Abstract
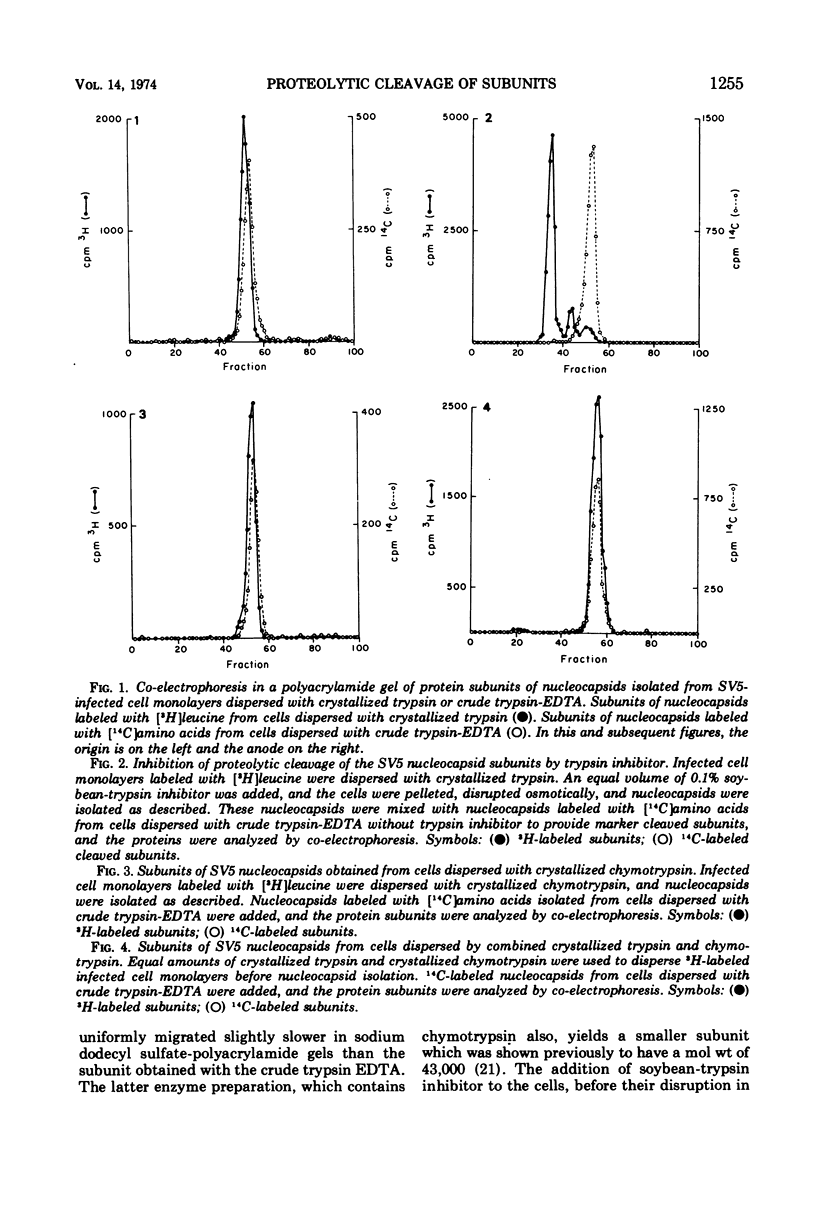
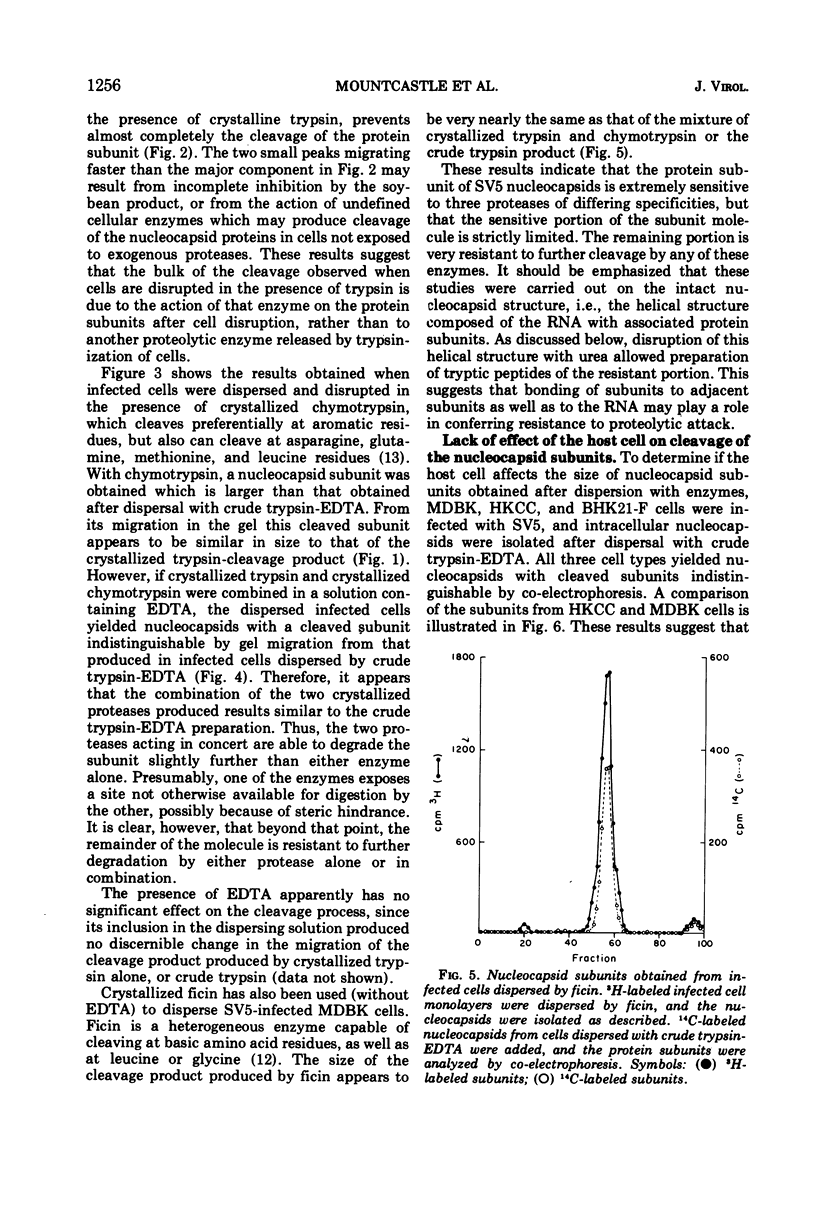
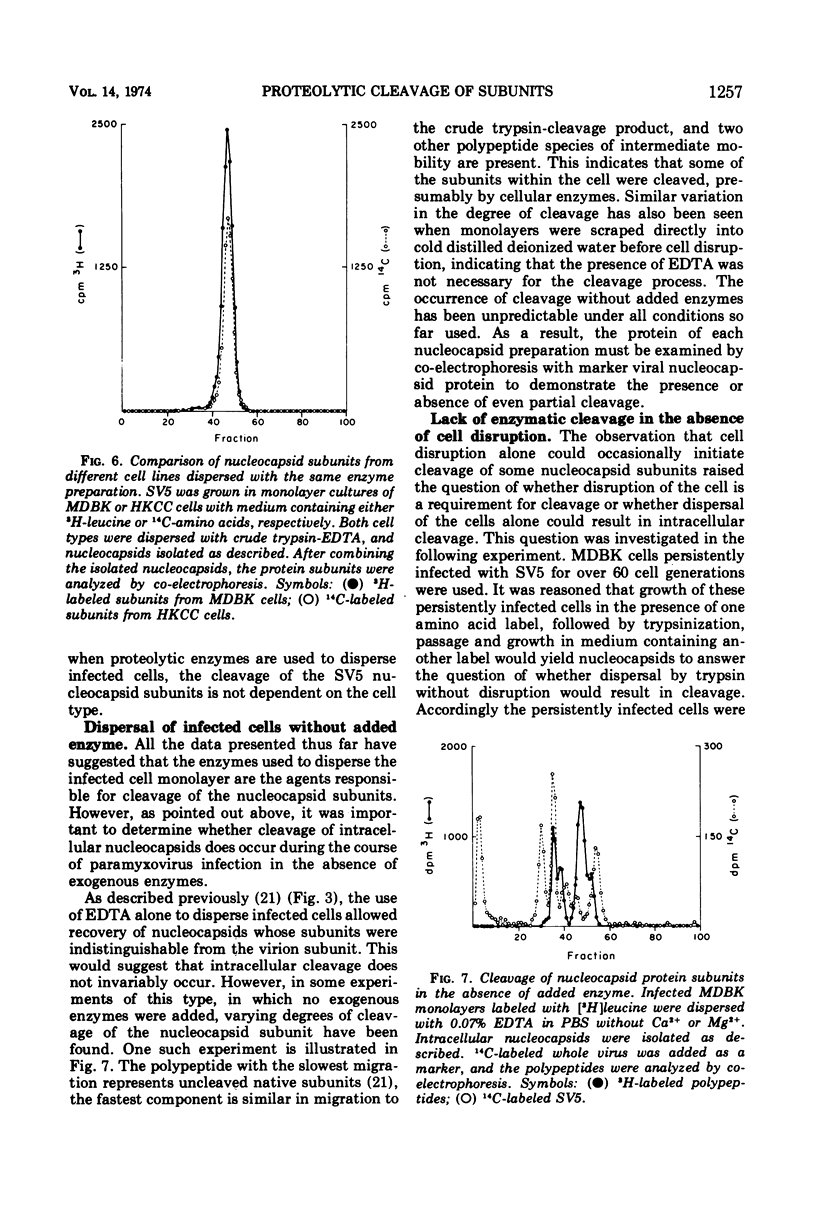
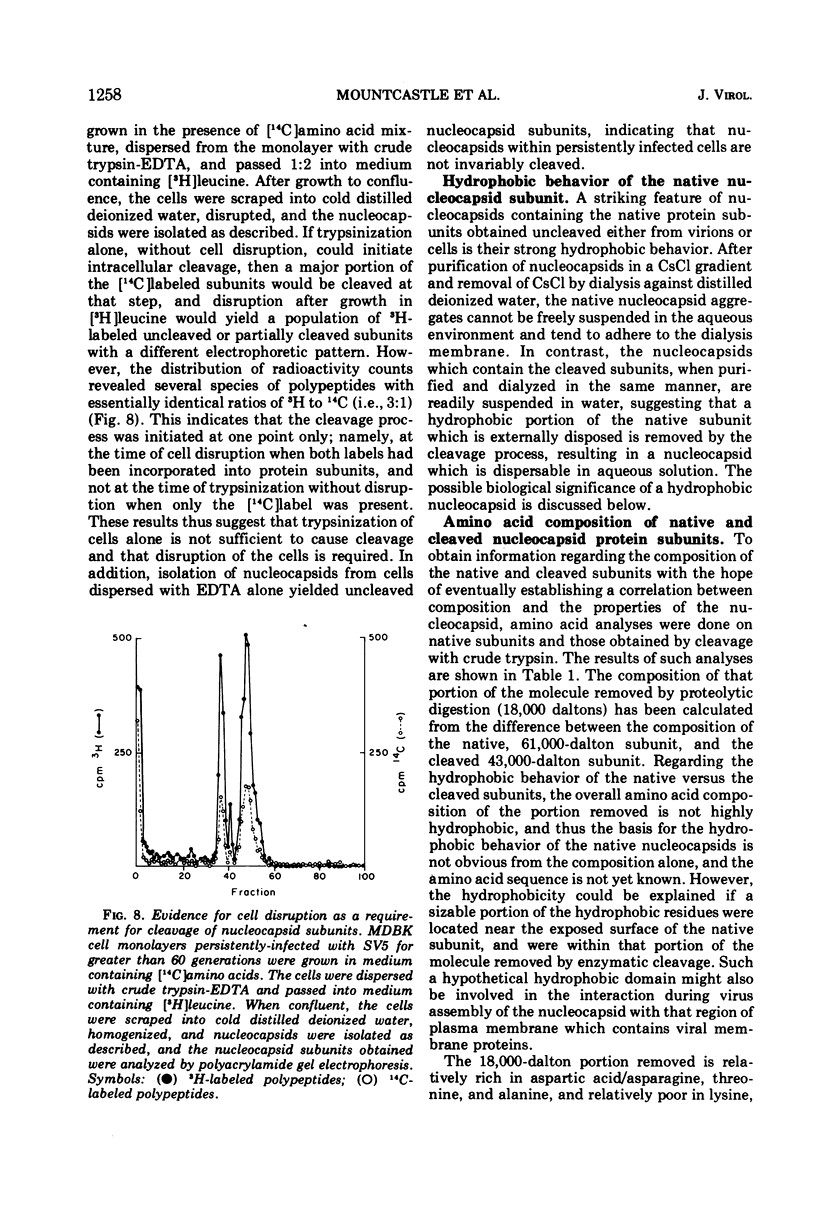
The protein subunits of the nucleocapsid of the parainfluenza virus simian virus 5 isolated from infected cells after dispersion with trypsin, chymotrypsin, or ficin are cleaved proteolytically. The molecular weights of the subunits which result from cleavage depend on the enzyme used, but are around 43,000, compared to the native subunit of 61,000. In most instances cleavage of the subunit appears to be due to the protease used to disperse the cell, and follows cell disruption. Nucleocapsids composed of native, uncleaved subunits can frequently be obtained from infected cells dispersed without a proteolytic enzyme; however, cleavage occasionally occurs even under those conditions, indicating that cellular proteases can at times cleave this protein. Nucleocapsids containing uncleaved subunits can be isolated from cells persistently infected with simian virus 5, indicating that persistent infection is not invariably associated with intracellular cleavage of this protein. Nucleocapsids composed of native subunits are hydrophobic, whereas those composed of the cleaved subunit can be dispersed in aqueous solution. It is suggested that the portion of the molecule removed by cleavage may be responsible for a specific interaction during virus assembly between the nucleocapsid and those areas of plasma membrane which contain the non-glycosylated viral membrane protein, which is also hydrophobic. An amino acid analysis of native and cleaved subunits has been done. The portion of the subunit removed by cleavage does not have a high proportion of hydrophobic residues, suggesting that those present are arranged together to form a hydrophobic domain.
