Abstract
Native tissues provide cells with complex, three dimensional (3D) environments comprised of hydrated networks of extracellular matrix proteins and sugars. By mimicking the dimensionality of native tissue while deconstructing the effects of environmental parameters, protein-based hydrogels serve as attractive, in vitro platforms to investigate cell-matrix interactions. For cell encapsulation, the process of hydrogel formation through physical or covalent crosslinking must be mild and cell compatible. While many chemical crosslinkers are commercially available for hydrogel formation, only a subset are cytocompatible; therefore, the identification of new and reliable cytocompatible crosslinkers allows for greater flexibility of hydrogel design for cell encapsulation applications. Here, we introduce tetrakis (hydroxymethyl) phosphonium chloride (THPC) as an inexpensive, amine-reactive, aqueous crosslinker for 3D cell encapsulation in protein-based hydrogels. We characterize the THPC-amine reaction by demonstrating its ability to react with primary and secondary amines of various amino acids. In addition, we demonstrate the utility of THPC to tune hydrogel gelation time (6.7 ± 0.2 to 27 ± 1.2 min) and mechanical properties (storage moduli ~250 Pa to ~2200 Pa) with a recombinant elastin-like protein. Lastly, we show cytocompatibility of THPC for cell encapsulation of two cell types, embryonic stem cells and neuronal cells, where cells exhibited the ability to differentiate and/or grow in elastin-like protein hydrogels.
Keywords: Crosslinker, Hydrogel, Cell Encapsulation, Amine-reactive
Within native tissue, cells often reside in complex, three-dimensional (3D) hydrated networks of extracellular matrix (ECM) proteins and sugars. To understand how cells respond and interact with these complex environments, 3D culture systems, developed in vitro, have been employed to recapitulate the dimensionality of native tissue while deconstructing the effects of environmental parameters. In particular, hydrogels, exhibiting high water content and tissue-like elastic properties, serve as attractive 3D cell culture platforms1. For cell encapsulation, the process of hydrogel formation through physical or covalent crosslinking must be mild and cell compatible, thus limiting the number of suitable materials.
Here, we focus on protein hydrogel formation through covalent crosslinking. Protein-based materials are comprised of amino acids, the building blocks of biological systems, and can provide cellular cues through micro-scale biological motifs2. Ionizable amino acid side groups (e.g., lysine’s ε-amine and cysteine’s sulfhydryl) provide site specific targets for crosslinking reactions and hydrogel formation. Covalent crosslinks offer precise control of hydrogel crosslinking density and allow for tunable mechanical properties that are capable of matching the stiffness of native tissues3–4. To date, a variety of chemical crosslinking chemistries have been utilized to form covalently crosslinked protein hydrogels. Amine-reactive N-hydroxysuccinimide (NHS) esters and their water-soluble analogs, sulfo-NHS esters, are commonly used homo-functional crosslinkers5–6 that demonstrate good reactivity at physiological pH, but are often susceptible to hydrolysis and degradation during the crosslinking reaction, which can lead to poor conjugation efficiency7. Typical gelation times for NHS ester systems are on the order of minutes to hours. Sulfhydryl groups have also been utilized to form disulfide crosslinks for hydrogel formation8–9. However, without the addition of external oxidizers, gelation is slow, requiring several hours. Additionally, generated disulfide crosslinks are sensitive to reducing agents that may be present in cell culture media. While several chemical crosslinkers are commercially available, only a subset is cytocompatible; therefore, the discovery of robust new cytocompatible crosslinkers promotes greater flexibility in hydrogel design for cell encapsulation applications.
Previously, Lim et al. introduced β-[tris(hydroxylmethyl) phosphino] propionic acid (THPP) as tri-functional crosslinker for polypeptide-based biomaterials. Through a Mannich-type condensation, THPP reacts with primary and secondary amines to form covalent crosslinks in aqueous solution at physiological pH. Single-suspension fibroblasts10, chondrocytes11, and human embryoid bodies12 were successfully encapsulated within THPP crosslinked hydrogels demonstrating cytocompatibility. Unfortunately, due to the complicated chemical synthesis of THPP, it is no longer readily available.
