Abstract
Subtilisin-like proteases have been proposed to play an important role for parasite survival in Toxoplasma gondii (Tg) and Plasmodium falciparum. The T. gondii subtilase TgSUB1 is located in the microneme, an apical secretory organelle whose contents mediate adhesion to the host during invasion. TgSUB1 is predicted to contain a glycosyl-phosphatidylinositol (GPI) anchor. This is unusual as Toxoplasma GPI-anchored proteins are targeted to the parasite's surface. In this study, we report that the subtilase TgSUB1 is indeed a GPI-anchored protein but contains dominant microneme targeting signals. Accurate targeting of TgSUB1 to the micronemes is dependent upon several factors including promoter strength and timing, accurate processing and folding. We analyzed the targeting domains of TgSUB1 using TgSUB1 deletion constructs and chimeras made between TgSUB1 and reporter proteins. The TgSUB1 prodomain is responsible for trafficking to the micronemes and is sufficient for targeting a reporter protein to the micronemes. Trafficking is dependent upon correct folding or other context-dependent conformation as the prodomain expressed alone is unable to reach the micromenes. Therefore, TgSUB1 is a novel example of a GPI-anchored protein in T. gondii that bypasses the GPI-dependent surface trafficking pathway to traffic to micronemes, specialized regulated secretory organelles.
Keywords: GPI protein, microneme, subtilase, Toxoplasma gondii, trafficking
Toxoplasma gondii (Tg) is an obligate intracellular parasite belonging to the phylum Apicomplexa. Other parasites in this phylum include Plasmodium, Cryptosporidium, Neospora and Eimeria, which are important human and animal pathogens. Although the host cell type varies among the Apicomplexa, the mechanism of invasion is remarkably similar. Common to this group of protozoa is an apical complex of secretory organelles: micronemes, rhoptries and dense granules. The successful completion of cell invasion is highly dependent on sequential secretion of the apical organelles (1,2).
The trafficking of secretory proteins to their respective organelles provides clues as to their possible functions. Regulation of intracellular transport of secretory proteins is proving to be complex.
Trafficking of proteins can be based on one or a combination of factors including specific sorting motifs, proteolytic cleavage and/or timing of expression. Toxoplasma glycosyl-phosphatidylinositol (GPI)-anchored proteins like surface antigen 1 (SAG1 or p30) are targeted to the parasite's surface through the GPI anchor. The GPI anchor sequence has been found to direct microneme proteins or heterologous reporters to the cell surface (3,4). Many microneme proteins (e.g. MIC2, MIC6 and MIC8) contain transmembrane domains with conserved tyrosine-based signal and acidic amino acid motifs within the cytoplasmic tail necessary for the microneme targeting (4,5). Moreover, soluble micronemal proteins (e.g. M2AP, MIC3, MIC4 and MIC1) associate with transmembrane micronemal proteins in a complex to be targeted to the microneme. Disruption of one of the proteins affects its partner's targeting (4,6).
Proper targeting of many proteases is dependent upon the prodomain. Prodomains can act as intramolecular chaperones (7,8). Thus, inefficient cleavage can perturb folding and/or association with trafficking partners, leading to retention or mistargeting (9). In some cases, specific sequences in the prodomain contain targeting signals (10). In T. gondii, the prodomain of several apical organelle proteins including MIC3, M2AP and ROP1 are important for apical targeting (11–13). Finally, subcellular localization of secretory proteins can also be promoter dependent (14,15). Localization and timing of expression of microneme, rhoptry and apicoplast proteins can be affected by the sequences driving expression (16,17).
TgSUB1 is a micronemal subtilisin-like serine protease that localizes to the cell surface after microneme secretion. Like other subtilases, TgSUB1 is cotranslationally proteolytically processed and undergoes further proteolytic maturation upon release from the micronemes (18). Subtilisins may play a critical role for parasite survival as serine protease inhibitors prevent invasion in T. gondii and Plasmodium falciparum (19,20) and block intracellular development (21) and egress from host cells (22). The specificity of function for proteases could be conferred by their substrate specificity or by localization and access to substrates. Therefore, trafficking determinants of proteases are of interest.
In this study, we report that TgSUB1 is associated to the parasite plasma membrane by a GPI anchor. Thus, TgSUB1 is distinctive in that it contains a GPI anchor, yet does not localize to the parasite's surface. TgSUB1 does not contain known microneme targeting motifs and has not been shown to associate with any other microneme proteins (unpublished data). Our studies illustrate that although many factors affect targeting of TgSUB1, the prodomain is necessary for trafficking to the micronemes.
Results
TgSUB1 is linked to the membrane through a GPI anchor
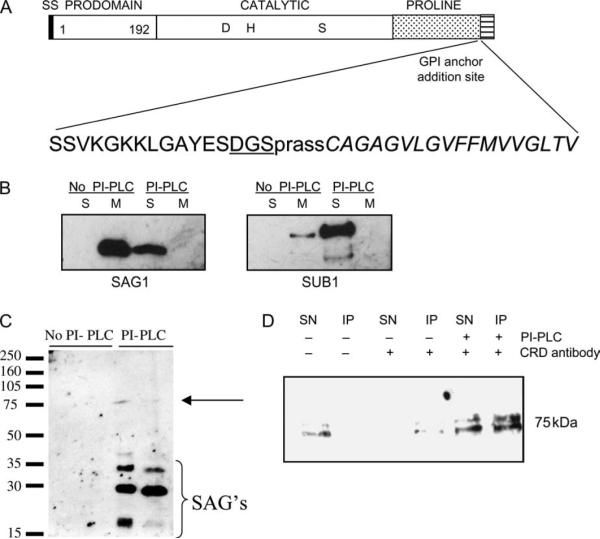
The amino acid coding sequence of TgSUB1 is predicted to have a GPI anchor addition site (Figure 1A) (18). We utilized phosphatidylinositol-specific phospholipase C (PI-PLC) and Triton-X-114 (TX-114) extraction to test whether TgSUB1 has a GPI anchor. PI-PLC specifically cleaves GPI-anchored proteins between the fatty acid group that tethers the protein to the membrane and the phosphatidylinositol group, thereby dissociating the protein from the membrane. Whole cell parasite lysate was incubated with and without PI-PLC, and products were extracted with TX-114. GPI-anchored proteins partition into the detergent phase but become soluble after treatment with the enzyme PI-PLC.
