributions may rely on a relatively advanced degree of reflection on the therapeutic process.
The fact that participants in the MBSCT condition who believed they took PBO endorsed the highest levels of confidence in maintaining their treatment gains compared to other groups suggests that combination medication and therapy trials may benefit from a tapering down of medication before therapy termination. Several medications, such as NTX, show promising outcomes for the treatment of alcohol problems, and results from the current study do not warrant the abandonment of such pharmacologic interventions. Rather, these findings suggest that clinicians should explore clients’ cognitive attributions of change when clients are engaged in combined treatment. Tapering down from medication during an active phase of psychotherapy may allow clients who are taking medication to develop self-efficacy in a medication-free context before they terminate all treatment. The optimal time during treatment that tapering should occur remains to be explored, and future randomized controlled trials of combined medication and psychotherapy for problem drinking should consider a taper down approach to evaluate its potential benefits and influence on self-efficacy.
It is noteworthy that we evaluated attributions and self-efficacy based on participant’s perception of their medication condition rather than actual medication condition. While this can be considered a limitation, choosing groups based on clients’ perceptions is relevant when evaluating cognitive attributions. In addition, we examined the relationship between actual medication condition and the attribution and confidence variables using a series of t-tests. Of the four primary outcome variables, only medication attributions showed a significant relationship to actual medication condition, and we entered actual medication condition as a covariate in the primary analysis of this variable. Furthermore, while 60.7% of our sample correctly guessed their medication condition, many participants did not guess their medication condition accurately, and these participants’ attributions of change would have been based on their hypothesized rather than actual medication condition prior to unblinding. This study indicates that client’s beliefs about their medication condition can influence their internal attributions along with their self-efficacy for continued changes, and that this relationship occurs independent of clients’ actual medication condition.
This study has several limitations, and it only begins to explore the relationship between treatment conditions, attributions for change, and self-efficacy for maintenance of treatment gains. One important comment on potential differences in self-efficacy and attributions based on MBSCT compared with BBCET includes that those in the MBSCT condition received significantly more interaction with professionals during the trial. Thus, we cannot ascribe differences between therapy groups exclusively to the therapeutic intervention. This comparison, however, should provide appropriate generalizability when comparing weekly psychotherapy to an enhanced medication management approach as practiced in the community.
Another limitation of this study includes the reliance on single-item self-report measures for analysis of specific attributions and self-efficacy. Due to the need to reduce participant burden, the MASE was kept to a few questions. To examine the validity of some key items, we correlated the confidence questions from the MASE with a validated measure of the degree to which individuals have the confidence to resist drinking in a variety of circumstances, the Situational Confidence Questionnaire – 39 (SCQ-39; Annis & Graham, 1988). This measure was administered at the same time point as the MASE, and both confidence to continue changes (r = .44) and confidence to continue changes without medication (r = .33) significantly correlated with overall scores on the SCQ-39. Even so, personal interpretations of the questions may have skewed the answers in ways we cannot ultimately discern. Therefore, our inability to thoroughly validate the measure remains a limitation of the study and a point of further exploration in future research.
This trial is the first investigation that we are aware of to examine attributions of change in a combined treatment for problem drinking, and the findings reported here require both replication and extension. Ultimately, studies should examine how these variables might mediate the long-term follow-up success of participants in a combined medication and psychotherapy trial for moderation of problem drinking. Participants in our trial were unblinded immediately after their post-treatment visit. Thus, participant’s attributions may have changed once they receive confirmation of their actual medication condition, making evaluation of this question difficult in our sample. Randomized controlled trials of combination treatments present a unique paradigm for examining attributions of change, and another interesting investigation would examine how participant’s attributions in randomized-controlled trials might change immediately before and after unblinding. In addition, it would be interesting to compare attributions in a sample that remains blind to their medication condition to those that are aware of their medication, like those who seek out medication in the community.
The current study provides preliminary evidence that treatment modality for problem drinking may influence clients’ attributions of change and confidence to maintain changes after treatment discontinuation. Altogether, the findings from this investigation add to a growing body of literature which suggests that engaging in psychotherapy without concurrent medication may provide a unique opportunity for developing internal attributions of change, increasing self-efficacy, and improving maintenance of treatment gains for a variety of conditions.
Figure 2.