As a potential replacement for THPP, we investigated the reactivity of tetrakis(hydroxymethyl) phosphonium chloride (THPC). THPC is a phosphonium salt used as a precursor to fire-retardant materials and is readily available in aqueous solution. Due to the similarity in structure, we hypothesized that THPC could be used as a tetra-functional crosslinker for cell encapsulation in protein-based hydrogels, where THPC would react with primary and secondary amines through a Mannich-type reaction. The objective of this study was to assess THPC as a chemical crosslinker for protein-based hydrogels and demonstrate its cytocompatibility for cell encapsulation applications.
In Schematic 1, we illustrate the suggested reaction mechanism of THPC and a primary amine. The Mannich-type reaction is initiated by the generation of formaldehyde (HCOH), which then reacts with the amine to yield an immonium ion. Subsequently, the THPC derivative reacts with the immonium ion to complete the hydroxymethyl arm replacement, resulting in the amine coupling. Reaction with additional amines can then occur in a similar manner with the remaining, unreacted hydroxymethyl arms.
Scheme 1.
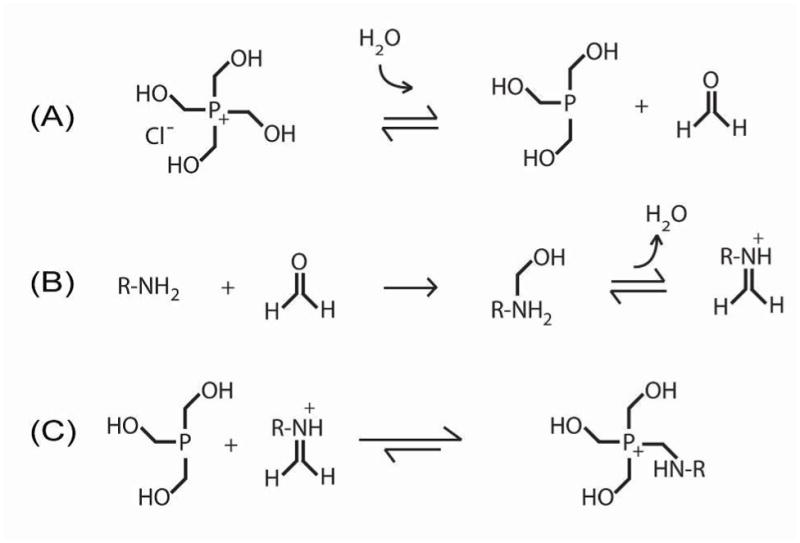
Suggested THPC reaction mechanism: (A) formation of formaldehyde to initiate hydroxymethyl arm replacement, (B) amine-formaldehyde reaction to yield an immonium ion in a Mannich-type reaction, and (C) phosphorus reaction with the immonium ion to complete the amine coupling.
Evidence to support a Mannich-type reaction rather than a simple condensation reaction with loss of water has been shown by others in similar reaction chemistries with hydroxymethyl phosphorus compounds13–14. Using a colorimetric formaldehyde detection assay based on a carbazole reaction15, we detected millimolar concentrations of formaldehyde in molar concentrations of THPC, where the quantity of formaldehyde detected positively correlated to THPC concentration (Figure 1A). Several amino acids (lysine, glycine, proline, and cysteine) were then individually reacted with THPC, using identical amino acid to THPC molar ratios. Formaldehyde generation was observed in all THPC reactions with the amino acids at levels significantly greater than that of the negative controls (i.e., amino acid alone), where no THPC was present (Figure 1B). After 20 minutes, we observed the greatest quantity of formaldehyde generation in the reaction with lysine, which contains two primary amines, followed by glycine, which has one primary amine, and then proline, which contains only a secondary amine. The smallest quantity of formaldehyde was detected in the reaction with cysteine; thus, cysteine’s sulfhydryl may interfere with the formaldehyde generation step. While these data do not provide detailed information about the kinetics of the reaction, they suggest that formaldehyde is an intermediate product in the THPC-amine reaction, which is consistent with the proposed Mannich-type reaction given in Schematic 1.
Figure 1.