Figure 1. TgSUB1 behaves like a GPI-anchored protein.
A) A schematic of TgSUB1 is shown indicating the signal sequence (ss), prodomain, catalytic domain, proline-rich domain and putative GPI anchor addition site. The amino acid residues encompassing the prodomain are indicated. The amino acids surrounding the proposed GPI addition site are illustrated. The GPI anchor addition site consists of a triad of amino acids (underlined) followed by a hydrophilic spacer region (lowercase) and a hydrophobic region (italics). The specific amino acids (aspartic acid, glycine and serine) of the GPI addition site and length of the subsequent domains are consistent with other known GPI-anchored proteins. B) TX-114 phase partitioning of TgSUB1. RH tachyzoite lysates were either treated or mock treated with PI-PLC, an enzyme that specifically cleaves GPI-anchored proteins inducing transition from a membrane-associated state (M) to a soluble state (S). SAG1, a known GPI-anchored protein, is shown for comparison. SAG1 is revealed with P30 antibody and TgSUB1 with AE653 antibody. Because of ongoing proteolysis of TgSUB1, AE653 antisera were used for all westerns. These antisera, which recognize a peptide adjacent to the GPI addition site, does not recognize TgSUB1 released into the media after secretion by micronemes (C). RH tachyzoite lysates treated or mock treated with PI-PLC were probed with the anti-CRD GPI-specific epitope antibody. Results are shown in duplicate. D) CRD antibody immunoprecipitates PI-PLC-cleaved TgSUB1. Immunoprecipitation was performed with CRD antibody. TgSUB1 was detected by western blot analysis with PfSUB1 antibody. Immunoprecipitations were performed (i) on untreated lysates with no primary CRD antibody, (ii) on untreated lysates with CRD antibody and (iii) with PI-PLC-treated tachyzoite lysates immunoprecipitated with CRD antibody. IP, precipitated protein fraction (precipitated with protein A beads); SN, supernatant containing non-precipitated proteins.
Aqueous and detergent phases were probed and examined for TgSUB1 and for the known GPI-anchored protein SAG1 (23). TgSUB1 and SAG1 were detected in the detergent phases upon TX-114 extraction without PI-PLC (Figure 1B). Upon addition of PI-PLC, SUB1 and SAG1 were no longer detected in the detergent phase but in the soluble phase. Because TgSUB1 can undergo proteolysis to be released from the membrane (18), we verified these results using other experimental tests.
Cleavage with PI-PLC results in the formation of a 1,2-cyclic phosphate ring left behind the cleaved GPI anchor. This specific epitope can be recognized with anti-cross-reacting determinant (CRD) antibodies purified from serum reactive to Trypanosoma brucei variant surface proteins. These antisera have previously been shown to recognize T. gondii GPI proteins after PI-PLC cleavage (23). Tachyzoite lysates were probed with CRD antibody following PI-PLC treatment. A faint band corresponding to the predicted size of cleaved TgSUB1 (75 kDa) could be detected with the CRD antibody (Figure 1C). To confirm the protein detected was TgSUB1, total T. gondii lysates were immunoprecipitated with CRD antibody. Western blots of the immunoprecipitated proteins were probed for TgSUB1 with PfSUB1 antisera. A small amount of CRD-reactive TgSUB1 was detected in untreated parasite lysates with enrichment of CRD-reactive TgSUB1 after PI-PLC treatment and immunoprecipitation (Figure 1D). Because of the abundance of other GPI-anchored proteins, CRD immunoprecipitations were not quantitative.
Metabolic labeling of anchor components is another method to identify GPI membrane anchors. [3H] ethanolamine and [3H] palmitate are incorporated into GPI-anchored surface proteins SAG1, 3 and 4 (24). [3H] ethanolamine can only be incorporated into a protein through a GPI anchor, whereas palmitate can be incorporated into GPI-anchored proteins or modify cysteine residues of acylated proteins through a thioester linkage. Palmitate-labeled GPI-anchored proteins can be distinguished from other palmitoylated proteins by treatment with hydroxylamine, which breaks thioester linkages of palmitate to acylated proteins without disrupting association of palmitate with GPI-anchored proteins. Extracellular tachyzoites were radiolabeled with [3H] ethanolamine or [3H] palmitate before immunoprecipitation with the PfSUB1 antibody. TgSUB1 immunoprecipitates were labeled with ethanolamine and with palmitate (Figure 2). In addition, palmitate labeling was stable in the presence of hydroxylamine. Altogether, these results show that TgSUB1 is membrane associated through a GPI anchor.
Figure 2. Biosynthetic labeling of the TgSUB1 GPI anchor.
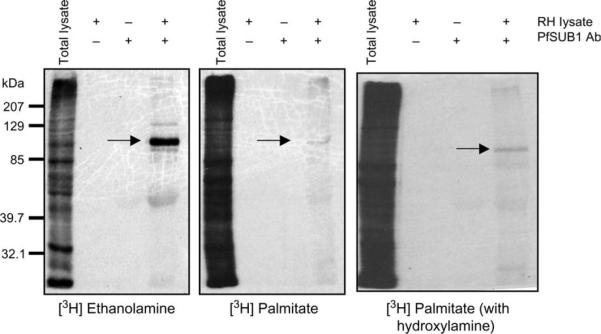
RH tachyzoites lysates were radiolabeled with [3H] ethanolamine and [3H] palmitate, markers of GPI anchors. Following radiolabeling, TgSUB1 was immunoprecipitated with the PfSUB1 antibody (arrow). Controls for immunoprecipitation were lysate incubated without antibody and antibody without lysate. The right panel represents palmitate-labeled proteins washed with hydroxylamine. Palmitate incorporated into GPI anchors is resistant to hydroxylamine treatment. Labeling of TgSUB1 with palmitate was sustained in the presence of hydroxylamine.