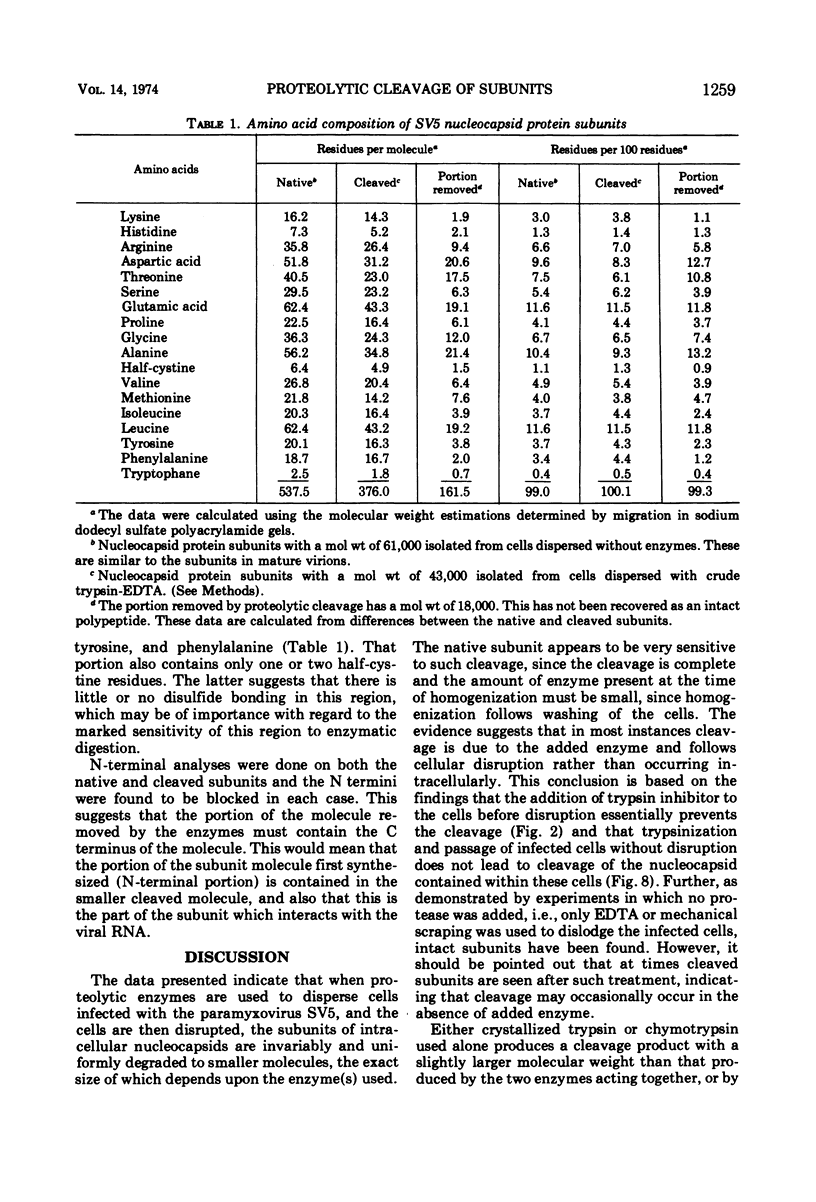
The N termini of both the native and cleaved subunits are blocked. This suggests that the portion of the molecule which is externally disposed and removed by cleavage contains the C terminus, and the cleaved subunit which reacts with the viral RNA contains the N terminus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W. MULTIPLICATION OF A MYXOVIRUS (SV5) WITH MINIMAL CYTOPATHIC EFFECTS AND WITHOUT INTERFERENCE. Virology. 1964 Jun;23:224–233. doi: 10.1016/0042-6822(64)90286-7. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc Natl Acad Sci U S A. 1967 Apr;57(4):949–956. doi: 10.1073/pnas.57.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. The nucleic acid of the parainfluenza virus SV5. Virology. 1968 Jun;35(2):289–296. doi: 10.1016/0042-6822(68)90269-9. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Mountcastle W. E., Choppin P. W. The sense of the helix of paramyxovirus nucleocapsids. J Mol Biol. 1972 Mar 14;65(1):167–169. doi: 10.1016/0022-2836(72)90499-8. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham A. C.H. The cause of irreversible polymerisation of tobacco mosaic virus protein. FEBS Lett. 1972 Sep 1;25(1):147–152. doi: 10.1016/0014-5793(72)80473-3. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt T. J., Seide R. K., Lackland H., Thunberg A. L. Serologic identity of the b4 allotypic determinants present on homogeneous rabbit light chains with different N-terminal amino acid sequences. J Immunol. 1972 Oct;109(4):735–741. [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Caliguiri L. A., Choppin P. W. Nucleocapsid protein subunits of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1970 Nov;6(5):677–684. doi: 10.1128/jvi.6.5.677-684.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagi S., ter Meulen V., Katz M., Koprowski H. Comparison of subacute sclerosing panencephalitis and measles viruses: an electron microscope study. J Virol. 1971 Jan;7(1):176–187. doi: 10.1128/jvi.7.1.176-187.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