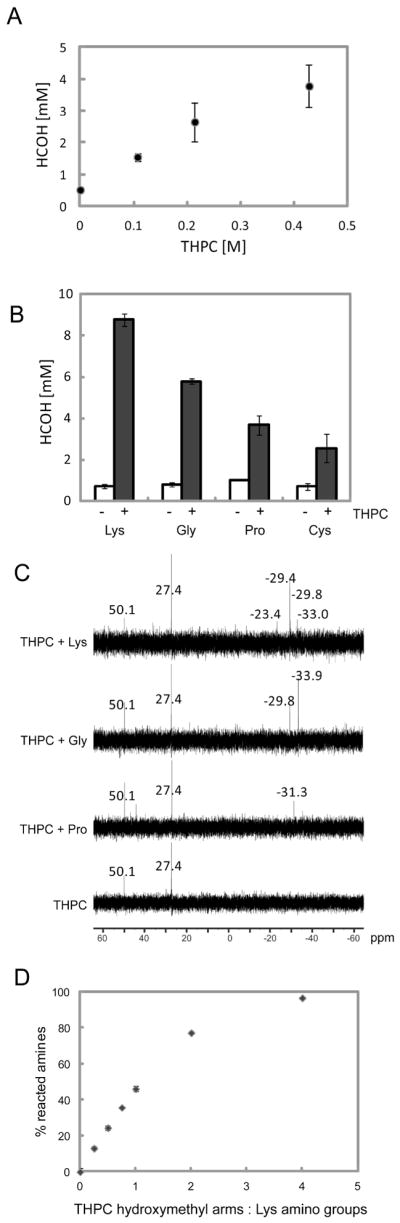
(A) Spontaneous formaldehyde (HCOH) generation positively correlates with THPC concentration. (B) Formaldehyde generation from various amino acids: lysine, glycine, proline, and cysteine alone (−) or reacted in the presence of 0.43 M THPC (+). (C) 31P NMR of THPC alone compared to THPC reacted with proline (Pro), glycine (Gly), or lysine (Lys), where chemical shifts are referenced to 85% phosphoric acid, which is assigned a chemical shift of 0. (D) Extent of THPC-lysine reaction as a function of THPC to lysine reactive group molar ratio.
From 31P NMR (Figure 1C), we see that within the THPC stock solution, the phosphorus resides in two different states, corresponding to THPC (50.1 ppm) and THPC minus a hydroxymethyl arm, or tris(hydroxymethyl)phosphine, (27.4 ppm) as expected (Schematic 1A). After reaction with lysine, glycine, or proline, we observe that the phosphorus signal shifts upfield, indicating a more electron-rich state. This suggests that both primary and secondary amines react with THPC (Figure 1C). In addition, Figure 1C shows that the α-amine (N-terminal) and the ε-amine (side chain) of lysine are both reactive (Supplementary Figure 1), as evidenced by the different chemical upshifts when compared to the reaction with glycine, which contains only the α-amine. Relative peak intensities also suggest greater reactivity of the lysine over glycine and proline, which is in agreement with Figure 1B. The extent of the THPC-lysine reaction was characterized using a 2,4,6-trinitrobenzene sulfonic acid (TNBSA) assay to detect free amino groups (Figure 1D). We observe that amino groups are being consumed by the reaction with THPC, and that by increasing the molar ratio of THPC reactive groups to amino groups, the reaction can be driven to near completion. The amine-reactivity of THPC allows us to exploit this small molecule as a covalent crosslinker for protein-based materials. The covalent crosslinks formed during the THPC-amine reaction are not susceptible to hydrolytic degradation; thus, the THPC crosslinker can be used to form stable crosslinked networks.
