Abstract
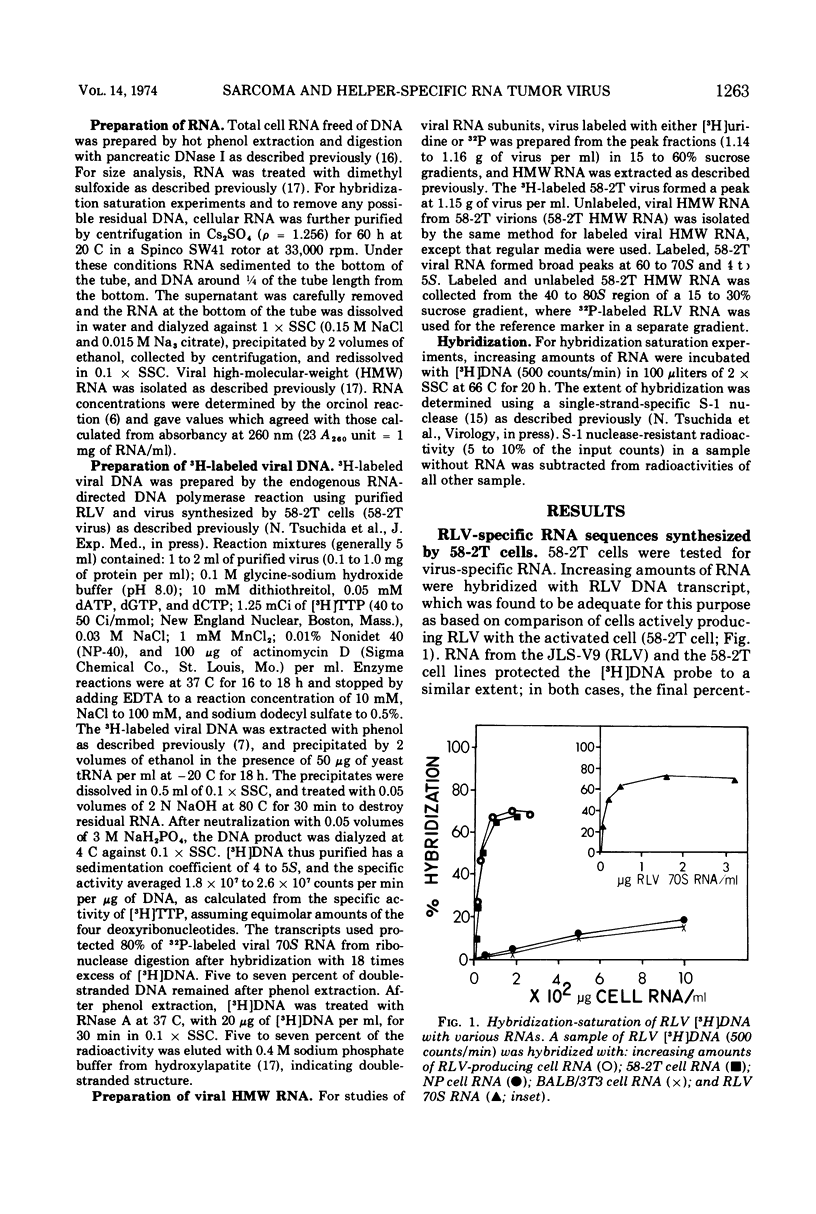
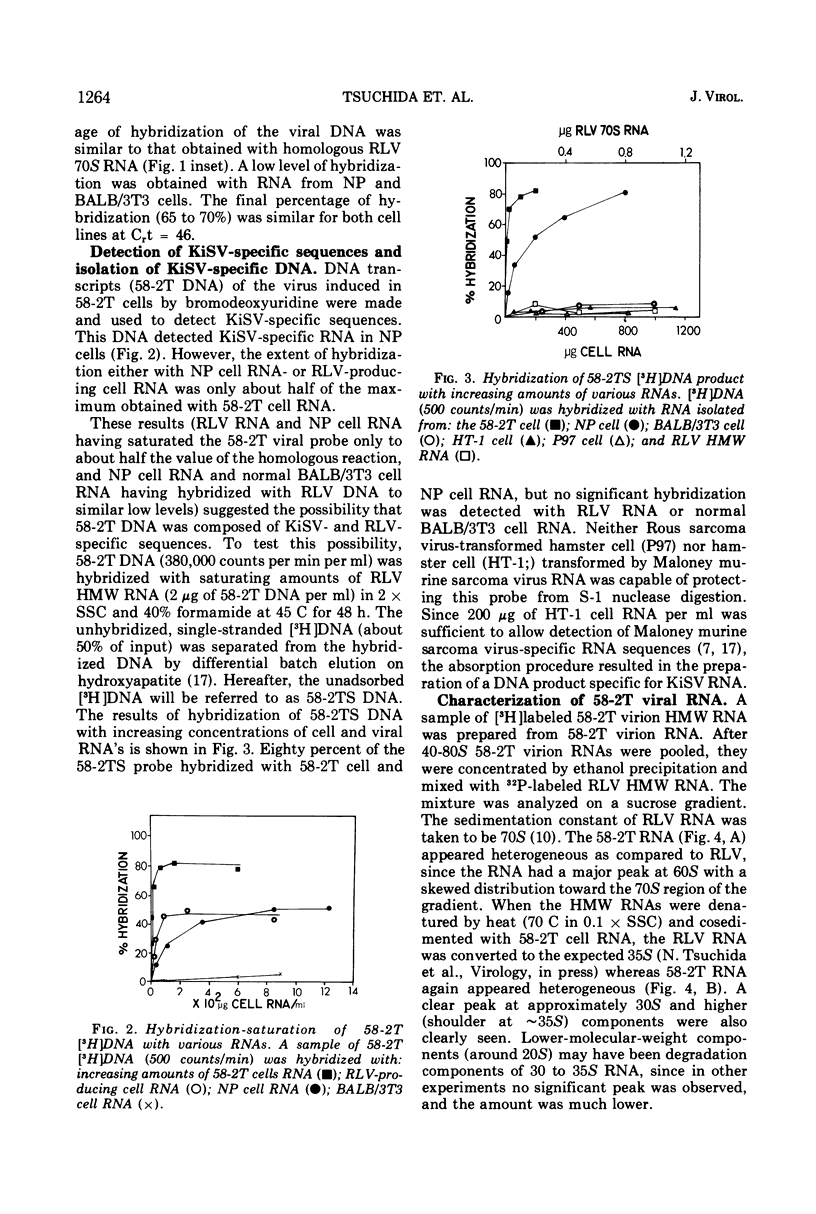
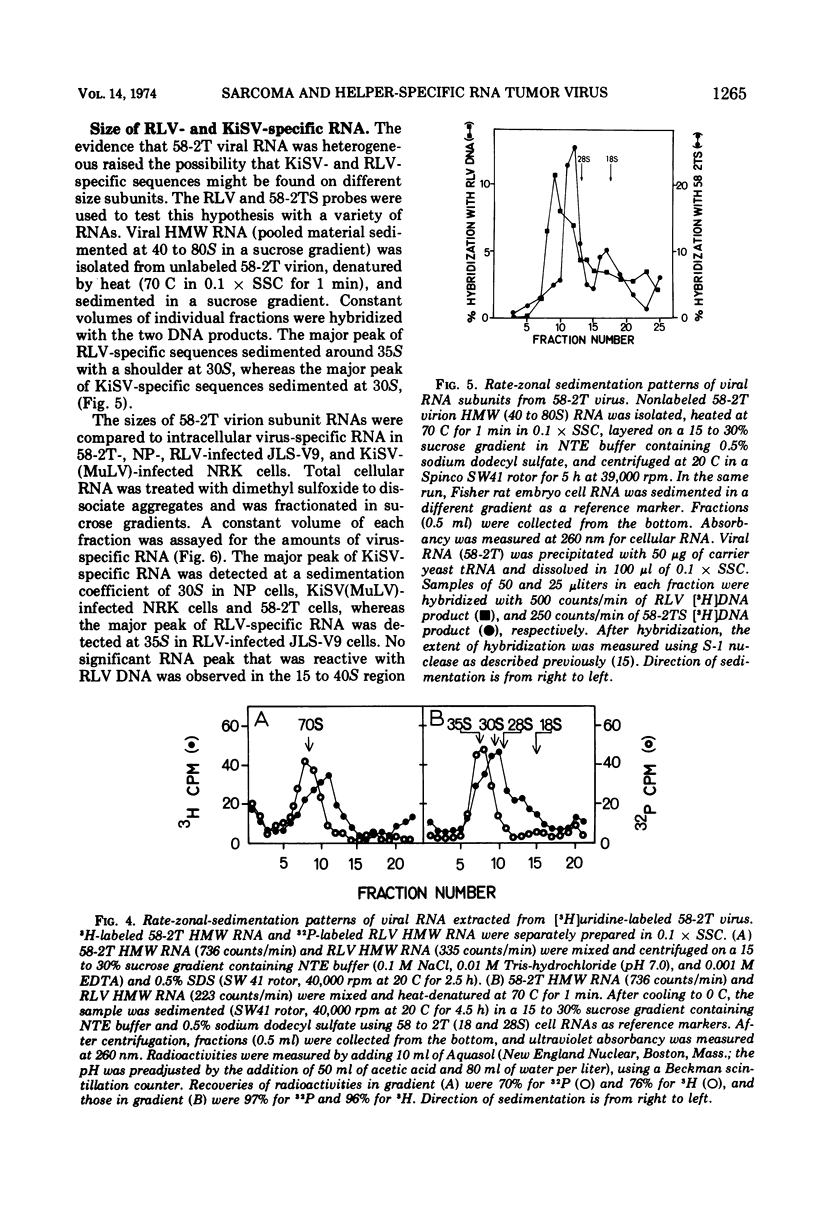
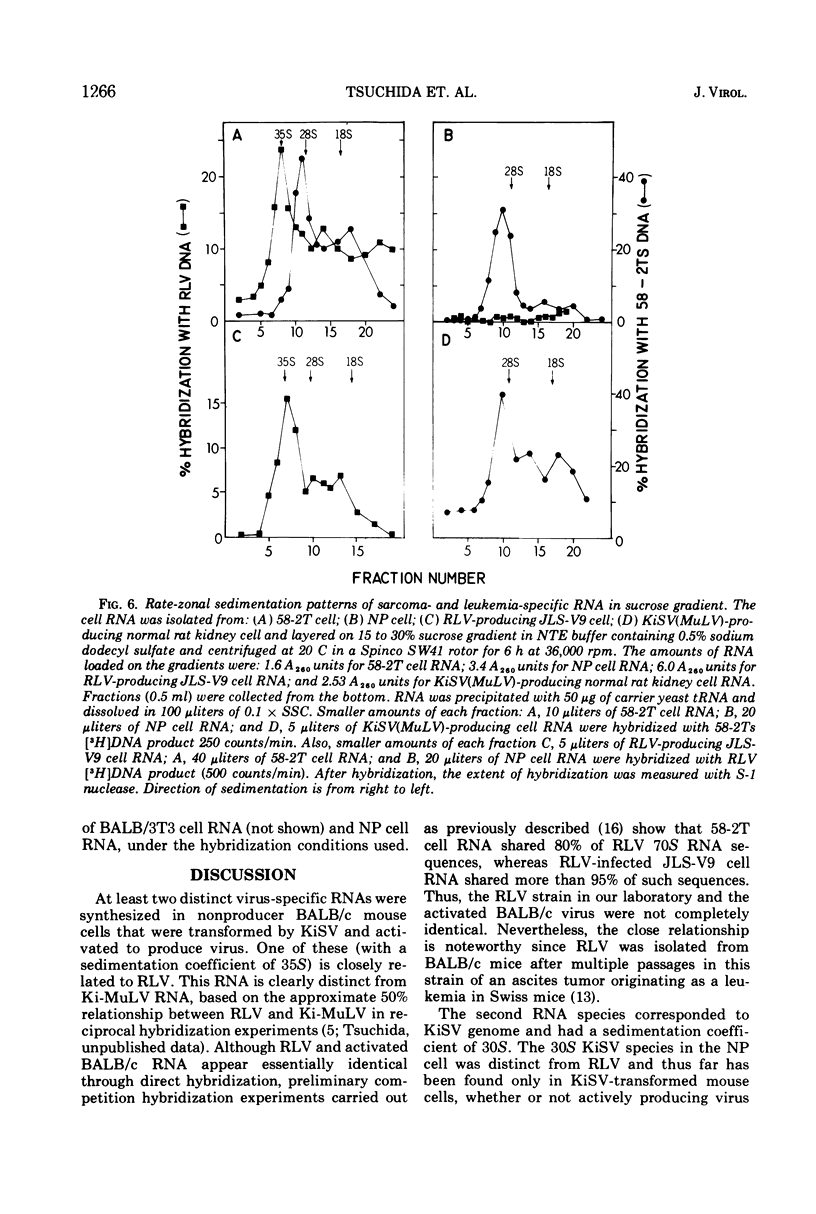
A tumor line (58-2T) was established from a slowly growing tumor in a BALB/c mouse inoculated with M58-2 cells. The latter clonal cell line was isolated after bromodeoxyuridine treatment as a flat variant from nonproducer BALB/3T3 cells transformed by the Kirsten sarcoma virus. The 58-2T cells produced type C virus with two discrete virus-specific RNA species. One of the species, which was probably an endogenous virus RNA subunit, had a sedimentation coefficient of 35S as the largest major subunit, and had sequences similar to Rauscher leukemia virus RNA based on nucleic acid hybridization. The other RNA species had 30S as the largest major subunit and corresponded to Kirsten sarcoma virus-specific RNA. These two RNA species formed heterogeneous, 60 to 70S, high-molecular-weight RNA in virions.