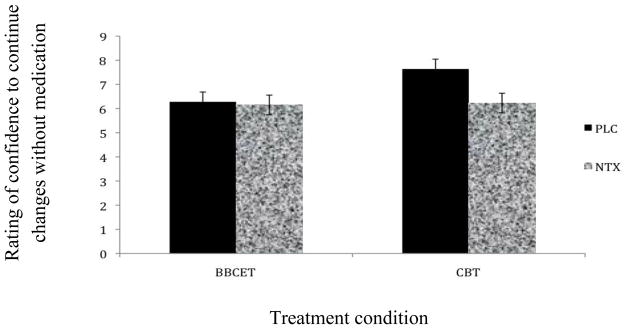
Between Group Differences on Confidence to Continue Changes Without Medication After Treatment.
Highlights.
We examine attributions of change in a treatment trial for problem drinking
Study includes a randomized controlled trial of medication and psychotherapy
Attributions differ based on therapy condition and perceived medication condition
Self-efficacy for maintenance of treatment gains relates to attributions
Acknowledgments
This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (R01 AA0115553-01).
Abbreviations
- MBSCT
Modified Behavioral Self-Control Therapy
- BBCET
Brief Behavioral Compliance Enhancement Treatment
- NTX
Naltrexone
- PBO
Placebo
- SMART
Selecting Moderate Alcohol-Related Targets
- MSM
Men who have sex with men
- MASE
Medication Attribution and Self-efficacy Questionnaire
- MED-Q
Medication Questionnaire
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katherine Schaumberg, Columbia Addiction Services and Psychotherapy Interventions Research, Columbia University Medical Center.
Alexis Kuerbis, Columbia Addiction Services and Psychotherapy Interventions Research, Columbia University Medical Center.
Jon Morgenstern, Columbia Addiction Services and Psychotherapy Interventions Research, Columbia University Medical Center.
Frederick Muench, Columbia University Medical Center; Jon Morgenstern, Columbia Addiction Services and Psychotherapy Interventions Research, Columbia University Medical Center.
References
- Annis HM, Graham JM. The Situational Confidence Questionnaire (SCQ-39) user’s guide. Toronto, Ontario, Canada: Addiction Research Foundation of Ontario; 1988. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. Journal of the American Medical Association. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Antonuccio DO, Danton WG, DeNelsky GY. Psychotherapy versus medication for depression: Challenging the conventional wisdom with data. Professional Psychology: Research and Practice. 1995;26:574–585. [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. Journal of the American Medical Association. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- Basoglu M, Marks IM, Killic C, Brewin CR, Swinson RP. Alprazolam and exposure for panic disorder with agoraphobia: Attribution of improvement to medication predicts subsequent relapse. British Journal of Psychiatry. 1994;164:652–659. doi: 10.1192/bjp.164.5.652. [DOI] [PubMed] [Google Scholar]
- Biondi M, Picardi A. Attribution of improvement to medication and increased risk of relapse of panic disorder with agoraphobia. Psychotherapy and Psychosomatics. 2003;72:110–111. doi: 10.1159/000068687. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, Rounsaville BJ. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Charney DA, Zikos E, Gill KJ. Early recovery from alcohol dependence: factors that promote or impede abstinence. Journal of Substance Abuse Treatment. 2010;38:42–50. doi: 10.1016/j.jsat.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the Composite International Diagnostic Interview Substance Abuse Module (CIDI-SAM): A comprehensive substance abuse interview. British Journal of Addiction. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Fairhurst SK, Piotrowski NA. Self-eficacy and addictive behaviors. In: Maddux JE, editor. Self-efficacy, adapatation, and adjustment: Theory, research and application. New York: Plenum; 1995. pp. 109–141. [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The Timeline Followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. American Journal of Psychiatry. 2005;162:151–161. doi: 10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh RR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goldbeck R, Myatt P, Aitchison T. End of treatment self-efficacy: A predictor of abstinence. Addiction. 1997;92:313–324. [PubMed] [Google Scholar]
- Hartzler B, Witkiewitz K, Villarroel N, Donovan D. Self-efficacy change as a mediator of associations between therapeutic bond and one-year outcomes in treatments for alcohol dependence. Psychology of Addictive Behaviors. 2011;25:269–278. doi: 10.1037/a0022869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Ilgen M, McKellar J, Tiet Q. Abstinence self-efficacy and abstinence 1 year after substance use disorder treatment. Journal of Consulting and Clinical Psychology. 2005;73:1175–1180. doi: 10.1037/0022-006X.73.6.1175. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Feinn R, Tennen H. Targeted naltrexone treatment moderates the relations between mood and drinking behavior among problem drinkers. Journal of Consulting and Clinical Psychology. 2004;72:317–327. doi: 10.1037/0022-006X.72.2.317. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, McCaul ME, Petry NM. Psychotherapy and pharmacotherapy in treatment of substance use disorders. In: Kranzler HR, Ciraulo DA, editors. Clinical manual of addiction psychopharmacology. Arlington, VA: American Psychiatric Publishing, Inc; 2005. pp. 339–363. [Google Scholar]
