Abstract
The dystrobrevin-binding protein 1 (DTNBP1) gene, which encodes the dysbindin-1 protein, is a potential schizophrenia susceptibility gene. Polymorphisms in the DTNBP1 gene have been associated with altered cognitive abilities. In the present study, dysbindin-1 null mutant (dys−/−), heterozygous (dys+/−), and wild-type (dys+/+) mice, on a C57BL/6J genetic background, were tested in either a match to sample or nonmatch to sample visual discrimination task. This visual discrimination task was designed to measure rule learning and detect any changes in response timing over the course of testing. Dys−/− mice displayed significant learning deficits and required more trials to acquire this task. However, once criterion was reached, there were no differences between the genotypes on any behavioral measures. Dys−/− mice exhibited increased compulsive and impulsive behaviors compared to control littermates suggesting the inability to suppress incorrectly-timed responses underlies their increased time to acquisition. Indeed, group comparisons of behavior differences between the first and last day of testing showed that only dys−/− mice consistently decreased measures of perseverative, premature, timeout, and total responses. These findings illustrate how some aspects of altered cognitive performance in dys−/− mice might be related to increased impulsive and compulsive behaviors, analogous to cognitive deficits in some individuals with psychiatric disorders.
Keywords: dysbindin, impulsivity, compulsivity, timing, schizophrenia, operant learning
1. Introduction
The dysbindin-1 protein is encoded by the dystrobrevin-binding protein 1 (DTNBP1) gene and is expressed widely in human and mouse brain [1]. Dysbindin-1 has been implicated in the regulation of vesicle formation and synaptic release [2] and it is a component of the biogenesis of lysosome-related organelles complex (BLOC-1) [3]. Recent studies in mice show that dysbindin-1 is more highly expressed during embryonic and early postnatal development compared to adulthood and BLOC-1 is involved in neurite outgrowth [4]. These findings highlight a potential role for dysbindin-1 in the normal development of brain structure and function. In addition to affecting neuron development, abnormalities in dysbindin-1 also affect human behavior, as variation in the DTNBP1 gene is associated with alterations in general cognitive ability [5–7].
The DTNBP1 gene was first identified as a schizophrenia susceptibility gene in an Irish population [8]. Subsequent studies have confirmed the link between DTNBP1 and schizophrenia in multiple, independent populations [9–10] and it remains among the leading candidate genes in meta-analyses [11]. Patients with schizophrenia have lower expression levels of dysbindin-1 mRNA and protein in the prefrontal cortex and hippocampus, regions associated with cognitive dysfunction in the disorder [12–15].
The so-called sandy (sdy) mouse harbors a spontaneous mutation in the DTNBP1 gene that results in the complete absence of dysbindin-1 protein [3]. The sdy mutation was originally discovered in mice with the DBA/2J genetic background. These mice exhibit impaired working memory in an operant delayed non-match-to-position task [16]. However, the DBA/2J genetic background itself produces dramatic locomotor and coordination deficits along with significant memory, genetic, and dopaminergic alterations that may confound the interpretation of cognitive phenotypes in the sdy mouse [17–19]. To circumvent the endogenous impairments found in the DBA/2J background, the sdy mutation has been transferred to the C57BL/6J background (dys−/−). Dys−/− mice are hyperactive in an open-field [20–21], display spatial memory deficits in the Morris water maze [20], working memory deficits in a discrete paired-trial T-maze task [21] and in a delayed nonmatch-to-position operant task [22]
Studies using cortical neuronal cultures taken from dys−/− mice have shown signaling alterations that may underlie the deficits in cognitive performance. In particular, dysbindin-1 has been associated with various aspects of synaptic function [20, 23], and the regulation of both dopamine (DA) and glutamate signaling in the brain (reviewed in [24]. In vitro experiments suggest that dysbindin-1 suppresses DA release [25] and cultured neurons from dys−/− mice have increased cell surface expression of the dopamine D2 receptor due to an increased membrane insertion rate [26]. Layer II/III pyramidal neurons from the prefrontal cortex (PFC) of dys−/− mice show increased activity at baseline, but decreased activity after D2 stimulation compared to wild-type mice and these effects may be due to D2-mediated alterations in the excitability of fast-spiking GABAergic interneurons [21, 26]. Reduced dysbindin-1 function also impairs glutamate signaling in neuronal culture [27]. In agreement with these in vitro studies, sdy mice display decreased excitability of glutamatergic neurons in the PFC and decreased release of glutamate in the hippocampus [2, 16]. Moreover, recent work demonstrates that dys−/− mice exhibit reduced NMDA-evoked currents and reduced NR1 mRNA expression in the PFC [22]. Taken together, these data implicate reduced dysbindin-1 function in behavioral and neurobiological effects that are thought to play a role in the development of cognitive abnormalities found in schizophrenia. Therefore, the dys−/− mouse serves as a useful genetic mouse model for the study of the cognitive domains affected by schizophrenia.