Disruption of the gene expressing TgSUB1
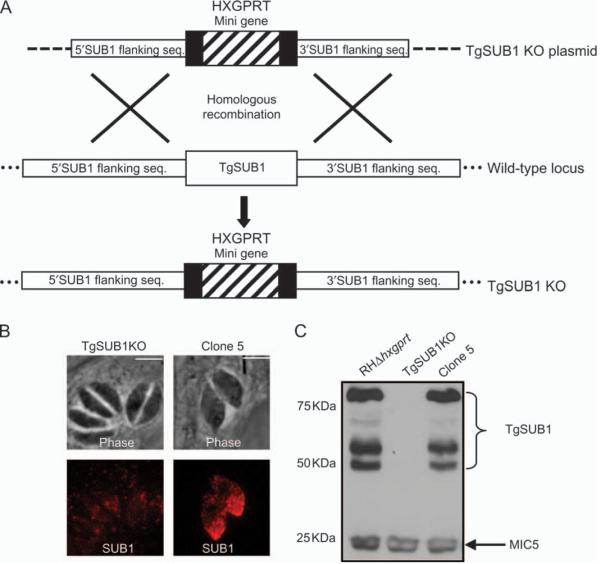
The processing and targeting of TgSUB1 were evaluated in a knockout (KO) strain generated by double homologous recombination. We generated two strains with disruption of TgSUB1 (RHgra1-GFP-tub-catΔhxgprt background and RHΔhxgprt background; Materials and Methods). Because the presence of GFP made dual labeling immunofluorescence analysis (IFA) experiments difficult, data presented are from the strain made in the RHΔhxgprt background, but results were similar in both KO strains. A single parasite clone (clone 5) containing the targeting construct, but expressing TgSUB1, was used as a control. Immunofluorescent staining and western blotting of TgSUB1KO parasites confirmed loss of expression of TgSUB1 with normal targeting and expression of other microneme proteins (Figures 3B,C, Figure S1A and data not shown). Neither KO strain had impairment in intracellular growth (Figure S1B and data not shown). Intracellular processing of microneme proteins was not affected during trafficking in the TgSUB1KO strain (proteins tested: M2AP, MIC6, MIC3, MIC5 and AMA1; Figure S2). Further phenotypic analysis of TgSUB1KO parasites is ongoing.
Figure 3. Generation of a TgSUB1 KO parasite.
A) A diagram of the strategy used to disrupt the TgSUB1 gene by homologous recombination in the RH background is shown. The TgSUB1KO plasmid is seen on the top line showing 2.9 kb of 5′ and 3′ DNA flanking the coding region of TgSUB1. Dashed lines indicate the rest of the targeting plasmid sequence. TgSUB1 is replaced by the HXGPRT cassette, which includes the HXGPRT gene surrounded by the DHFR promoter and terminator sequences. Dots signify the rest of the genomic locus. B and C) IFA and western blot analysis of TgSUB1KO and clone 5 parasites (TgSUB1 was revealed with AE653 antibody). MIC5 is used as internal control for western blot loading. TgSUB1 immunofluorescence signal depicts the conventional apical microneme labeling. Scale bar: 5 μm.
Microneme-specific localization of TgSUB1 is promoter specific
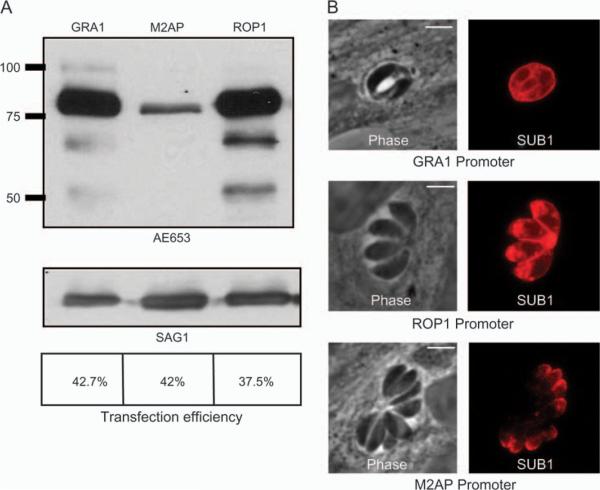
We and others have observed that overexpression of secreted proteins can lead to mistrafficking (16). To follow TgSUB1 localization, a hemagglutinin (HA) epitope tag was inserted just before the TgSUB1 GPI anchor addition site to avoid disruption of membrane association (25). We tested expression of the full-length HA-tagged TgSUB1 (FL5SUB1-HA) construct under three different promoters driving expression of three different secretory organelle proteins: GRA1 (dense granules), ROP1 (rhoptries) and M2AP (micronemes). TgSUB1 levels were higher as detected by western blot analysis when expression was driven by ROP1 and GRA1 (Figure 4A). TgSUB1 expression driven by the GRA1 promoter resulted in localization to the parasitophorous vacuole and parasite surface (Figure 4B and Figure S2). When TgSUB1 was expressed using the ROP1 promoter, some TgSUB1 was located apically with variable colocalization with micronemal markers (Figure 4B and Figure S2). In some tachyzoites, TgSUB1 was also targeted to the cell surface (Figure 4B and Supplementary Data S2). Expression driven by the M2AP promoter showed that TgSUB1 properly localized to the micronemes as verified by the colocalization with other micronemal proteins (Figure 4B and Figure S2). Expression of TgSUB1 using the SAG1 promoter was similar to results with M2AP, but results were less consistent (data not shown). All other targeting constructs were driven by the M2AP promoter.
Figure 4. TgSUB1 localization depends upon the promoter.
A) Western blot analysis of tachyzoites transiently transfected with TgSUB1-HA (FL5SUB1-HA) driven by the M2AP, GRA1 and ROP1 promoters. Blots were probed with anti-TgSUB1 AE653 antibody or probed with antibodies against SAG1 to ensure that equivalent levels of parasite proteins were being compared. Transfection efficiencies are indicated. B) Immunofluorescence of tachyzoites transiently transfected with TgSUB1-HA driven by the M2AP, GRA1 and ROP1 promoters. Antibody AE653 was used to show localization of the different TgSUB1 constructs. Scale bar: 5 μm.