DNA transcripts (58-2T DNA) from the activated virus contained base sequences complementary to Rauscher leukemia virus and Kirsten sarcoma virus. The Kirsten sarcoma virus-specific DNA sequences (58-2TS) were purified from 58-2T DNA by eliminating RLV-specific sequences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Chemical induction of focus-forming virus from nonproducer cells transformed by murine sarcoma virus. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3069–3072. doi: 10.1073/pnas.68.12.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M. Biochemical studies on adenovirus multiplication. 1. Stimulation of phosphorus incorporation into deoxyribonucleic acid and ribouncleic acid. Virology. 1959 Nov;9:343–358. doi: 10.1016/0042-6822(59)90127-8. [DOI] [PubMed] [Google Scholar]
- Green M., Rokutanda H., Rokutanda M. Virus specific RNA in cells transformed by RNA tumour viruses. Nat New Biol. 1971 Apr 21;230(16):229–232. doi: 10.1038/newbio230229a0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Klein R., Lomg C. W., Gilden R. Mutants of nonproducer cell lines transformed by murine sarcoma virus. II. Relationship of tumorigenicity to presence of viral markers and rescuable sarcoma genome. J Exp Med. 1973 Aug 1;138(2):364–372. doi: 10.1084/jem.138.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Klein R., Toni R., Walker J., Gilden R. Mutants of nonproducer cell lines transformed by murine sarcoma viruses. I. Induction, isolation, particle production, and tumorigenicity. J Exp Med. 1973 Aug 1;138(2):356–363. doi: 10.1084/jem.138.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luborsky S. W. Sedimentation equilibrium analysis of the molecular weight of a tumor virus RNA. Virology. 1971 Sep;45(3):782–787. doi: 10.1016/0042-6822(71)90195-4. [DOI] [PubMed] [Google Scholar]
- Maisel J., Klement V., Lai M. M., Ostertag W., Duesberg P. Ribonucleic acid components of murine sarcoma and leukemia viruses. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3536–3540. doi: 10.1073/pnas.70.12.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe H., Gilden R. V., Hatanaka M. Specificity of the DNA product of RNA-dependent DNA polymerase in type C viruses. II. Quantitative analysis. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3923–3927. doi: 10.1073/pnas.70.12.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUSCHER F. J. A virus-induced disease of mice characterized by erythrocytopoiesis and lymphoid leukemia. J Natl Cancer Inst. 1962 Sep;29:515–543. [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Green M. Intracellular and virion 35 S RNA species of murine sarcoma and leukemia viruses. Virology. 1974 May;59(1):258–265. doi: 10.1016/0042-6822(74)90221-9. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]