- Madson MB, Campbell TC. Measures of fidelity in motivational enhancement: A systematic review. Journal of Substance Abuse Treatment. 2006;31:67–73. doi: 10.1016/j.jsat.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Marks l, Swinson RP, Basoglu M, Kuck K, Noshirvani H, Sullivan G. Alprazolam and exposure alone and combined in panic disorder with agoraphobia: A controlled study in London and Toronto. British Journal of Psychiatry. 1993;162:776–787. doi: 10.1192/bjp.162.6.776. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Stauffer R, Lee SM, Rohde CA. Naltrexone dampens ethanol-induced cardiovascular and hypothalamic-pituitary-adrenal axis activation. Neuropsychopharmacology. 2001;25:537–547. doi: 10.1016/S0893-133X(01)00241-X. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Kuerbis A, Kahler CW, Bux DA, Kranzler H. Testing naltrexone and behavioral therapy for problem drinking men-who-have-sex-with-men. Journal of Consulting and Clinical Psychology. doi: 10.1037/a0028615. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench F, Blain L, Morgenstern J, Irwin TW. Self-efficacy and attributions about change in persons attempting to reduce compulsive sexual behavior with medication vs. placebo. Sexual Addiction & Compulsivity. 2011;18:232–242. doi: 10.1080/10720162.2011.593420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J, Irwin TW, Wainberg ML, Parsons JT, Muench F, Bux DA, et al. A randomized controlled trial of goal choice interventions for alcohol use disorders among men-who-have-sex-with-men. Journal of Consulting and Clinical Psychology. 2007;75:72–84. doi: 10.1037/0022-006X.75.1.72. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, McKay J. Rethinking the paradigms that inform behavioral treatment research for substance use disorders. Addiction. 2007;102:1377–1389. doi: 10.1111/j.1360-0443.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. Journal of the American Medical Association. 1999 Mar;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, et al. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Archives of General Psychiatry. 1996;53:217–224. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville BJ. Naltrexone and coping skills therapy for alcohol dependence: A controlled study. Archives of General Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Powers MB, Smits JA, Whitley D, Bystritsky A, Telch MJ. The effect of attributional processes concerning medication taking on return of fear. Journal of Consulting and Clinical Psychology. 2008;76:478–490. doi: 10.1037/0022-006X.76.3.478. [DOI] [PubMed] [Google Scholar]
- Sanchez-Craig M, Annis HM, Bornet AR, MacDonald KR. Random assignment to abstinence and controlled drinking: Evaluation of a cognitive-behavioral program for problem drinkers. Journal of Consulting and Clinical Psychology. 1984;52:390–403. doi: 10.1037//0022-006x.52.3.390. [DOI] [PubMed] [Google Scholar]
- Schwarzer R, Fuchs R. Self-efficacy and health behaviors. In: Conner M, Norman P, editors. Predicting health behavior. Buckingham: Open University Press; 1996. pp. 163–196. [Google Scholar]
- Vielva I, Iraugi I. Cognitive and behavioural factors as predictors of abstinence following treatment for alcohol dependence. Addiction. 2001;96:297–303. doi: 10.1046/j.1360-0443.2001.96229713.x. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayasguda M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Donovan DM, Hartzler B. Drink refusal training as part of a combined behavioral intervention: Effectiveness and mechanisms of change. Journal of Consulting and Clinical Psychology. 2012;80:440–449. doi: 10.1037/a0026996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweben A. Integrating pharmacotherapy and psychosocial interventions in the treatment of individuals with alcohol problems. Journal of Social Work Practice in the Addictions. 2001;1:65–80. [Google Scholar]