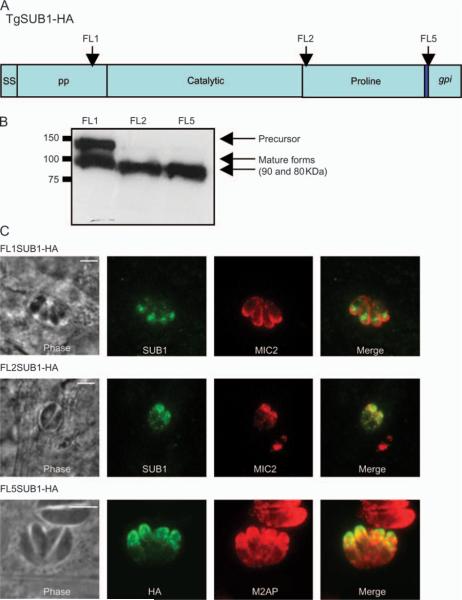
The prodomain processing is important for the targeting
An HA tag was inserted in various domains of the protein and their localization monitored in the TgSUB1KO background (Figure 5). Most TgSUB1 constructs were correctly processed to the lower molecular weight forms (Figure 5B) and were targeted to the micronemes (Figure 5C). However, the HA tag inserted in the prodomain (FL1SUB1-HA) results in incomplete processing as reflected in accumulation of the precursor (Figure 5B). The construct was less efficiently targeted to the micronemes and seemed to be arrested in within vesicles within the secretory pathway (Figure 5C). Prior pulse–chase studies have shown that the initial processing occurs rapidly and is Brefeldin A insensitive, consistent with the prodomain processing occurring cotranslationally in the endoplasmic reticulum (ER) as is typical of subtilases (18). Therefore, the impairment of processing is unlikely to be ascribed to inaccessibility to the compartment required for prodomain processing. Rather, proper autocatalytic prodomain processing appears to facilitate trafficking.
Figure 5. Effect of position of the HA tag in TgSUB1 upon microneme localization.
A) A schematic of TgSUB1-HA is represented with different domains (ss, signal sequence; pp, prodomain; catalytic, catalytic site; proline, proline-rich domain and gpi, C-terminal part containing GPI anchor signal). The dark blue rectangle represents the HA tag in FL5SUB1-HA. The arrows indicate other positions where the HA tag has been inserted. B) Western blot analysis of M2AP-driven TgSUB1-HA tagged constructs after transient transfection of RH TgSUB1KO tachyzoites. Membranes were probed with a rat anti-HA antibody. C) IFA of tachyzoites transfected with different TgSUB1-HA constructs. TgSUB1 expression was detected with a rat anti-HA antibody or the AE653 anti-TgSUB1 antibody. MIC2 and M2AP were used as markers for micronemes and were visualized with proper antibodies. Scale bar: 5 μm.
Importance of the propeptide of TgSUB1 in its intracellular localization
To examine the effect of individual domains on the localization of TgSUB1, we created deletion constructs lacking the propeptide, the catalytic domain, the proline-rich domain or the GPI anchor (Figure 6). The GPI anchor of SAGs has been shown to be necessary for surface localization (3,4). A TgSUB1 construct lacking the GPI anchor goes to the micronemes (Figure 6A). Moreover, when the TgSUB1 GPI anchor was replaced by the SAG1 GPI anchor addition site, the TgSUB1 chimera construct still targeted to the micronemes, although in some cases colocalization with another microneme marker was incomplete (Figure 6B).
Figure 6. Expression of TgSUB1-HA deletion constructs.
Deletion constructs were transiently transfected into RH TgSUB1KO parasites. The dark blue rectangle indicates the HA tag (A, C–E). Immunofluorescence of intracellular tachyzoites containing the constructs was performed with a rat anti-HA antibody. In (B), the GPI domain corresponds to the C-terminal part of SAG1 containing the GPI anchor signal fused to the full length of TgSUB1 sequence. The construct was visualized with the AE653 anti-TgSUB1 antibody. M2AP and MIC2 were used as markers for micronemes. Scale bar: 5 μm.
We hypothesized that the TgSUB1 sequence might contain a domain, allowing its microneme targeting and thus bypassing the GPI routing to the parasite surface. As prolines are frequently critical in other protein–protein interactions, we tested if TgSUB1's proline-rich domain was responsible for microneme targeting. A construct lacking the proline-rich domain still goes to the micronemes (Figure 6C). Fusion of the proline-rich domain to SAG1 did not alter SAG1 targeting to the cell surface (Figure S3). Surprisingly, a TgSUB1 protein lacking the catalytic domain still localizes to micronemes (Figure 6D). However, a construct lacking the prodomain remained in an area surrounding the nucleus of the parasite (Figure 6E). Altogether, these results showed that the GPI anchor, the proline-rich domain and catalytic domain are not involved for the microneme targeting, whereas the prodomain appears to be essential for trafficking.
Prodomain folding is essential for microneme targeting
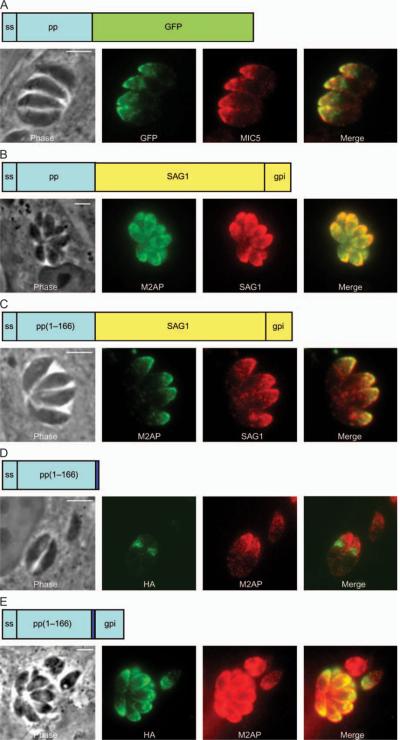
To test if the TgSUB1 prodomain contains a microneme targeting signal, we fused the TgSUB1 prodomain to the heterologous reporter genes GFP and SAG1. Both of these proteins have been successfully used to dissect targeting motifs in T. gondii (4,5,12). SAG1, the major tachyzoite surface antigen or SAG, is targeted to the surface through its GPI anchor (24). The two chimera constructs were correctly targeted to the micronemes (Figure 7A,B). To further map the targeting domain, we deleted 26 residues from the C-terminal portion of the predicted prodomain and fused it to SAG1. This pp(1–166)-SAG1 construct was also targeted correctly to the micronemes (Figure 7C).