Previously our laboratory has reported the design and synthesis of a modular recombinant elastin-like protein (ELP) containing bioactive domains16 with specified lysines to act as amine-reactive crosslinking sites for formation of thin films and 2D cell scaffolds (Figure 2A). To demonstrate the ability of THPC to tune the properties of 3D protein-based hydrogels, solutions of ELP (5 wt% in phosphate-buffered saline) were crosslinked with various stoichiometric ratios of THPC. Gelation time was determined using an oscillatory rheometer (ARG2, TA Instruments). By exploiting the fixed stress experimental setup, the gelation point can be determined from the sample strain curve over time. With gelation, the sample strain rapidly decreases due to an increase in modulus. Gelation time was defined as the time at which the sample strain curve reached an inflection point, providing a good estimate of the gelation point. In varying THPC to ELP protein reactive group stoichiometry, gelation time could be varied in a biphasic manner, where the fastest gelation time for a 5 wt% ELP hydrogel was 6.7 min at a 1:1 THPC to ELP reactive group molar ratio (Figure 2B). As expected, both decreases (e.g., 0.5:1, 0.75:1) and increases (e.g., 2:1, 4:1) in this ratio result in longer gelation times likely due to under-saturated or over-saturated amine reaction sites, respectively. As the amount of THPC reactive sites is increased to match the amount of amine reactive groups, the probability of the crosslinking reaction occurring is increased, resulting in faster gelation times17. However, when the amount of THPC reactive sites exceeds the number of amine sites, more THPC molecules may achieve single-arm binding events, consuming the free amines without forming effective crosslinks, as opposed to multiple-arm binding events, which are required for crosslink formation. Thus, as observed, longer gelation time and more compliant hydrogels are expected when the amine reactive sites are over-saturated with a stoichiometric excess of THPC reactive sites.
Figure 2.
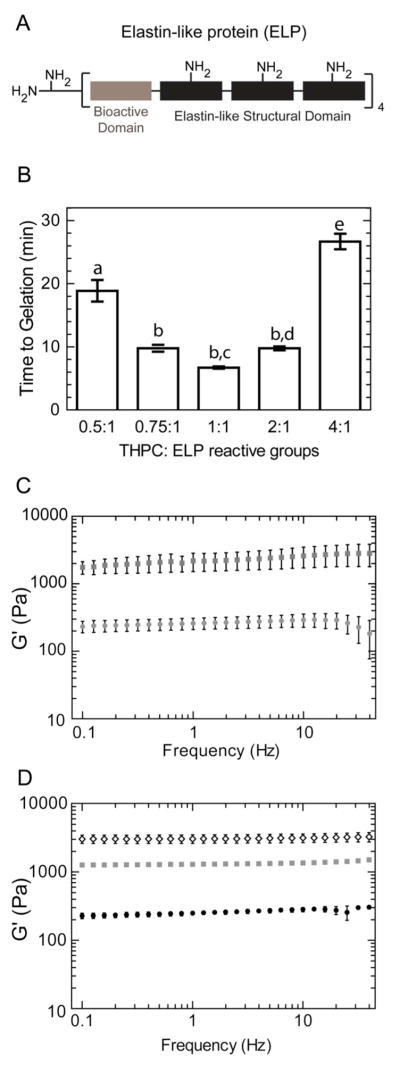
(A) Schematic of elastin-like protein (ELP). (B) Time to gelation of 5 wt% ELP hydrogels with various THPC:ELP reactive group molar ratios. Statistical significance is represented by letters above each column, with different letters signifying distinct statistical groups, p < 0.05. (C) Storage modulus, G′, of 5 wt% ELP hydrogels for 0.5:1 (gray circle) and 1:1 (gray squares) crosslinking density groups during frequency sweeps at a shear strain of 0.7%, which was determined to be within the linear viscoelastic regime. (D) Storage moduli, G′, of ELP hydrogels containing 3 (black circles), 5 (gray squares), and 10 (clear diamonds) weight percent ELP at 1:1 crosslinking density.
According to elasticity theory, elastic free-energy is dependent on the number of active polymer chains between crosslinks in a network18. Thus, changes in hydrogel crosslinking density, tuned by varying crosslinker to protein reactive group stoichiometry, are reflected in hydrogel mechanics. The frequency dependency of the storage modulus (G′) for the ELP hydrogels was determined in the range of 0.1 to 100 Hz using a constant 0.7% strain, which was verified to be in the linear viscoelastic regime. For 5 wt% ELP hydrogels with 1:1 crosslinking density, a plateau storage modulus of ~2200 Pa was found. By decreasing the crosslinking density, using a 0.5:1 THPC to protein reactive group stoichiometry, the plateau storage modulus could be reduced to ~250 Pa (Figure 2C). Similar to other biopolymer hydrogels, increasing the protein weight percent of the ELP hydrogel while keeping the crosslinker to protein stoichiometry the same also resulted in increased hydrogel storage moduli (Figure 2D). Increasing macromer content from 3 wt% to 5 wt% to 10wt% in ELP hydrogels resulted in increases in storage moduli from 300 ± 22 to 1320 ± 23 to 3090 ± 16 Pa, respectively.