Figure 7. Expression of TgSUB1 prodomain constructs.
Chimeras containing GFP and SAG1 fused to the signal sequence and prodomain of TgSUB1 were transiently transfected into RH TgSUB1KO and RH SAG1KO parasites, respectively. The GFP reporter construct was detected by fluorescence of the GFP protein (A), whereas the SAG1 reporter construct was detected with a SAG1 antibody (B, C). The prodomain–HA and the prodomain–HA–GPI constructs were detected with a rat anti-HA antibody (D, E). Colocalizations performed with antibodies specific for microneme proteins M2AP and MIC5. All the TgSUB1 prodomain reporter constructs consist of the full length of the prodomain with the cleavage site except for the constructs consisting of the prodomain lacking the last 26 amino acids: pp(1–166) (C–E). Scale bar: 5 μm.
In parallel, we examined the localization of the prodomain alone. Surprisingly, the pp(1–166)-HA became stuck in a post-ER compartment and did not progress to the micronemes (Figure 7D), suggesting either that it did not contain a targeting motif or that recognition of the targeting motif by the microneme targeting machinery is dependent upon conformation. To test this hypothesis, we fused the pp(1–166)-HA to the C-terminal TgSUB1 sequence before the GPI anchor addition site. This construct was correctly targeted to the micronemes (Figure 7E). Altogether, these results show that the prodomain is sufficient to induce and redirect trafficking to the micronemes but that conformation of the prodomain sequence is critical for the prodomain to fulfill its role.
Discussion
The microneme protease TgSUB1 is unique in that it is the first example of a GPI-anchored protein localizing to a secretory organelle in T. gondii. GPI-anchored proteins of T. gondii and other protozoa are typically SAGs. Although the exact sorting mechanism through the secretory pathway is not known, the GPI anchor functions as a sorting and targeting signal sufficient to target reporters or microneme proteins to the cell surface (3,4).
Although the microneme targeting signals in the TgSUB1 prodomain are dominant, the trafficking machinery is saturable with overexpression, leading to surface expression. Microneme targeting of TgSUB1 is promoter dependent, and overexpression of TgSUB1 leads to expression on the cell surface. TgSUB1 driven by the SAG1 promoter was expressed at levels similar to that driven by the M2AP promoter. Despite this, SAG1-driven TgSUB1 was not as consistently targeted to the micronemes. Thus, it appears that both proper timing and level of expression are important for efficient sorting to the micronemes.
Subtilase prodomains are essential regulators of protease activity. After autocatalytic cleavage, the prodomain remains noncovalently associated to the catalytic site, acting as an intramolecular chaperone. The prodomain is required for proper folding of the protease and acts as an inhibitor of catalytic activity. Once in the proper environment (typically regulated by pH and/or calcium), the prodomain dissociates from the catalytic domain, leaving an active subtilisin. Prior studies with mammalian subtilases have revealed that cleavage of the prodomain is required for transit through the secretory pathway (9). The FL1SUB1-HA was incompletely cleaved because of the placement of the tag in the prodomain. Prior studies revealed that a catalytically dead mutant TgSUB1S999A was incorrectly cleaved (25). Both these incompletely processed TgSUB1 constructs were only partially localized in the micronemes, with a substantial fraction retained within the secretory pathway, suggesting that the TgSUB1 prodomain cleavage is required for an efficient trafficking.
Unlike other microneme proteins, TgSUB1 appears to have all components required for transit to the micronemes. Other known microneme proteins traffic within macromolecular complexes in which one component acts as a chaperone and another as an escorter for the complex (4–6). The signals for escorter function lie within the cytosolic region of transmembrane proteins (e.g. MIC2, MIC6 and MIC8). Typically, one component of the complex is proteolytically cleaved during assembly and transit through the secretory pathway, and this prodomain is required for trafficking of the entire complex (13). Sequence alignment of the prodomains involved in microneme protein targeting (AMA1, M2AP and MIC3) with TgSUB1's prodomain did not showing any conserved motifs. TgSUB1 also does not contain the T. cruzi cathepsin prodomain trafficking motif (10).
We have not been able to detect any proteins that associate with TgSUB1 in coimmunoprecipitation experiments. Instead, the prodomain of TgSUB1 appears to confer all information necessary for targeting of TgSUB1 or a heterologous protein to the micronemes. Proper proteolytic processing of TgSUB1 is not essential for microneme localization, but correct processing does appear to facilitate efficient targeting, possibly by exposing a domain necessary for microneme targeting. As for other subtilases, the prodomain is likely critical for correct folding of TgSUB1 as well as progression in the secretory pathway (7–9). Recognition of the prodomain by the trafficking machinery is dependent upon correct conformation of the prodomain as the prodomain sequence expressed alone was retained in the secretory pathway.
RAMA, a rhoptry protein of P. falciparum is GPI anchored and has been proposed to act as a nucleus for sorting to the rhoptries by associating with lipid rafts through its GPI anchor (26). A second P. falciparum GPI-anchored rhoptry protein, Pf34, has also been described (27). Surprisingly, Pf34 and RAMA differ subtly in their localization with RAMA associated with the rhoptry body and Pf34 to the rhoptry neck (RON), but both are enriched in detergent-resistant microdomains (DRMs). The T. gondii RON proteins are predicted to have a GPI anchor as well, although this is not been experimentally verified (28). The prodomains of prohormone convertase 2 and 3 are reported to facilitate trafficking due on the basis of their ability to associate with lipid rafts (29,30). Thus, it appears that in the Apicomplexa, GPI-anchored proteins can be trafficked to the cell surface, rhoptries or micronemes. These GPI-anchored proteins may traffic together in DRMs and be sorted subsequently to their respective compartments. Alternatively, these GPI-anchored proteins may assemble in organelle-specific macromolecular complexes within DRMs. TgSUB1 does not have a cytoplasmic tail and does not coprecipitate with other microneme proteins, so it is unclear whether a protein motif is responsible for its micronemal targeting. Further studies will be needed to determine if TgSUB1 is associated with DRMs through its prodomain or GPI moiety. Although GPI anchors may facilitate assembly of some secretory compartments, our studies indicate that the TgSUB1 prodomain is the primary determinant of its micronemal localization.
What other possible roles could the GPI anchor serve if not for targeting? TgSUB1 is displayed upon the parasite surface after microneme secretion and is cleaved off the surface during invasion (18). Perhaps, the GPI anchor provides a mechanism to fine-tune regulation of proteolysis during invasion by transiently stabilizing it on the cell surface. After microneme secretion, the GPI anchor would tether TgSUB1 to the parasite plasma membrane, prolonging the duration of TgSUB1 access to substrates in close proximity to the parasite surface. These substrates could be other microneme contents released onto the cell surface, resident cell surface proteins or unidentified host cell substrates cleaved during invasion or tissue penetration. These possibilities are now being actively investigated.
Materials and Methods
Parasite maintenance
T. gondii RHΔhxgprt strain and recombinants SUB1KO and SAG1KO (gift of John Boothroyd, Stanford University) were cultured in human foreskin fibroblast (HFF) cells with DMEM (Gibco) containing 10% FBS, 1% penicillin/streptomycin and 1% glutamine.
Western blotting
Reduced protein lysates were loaded on an 8% sodium dodecyl sulfate gel and then transferred to nitrocellulose membrane. Membranes were probed with a 1:5000 rabbit primary antibody dilution (for detection of TgSUB1: rabbit antisera AE653 made to a peptide immediately adjacent to the GPI anchor addition site and rabbit antisera to PfSUB1, gift from Michael Blackman, National Institute for Medical Research, UK; SAG1-P30 rabbit antisera, gift from Lloyd Kasper, Dartmouth Medical School, and rabbit antisera to MIC5, gift of Vern Carruthers, University of Michigan Medical School) and a 1:5000 anti-rabbit horseradish peroxidase antibody. Signals were revealed with a peroxidase enhanced chemiluminescence western blot detection reagent (Pierce). Prior studies have shown that PfSUB1 antisera are specific for TgSUB1 (18).
TX-114 phase partitioning and PI-PLC digestion
TX-114 phase partitioning and PI-PLC digestion of cell lysates were performed as described (31). Freshly lysed parasites were either treated or mock treated with 5 μL of PI-PLC (Molecular Probes) for 2 h at 37°C. The parasite suspension was lysed with 1/10 the volume of precondensed TX-114 (Pierce) and incubated on ice for 30 min. Samples were centrifuged, and the cleared supernatant was incubated for 5 min at 30°C. The detergent and aqueous phases were separated by centrifugation for 5 min at 300 × g and subsequently re-extracted. The final phases of both were acetone precipitated. Pellets from each phase were resuspended in an appropriate amount of 2× sample buffer and subsequently resolved by SDS-PAGE. Alternatively, tachyzoites lysates were PI-PLC treated and then immunoprecipitated with CRD antibody (gift of Jay Bangs, University of Wisconsin).
[3H] metabolic labeling
Freshly lysed extracellular tachyzoites were filtered, centrifuged and resuspended in 2 mL of labeling media (5% dialyzed FBS and 1% penicillin/streptomycin in DMEM media). About 500 μCi of [3H] palmitate or 1 mCi of [3H] ethanolamine (Amersham Biosciences) were added to the tubes and incubated at 37°C. After 4 h, samples were pelleted, resuspended in lysis buffer with protease inhibitor cocktail (Roche) and incubated on ice for 30 min. Samples were centrifuged at 4°C for 30 min and assayed for TgSUB1 expression by immunoprecipitation.
Immunoprecipitation
PI-PLC-treated lysates and [3H] palmitate- and [3H] ethanolamine-labeled samples were precleared with ImmunoPure Plus Immobilized Protein A (Pierce). Samples were then immunoprecipitated with CRD antibody (PIPLC-treated or untreated tachyzoite lysates) or with PfSUB1 antibody ([3H] palmitate–ethanolamine-labeled samples) and incubated overnight at 4°C. ImmunoPure Plus Immobilized Protein A beads were added to all immunoprecipitates and incubated for 3 h. Samples were pelleted and resuspended in 2× sample buffer. For PI-PLC-treated lysates, samples were analyzed by western blot analysis probing with PfSUB1 antibody. For [3H] palmitate- and [3H] ethanolamine-labeled samples, gels were incubated in either hydroxylamine or Tris, dried onto Whatman paper and exposed to Kodak film for approximately 6 months.
Targeted disruption of TgSUB1
Approximately 3 kb of 5′ and 3′ sequences flanking the coding region of TgSUB1 were identified by searching the Toxoplasma genome database (www.toxodb.org). Sequences were amplified by polymerase chain reaction (PCR) from genomic DNA and cloned into a plasmid containing the HXGPRT minigene using standard cloning techniques. This TgSUB1-HXGPRT minigene construct was transfected into either RHgra1-GFP-tubcatΔhxprt or RHΔhxgprt strain parasites (32), and drug selection was applied with 25 μg/mL mycophenolic acid and 50 μg/mL xanthine. Single clones were obtained after one cycle of lysis by limiting dilution and screened for loss of TgSUB1 expression by western blotting and IFA. The targeted disruption was successfully obtained in both cell lines. In each case, a clone that was successfully transfected but did not lead to KO of TgSUB1 was obtained in parallel. Both KO cell lines gave the same results when tested in western blot and IFA experiments.