In Figures 1 and 2, THPC has been shown to react with primary and secondary amines of various amino acids, where this covalent coupling reaction can be utilized to crosslink protein-based polymers containing free amines. While THPC concentration has been shown here to modulate hydrogel mechanics of a custom-designed recombinant protein, THPC can also be added to other protein-based, physical hydrogel systems, like collagen, to significantly stiffen the matrix by adding additional crosslinks into the network without changing the protein concentration (Supplementary Figure 2).
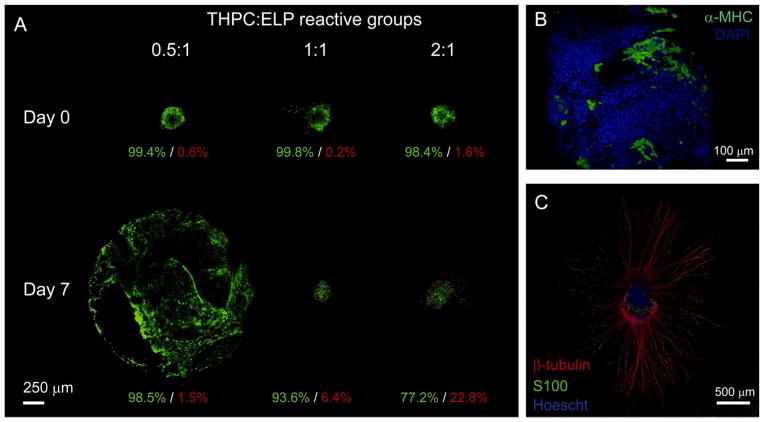
Lastly to demonstrate THPC’s compatibility for 3D cell encapsulation, mouse embryoid bodies were encapsulated in 5 wt% ELP hydrogels varying THPC crosslinker to protein reactive group ratios (0.5:1, 1:1, and 2:1). Mouse embryoid bodies were formed from embryonic stem cells through the hanging drop method19–20. Each embryoid body was encapsulated in a single ELP hydrogel that contained RGD, cell-adhesive bioactive domains. Hydrogels were formed by incubating at room temperature for 10 minutes followed by 37°C for 10 minutes. Live/dead cytotoxicity assay (Invitrogen) was used to assess cell viability immediately after encapsulation and after 7 days of culture (Figure 3A). Over 98% cell viability was observed in all hydrogels on day 0 immediately after crosslinking. After one week, viability ranged from 77–98%, with extensive cellular outgrowth from embryoid bodies encapsulated in hydrogels using the lowest crosslinking density. The phenomenon of greater cellular outgrowth in hydrogels with lower crosslinking density agrees with cell outgrowth and spreading observations in other cell-hydrogel systems21–22. Even though good viability was observed under these conditions, free amines located on the cell surface are susceptible to crosslinking with THPC and may affect cell function. This is true for all covalent crosslinkers that target primary amines; thus, it is important to assess cell viability for each crosslinker, cell type, and hydrogel combination. Additionally, the THPC-amine reaction generates a formaldehyde intermediate, which at high concentrations can be cytotoxic. At the millimolar concentrations of THPC (~2.3–9.2 mM for a 5 wt% ELP hydrogel at 0.5:1 to 2:1 crosslinking density) used for cell encapsulation in these studies, only micromolar concentrations of formaldehyde may be present and do not appear to result in significant cell death. However, THPC concentration should be minimized for a desired hydrogel stiffness to minimize potential cytotoxic effects of formaldehyde.
Figure 3.