Generation of TgSUB1 targeting constructs and parasite transfection
Constructs were created by standard cloning procedures utilizing site-directed mutagenesis (Quikchange Site-Directed Mutagenesis; Stratagene) or splice overlap extension (SOE) PCR techniques, driven by the M2AP, ROP1, GRA1 or SAG1 promoters. For HA insertion, Bg/II sites were introduced into TgSUB1 before the GPI anchor and after the catalytic sites by site-directed mutagenesis. The TgSUB1-HA construct was made by annealing HA oligos together (containing Bg/II restriction sites on either end) and ligating into the mutated TgSUB1. For insertion of an HA tag in the prodomain, the HA tag flanked with PstI sites was ligated in the prodomain that contains a single PstI site. The TgSUB1-HA version without the GPI or without the proline-rich domain was made by amplifying corresponding sequences with primers containing NsiI and NheI restriction sites and ligated into a vector containing the HA tag flanked by a NheI site in its N-terminal part. The TgSUB1 construct HA tagged before the GPI anchor was used as a template. TgSUB1 constructs without the catalytic domain or the prodomain were made by SOE PCR using overlapping primers. For the TgSUB1 prodomain–HA-tagged construct, the prodomain lacking the last 26 amino acids was amplified with primers containing NsiI and NheI restriction sites and ligated into a vector containing the HA tag [pp(1–166)-HA]. The TgSUB1 prodomain–HA fused to TgSUB1 GPI [pp(1–166)-HA–GPI] construction was made by SOE PCR using overlapping primers between the SUB1 prodomain–HA construct and the TgSUB1 13 residues of the C-terminal sequence containing the GPI anchor signal. Bg/II sites were also created in SAG1 before the C-terminal part containing the anchor signal. The TgSUB1 construct with SAG1's GPI anchor was then generated by standard restriction endonuclease digestions and ligation techniques utilizing the internal Bg/II sites in each gene. The TgSUB1 prodomain reporter constructs (SUB1ppGFP and SUB1ppSAG1) were made by SOE PCR using overlapping primers. Finally, the TgSUB1-pp(1–166)-SAG1 was made by amplifying the SAG1 gene with a forward primer containing a PstI site and ligated in the unique TgSUB1 prodomain PstI site. All constructs were then inserted into a vector containing the desired promoter using standard restriction endonuclease digestions and ligation techniques. Primer sequences are available in Table S1. All constructs were verified by DNA sequence analysis.
Parasite transfection was performed by electroporation. Freshly lysed parasites were resuspended in cytomix [120 mm KCl, 0.15 mm CaCl2, 10 mm K2HPO4–KH2PO4 (pH 7.6), 25 mm HEPES (pH 7.6), 2 mm EGTA, 5mm MgCl2,2 mm ATP and 5 mm glutathione) with 100 μg of supercoiled DNA and electroporated (BTX Electro Cell Manipulator 600; settings: R3, 2 kV and 50 W). Transfected parasites were transferred to HFF confluent cells in T25 flasks for western blot analysis or in glass coverslip for microscopy.
Immunofluorescence analysis
HFF monolayers were grown on glass coverslips in 24-well plates until confluent. Transiently transfected tachyzoites were allowed to infect confluent cells for 20–24 h. Cells were fixed for 20 min in 3% paraformaldehyde and permeabilized for 10 min in 0.2% Triton-X-100 in PBS. A PBS1 ×/BSA3%/Triton-X-100 0.2% solution was used for blocking and staining. All washes were performed with a PBS1 ×/Triton-X-100 0.2% solution. Antibody dilutions used were rabbit anti-SUB1 AE653 (1:500), mouse anti-SAG1 (1:500; gift of Lloyd Kasper, Dartmouth Medical School), mouse anti-MIC2-6D10, rabbit anti-M2AP and MIC5 (1:500; gift from Vern Carruthers) and rat anti-HA (1:100; Roche). Alexa Fluor-conjugated secondary antibodies (Molecular Probes) were used at 1:2000 dilution. Coverslips were mounted on slides with Fluoromount-G (Southern Biotech) and viewed with an Olympus Digital Microscope. Staining with SAG1 antibody was performed in SAG1KO RH stain parasites. Staining of TgSUB1-HA was similar when detected with either TgSUB1 or PfSUB1 antisera (rabbit) or HA antibodies (rat).
Supplementary Material
Acknowledgments
This study has been supported by National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) grant RO1-A146985 (K. K.) and NIH NIAID training grant 5T32-A107506 (E. M. B.). Some of the data in this paper were published as part of a thesis submitted by E. M. B. in partial fulfillment of the requirements for the Degree of Doctor of Philosophy in the Sue Golding Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University.
References
- 1.Carruthers V, Sibley L. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 2.Carruthers V, Boothroyd J. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2006;9:1–7. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Karsten V, Qi H, Beckers C, Reddy A, Dubremetz J, Webster P, Joiner K. The protozoan parasite Toxoplasma gondii targets proteins to dense granules and the vacuolar space using both conserved and unusual mechanisms. J Cell Biol. 1998;141:1323–1333. doi: 10.1083/jcb.141.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiss M, Viebig N, Brecht S, Fourmaux M, Soete M, Di Cristina M, Dubremetz J, Soldati D. Identification and characterization of an escorter for two secretory adhesins in Toxoplasma gondii. J Cell Biol. 2001;152:563–578. doi: 10.1083/jcb.152.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Cristina M, Spaccapelo R, Soldati D, Bistoni F, Crisanti A. Two conserved amino acid motifs mediate protein targeting to the micronemes of the apicomplexan parasite Toxoplasma gondii. Mol Cell Biol. 2000;20:7332–7341. doi: 10.1128/mcb.20.19.7332-7341.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huynh M, Rabenau K, Harper J, Beatty W, Sibley L, Carruthers V. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 2003;22:2082–2090. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eder J, Rheinnecker M, Fersht A. Folding of subtilisin BPN': role of the pro-sequence. J Mol Biol. 1993;233:293–304. doi: 10.1006/jmbi.1993.1507. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Hu Z, Jordan F, Inouye M. Functional analysis of the propeptide of subtilisin E as an intramolecular chaperone for protein folding. Refolding and inhibitory abilities of propeptide mutants. J Biol Chem. 1995;270:25127–25132. doi: 10.1074/jbc.270.42.25127. [DOI] [PubMed] [Google Scholar]