(A) Representative live (green)/dead (red) projections of encapsulated mouse embryoid bodies in 5 wt% ELP hydrogels (2 mm in diameter, 0.5 mm in height) of varying THPC:ELP reactive group molar ratios immediately after encapsulation and after 7 days of culture. Hydrogels with 0.5:1, 1:1, and 2:1 crosslinking density correspond to storage moduli of ~260, 2200, and 1400 Pa, respectively. By day 7, cellular outgrowth of the lowest crosslinking density hydrogel (0.5:1) encompassed the entire hydrogel. Scale bar = 250 microns. (B) Cardiomyocyte differentiation of an encapsulated mouse embryoid body, in a 5 wt% ELP hydrogel with 0.5:1 crosslinking density, was visualized by expression of GFP-tagged alpha-myosin heavy chain (α-MHC) reporter (green) and counterstained with DAPI (blue) for nuclei visualization after 8 days of culture. Scale bar = 100 microns. (C) Encapsulated chick dorsal root ganglion (DRG), in a 3 wt% ELP hydrogel with 1:1 crosslinking density (modulus ~1500 Pa), cultured for 7 days, and stained for neuronal marker, β-tubulin (red), and glial marker, S100 (green), with Hoescht (blue) for nuclei visualization. Scale bar = 500 microns.
To show that THPC crosslinking is not only cell compatible but supports the retention of cell function, encapsulated embryoid bodies were also monitored for cardiomyocyte differentiation. Using a mouse embryonic stem cell line containing a GFP-tagged α-myosin heavy chain reporter23, cardiomyocyte differentiation could be visualized within the THPC-crosslinked 5 wt% ELP hydrogels (Figure 3B). As further evidence of cell compatibility and potential utility for cultures of a variety of different tissue types, dorsal root ganglia (DRGs), isolated from E9 chick embryos, were encapsulated in more compliant 1:1 THPC-crosslinked 3 wt% ELP hydrogels. After 7 days of culture, DRGs stained for neuronal and glial markers (β-tubulin and S100, respectively), demonstrating the ability of THPC-crosslinked ELP hydrogels to support neuronal axon growth into the hydrogel (Figure 3C).
CONCLUSION
Protein-based hydrogel systems serve as attractive in vitro systems to investigate cell response to environmental cues. The identification of new, cytocompatible crosslinkers allows for greater flexibility of hydrogel design. Here, we have introduced THPC as an inexpensive, aqueous crosslinker for 3D cell encapsulation in protein-based hydrogels. We have characterized the THPC-amine reaction, demonstrated the use of THPC in tuning hydrogel properties, and showed cytocompatibility with retention of cell growth and phenotype in an ELP hydrogel system.
Supplementary Material
SYNOPSIS.
The primary goal of this communication is to report the identification and utility of tetrakis (hydroxylmethyl) phosphonium chloride (THPC) as an inexpensive but widely applicable crosslinker for protein-based materials. We confirm THPC specificity for amine residues, measure reaction efficiency, and demonstrate the ability to use THPC to tune hydrogel mechanics. Furthermore, we demonstrate cell compatibility of THPC for three-dimensional cell encapsulation within elastin-like protein hydrogels with two different cell types.
Acknowledgments
The authors would like to thank Andrew T. Higgs from the Sellinger lab at Stanford University for his help in 31P NMR experiments, the Kamm lab from Massachusetts Institute of Technology for the generous gift of mouse embryonic stem cells with GFP-tagged α-myosin heavy chain reporter, and Debanti Sengupta and Dr. Justin Du Bois for helpful discussion about THPP reaction mechanism. The authors would also like to acknowledge funding support from AHA 10POST4190103 (CC), NIH NRSA F32NS076222 (KJL), NSF EFRI-CBE 0735551 (SCH), NIH DP2OD006477 (SCH), NIH R21 AR062359 (SCH), NSF DMR 0846363 (SCH), Stanford Bio-X IIP5-85 (SCH), and Stanford Cardiovascular Institute Younger Grant (SCH).