- 9.Creemers J, Vey M, Schafer W, Ayoubi T, Roebroek A, Klenk H, Garten W, Van de Ven W. Endoproteolytic cleavage of its propeptide is a prerequisite for efficient transport of furin out of the endoplasmic reticulum. J Biol Chem. 1995;270:2695–2702. doi: 10.1074/jbc.270.6.2695. [DOI] [PubMed] [Google Scholar]
- 10.Huete-Perez JA, Engel JC, Brinen LS, Mottram JC, McKerrow JH. Protease trafficking in two primitive eukaryotes is mediated by a prodomain protein motif. J Biol Chem. 1999;274:16249–16256. doi: 10.1074/jbc.274.23.16249. [DOI] [PubMed] [Google Scholar]
- 11.Bradley P, Boothroyd J. The pro region of Toxoplasma ROP1 is a rhoptry-targeting signal. Int J Parasitol. 2001;31:1177–1186. doi: 10.1016/s0020-7519(01)00242-9. [DOI] [PubMed] [Google Scholar]
- 12.Striepen B, Soldati D, Garcia-Reguet N, Dubremetz J, Roos DS. Targeting of soluble proteins to the rhoptries and micronemes in Toxoplasma gondii. Mol Biochem Parasitol. 2001;113:45–53. doi: 10.1016/s0166-6851(00)00379-0. [DOI] [PubMed] [Google Scholar]
- 13.Harper J, Huynh M, Coppens I, Parussini F, Moreno S, Carruthers VB. A cleavable propeptide influences Toxoplasma infection by facilitating the trafficking and secretion of the TgMIC2-M2AP invasion complex. Mol Biol Cell. 2006;17:4551–4563. doi: 10.1091/mbc.E06-01-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocken C, van der Wel A, Dubbeld M, Narum D, van de Rijke F, van Gemert G, van der Linde X, Bannister L, Janse C, Waters A, Thomas A. Precise timing of expression of a Plasmodium falciparum-derived transgene in Plasmodium berghei is a critical determinant of subsequent subcellular localization. J Biol Chem. 1998;273:15119–15124. doi: 10.1074/jbc.273.24.15119. [DOI] [PubMed] [Google Scholar]
- 15.Rug M, Wickham M, Foley M, Cowman A, Tilley L. Correct promoter control is needed for trafficking of the ring-infected erythrocyte surface antigen to the host cytosol in transfected malaria parasites. Infect Immun. 2004;72:6095–6105. doi: 10.1128/IAI.72.10.6095-6105.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soldati D, Dubremetz J, Lebrun M. Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int J Parasitol. 2001;31:1293–1302. doi: 10.1016/s0020-7519(01)00257-0. [DOI] [PubMed] [Google Scholar]
- 17.Karnataki A, Derocher A, Coppens I, Nash C, Feagin J, Parsons M. Cell cycle-regulated vesicular trafficking of Toxoplasma APT1, a protein localized to multiple apicoplast membranes. Mol Microbiol. 2007;63:1653–1668. doi: 10.1111/j.1365-2958.2007.05619.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller SA, Binder EM, Blackman MJ, Carruthers VB, Kim K. A conserved subtilisin-like protein TgSUB1 in microneme organelles of Toxoplasma gondii. J Biol Chem. 2001;276:45341–45348. doi: 10.1074/jbc.M106665200. [DOI] [PubMed] [Google Scholar]
- 19.Blackman M. Proteases involved in erythrocyte invasion by the malaria parasite: function and potential as chemotherapeutic targets. Curr Drug Targets. 2000;1:59–83. doi: 10.2174/1389450003349461. [DOI] [PubMed] [Google Scholar]
- 20.Conseil V, Soete M, Dubremetz J. Serine protease inhibitors block invasion of host cells by Toxoplasma gondii. Antimicrob Agents Chemother. 1999;43:1358–1361. doi: 10.1128/aac.43.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw M, Roos D, Tilney L. Cysteine and serine protease inhibitors block intracellular development and disrupt the secretory pathway of Toxoplasma gondii. Microbes Infect. 2002;4:119–132. doi: 10.1016/s1286-4579(01)01520-9. [DOI] [PubMed] [Google Scholar]
- 22.Yeoh S, O'Donnell R, Koussis K, Dluzewski A, Ansell K, Osborne S, Hackett F, Withers-Martinez C, Mitchell G, Bannister L, Bryans J, Kettleborough C, Blackman M. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell. 2007;131:1072–1083. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 23.Nagel S, Boothroyd J. The major surface antigen, P30, of Toxoplasma gondii is anchored by a glycolipid. J Biol Chem. 1989;264:5569–5574. [PubMed] [Google Scholar]
- 24.Tomavo S, Schwarz R, Dubremetz J. Evidence for glycosyl-phosphatidylinositol anchoring of Toxoplasma gondii major surface antigens. Mol Cell Biol. 1989;9:4576–4580. doi: 10.1128/mcb.9.10.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binder EM, Kim K. Location, location, location: trafficking and function of secreted proteases of Toxoplasma and Plasmodium. Traffic. 2004;5:914–924. doi: 10.1111/j.1600-0854.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- 26.Topolska A, Lidgett A, Truman D, Fujioka H, Coppel R. Characterization of a membrane-associated rhoptry protein of Plasmodium falciparum. J Biol Chem. 2004;279:4648–4656. doi: 10.1074/jbc.M307859200. [DOI] [PubMed] [Google Scholar]
- 27.Proellocks N, Kovacevic S, Ferguson D, Kats L, Morahan B, Black C, Waller K, Coppel R. Plasmodium falciparum Pf34, a novel GPI-anchored rhoptry protein found in detergent-resistant microdomains. Int J Parasitol. 2007;37:1233–1241. doi: 10.1016/j.ijpara.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley P, Ward C, Cheng S, Alexander D, Coller S, Coombs G, Dunn J, Ferguson D, Sanderson S, Wastling J, Boothroyd J. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J Biol Chem. 2005;280:34245–34258. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- 29.Blázquez M, Thiele C, Huttner W, Docherty K, Shennan K. Involvement of the membrane lipid bilayer in sorting prohormone convertase 2 into the regulated secretory pathway. Biochem J. 2000;349:843–852. doi: 10.1042/bj3490843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blázquez M, Docherty K, Shennan K. Association of prohormone convertase 3 with membrane lipid rafts. J Mol Endocrinol. 2001;27:107–116. doi: 10.1677/jme.0.0270107. [DOI] [PubMed] [Google Scholar]
- 31.Brusca J, Radolf J. Isolation of integral membrane proteins by phase partitioning with Triton X-114. Methods Enzymol. 1994;228:182–193. doi: 10.1016/0076-6879(94)28019-3. [DOI] [PubMed] [Google Scholar]
- 32.Donald RGK, Roos DS. Gene knock-outs and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit and run mutagenesis. Mol Biochem Parasitol. 1998;91:295–305. doi: 10.1016/s0166-6851(97)00210-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.