ABBREVIATIONS
- 3D
three dimensional
- α-MHC
alpha-myosin heavy chain
- DRG
dorsal root ganglia
- ECM
extracellular matrix
- ELP
elastin-like protein
- HCOH
formaldehyde
- NHS
N-hydroxysuccinimide
- THPC
tetrakis(hydroxymethyl)phosphonium chloride
- THPP
tris(hydroxymethyl) phosphino propionic acid
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
ASSOCIATED CONTENT
Supporting Information. Detailed methods are provided. Zoomed in image of 31P NMR chemical shifts for THPC-Lys reaction (Figure S1). Storage (G′) and loss moduli (G″) of THPC-stiffened collagen hydrogels (Figure S2). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng, Part B. 2008;14(2):149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romano NH, Sengupta D, Chung C, Heilshorn SC. Protein-engineered biomaterials: Nanoscale mimics of the extracellular matrix. Biochim Biophys Acta, Gen Subj. 2011;1810(3):339–349. doi: 10.1016/j.bbagen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lampe KJ, Mooney RG, Bjugstad KB, Mahoney MJ. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. J Biomed Mater Res, Part A. 2010;94A(4):1162–1171. doi: 10.1002/jbm.a.32787. [DOI] [PubMed] [Google Scholar]
- 4.Chung C, Burdick JA. Influence of Three-Dimensional Hyaluronic Acid Microenvironments on Mesenchymal Stem Cell Chondrogenesis. Tissue Eng, Part A. 2009;15(2):243–254. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Zio K, Tirrell DA. Mechanical properties of artificial protein matrices engineered for control of cell and tissue behavior. Macromolecules. 2003;36(5):1553–1558. [Google Scholar]
- 6.Trabbic-Carlson K, Setton LA, Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules. 2003;4(3):572–580. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- 7.Hermanson GT. Bioconjugate techniques. 2. Academic Press, Inc; San Diego: 2008. [Google Scholar]
- 8.Shu XZ, Liu YC, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3(6):1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 9.Asai D, Xu D, Liu W, Quiroz FG, Callahan DJ, Zalutsky MR, Craig SL, Chilkoti A. Protein polymer hydrogels by in situ, rapid and reversible self-gelation. Biomaterials. 2012;33(21):5451–5458. doi: 10.1016/j.biomaterials.2012.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim DW, Nettles DL, Setton LA, Chilkoti A. Rapid cross-linking of elastin-like polypeptides with (hydroxymethyl)phosphines in aqueous solution. Biomacromolecules. 2007;8(5):1463–1470. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nettles DL, Haider MA, Chilkoti A, Setton LA. Neural Network Analysis Identifies Scaffold Properties Necessary for In Vitro Chondrogenesis in Elastin-like Polypeptide Biopolymer Scaffolds. Tissue Eng, Part A. 2010;16(1):11–20. doi: 10.1089/ten.tea.2009.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung C, Anderson E, Pera RR, Pruitt BL, Heilshorn SC. Hydrogel crosslinking density regulates temporal contractility of human embryonic stem cell-derived cardiomyocytes in 3D cultures. Soft Matter. 2012;8(39):10141–10148. doi: 10.1039/C2SM26082D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank AW, Daigle DJ, Vail SL. Chemistry of hydroxymethyl phosphorus-compounds: phosphines, phosphine oxides, and phosphonium hydroxides. Text Res J. 1982;52(12):738–750. [Google Scholar]
- 14.Vullo WJ. Hydroxymethyl replacement reactions of tetrakis (hydroxymethyl)-phosphonium chloride. Ind Eng Chem Prod Res Dev. 1966;5(4):346. [Google Scholar]
- 15.Taylor KA. A colorimetric formaldehyde assay. Appl Biochem Biotechnol. 1997;68(1–2):81–93. [Google Scholar]
- 16.Straley KS, Heilshorn SC. Independent tuning of multiple biomaterial properties using protein engineering. Soft Matter. 2009;5(1):114–124. [Google Scholar]
- 17.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, 3rd, Gorman RC, Burdick JA. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010;107(25):11507–12. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowman AM, Peppas NA. Hydrogels. In: Mathiowitz E, editor. Encyclopedia of Controlled Drug Delivery. Wiley; New York: 1999. pp. 397–418. [Google Scholar]
- 19.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78(4):442–453. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 20.Keller GM. In-vitro differentiation of embryonic stem-cells. Curr Opin Cell Biol. 1995;7(6):862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 21.Khetan S, Burdick JA. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials. 2010;31(32):8228–34. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 22.Lampe KJ, Antaris AL, Heilshorn SC. Design of 3D engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. doi: 10.1016/j.actbio.2012.10.033. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107(14):1912–1916. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.