Altered dopamine release combined with increased expression of D2 receptors in dys−/− mice may contribute to alterations in reward-based learning and decision making. In fact, mice that overexpress D2 receptors in the striatum exhibit motivation and timing deficits, along with working memory impairments, in operant tasks [28–29]. Interestingly, loss of dysbindin-1 in sdy mice leads to decreased KCl-induced DA release in the PFC [30] suggesting that loss of dysbindin-1 can produce a hypodopaminergic state in the prefrontal cortex that may disrupt cognitive processes. Prefrontal cortical hypodopaminergic function has been thought to underlie some of the cognitive deficits in schizophrenia [31]. In the current study, we tested the performance of dys−/− mice, wild-type (dys+/+) and heterozygous (dys+/−) littermates in an automated, operant learning paradigm. We focused our analysis on performance during the acquisition of the task in order to determine if there were inherent genotype-specific differences in the response pattern that could affect overall performance. We specifically wanted to address a gap in the literature concerning response timing in operant learning tasks. Response timing deficits serve to identify putatively abnormal compulsive or impulsive behavior. The task we used in this experiment is a simple visual-cue discrimination task adapted from previous studies in rats [32–33]. Learning in similar tasks utilizing mice has been shown to be dependent on intact signaling in the dorsal striatum and specifically intact dopaminergic signaling [34–35]. In the current study, we present evidence that dys−/− mice exhibit impaired performance in a reward-based operant task due to increased compulsive and impulsive behavior.
2. Materials and Methods
2.1. Animals
All procedures were approved by the National Institute of Mental Health Animal Care and Use committee and followed the National Institutes of Health manual Using Animals in Intramural Research. The dys−/−, along with their dys+/− and dys+/+ littermates used in this study, were derived from sdy mice backcrossed with C57BL/6J mice (>11 backcrosses) and were produced using a heterozygous (dys+/−) breeding strategy as previously described [21]. Mice were identified by PCR analysis of tail DNA. One week prior to test preparations, mice were moved from the animal colony room to a climate-controlled holding room (21±1 C), weighed, singly housed, and maintained on a 12-hour reverse light/dark cycle with free access to food and water. All testing was conducted using male mice between 3 and 6 months of age and experiments were conducted during the first 2 hours of the dark cycle.
2.2. Preparation
Following a week of acclimation to single-housing and the holding room, body weight and 24 h food intake were recorded for three consecutive days. Mice were then food restricted throughout the experiment to maintain 85% of their free-feeding body weight. Mice were habituated to the reward pellets (5TUL 14mg rodent tablets, TestDiet, Richmond, IN, USA) used in subsequent testing during this initial food restriction phase.
2.3. Operant Task
2.3.1. Apparatus
Mice were tested in operant chambers (ENV-307W; Med Associates, St. Albans, VT, USA), housed inside sound attenuating boxes, and connected to a PC equipped with MED-PC IV software (Med Associates, St. Albans, VT, USA). The operant chambers contained two nose poke holes (1cm in diameter), each outfitted with a recessed LED stimulus light (ENV-115C). Nose pokes were detected by an infrared photocell beam. A food magazine (ENV-303W) was located between the two nose poke holes. The house light was located 7 cm above the food magazine.
2.3.2. Habituation Phase
Mice were trained to poke into the nose poke holes and retrieve the reward pellet from the magazine. Each training day consisted of a 5-minute acclimation period in which the mice were allowed to explore the operant chamber. The house light and stimulus lights were off during acclimation. After the acclimation period, a reward pellet was dispensed and a 40-minute training session began. The training session consisted of alternating light and dark trials. During the light trials, both stimulus lights were illuminated for up to 10 seconds. If the mouse poked into either hole, the lights were immediately extinguished and a reward pellet was dispensed. The mouse had four seconds to eat the pellet. After a 5-second intertrial delay, a dark trial was conducted. During dark trials, all lights in the chamber were off and the mouse again had 10 seconds to poke into either nose poke hole and receive a reward pellet. After 10 seconds, if the mouse had not poked in either hole, no reward pellet was dispensed and an omission was recorded. Alternating light/dark trials continued for the entire 40-minute session. Total number of pokes and omissions were recorded along with the latency to poke. Habituation continued until a criterion of three consecutive days of at least 20 light trial pokes and 20 dark trial pokes was reached.
2.3.3. Testing Phase
Testing sessions, like habituation sessions, were 40 minutes long. During the testing phase each mouse was randomly assigned one of two possible tasks: poke in the nose poke hole associated with the stimulus light, or poke in the nose poke hole not associated with the stimulus light, in order to receive a reward pellet. Whether the right or left hole was illuminated was varied in a semi-random fashion so that the same hole was not illuminated more than two trials in a row. There were no differences in performance between the groups based on whether they were rewarded for poking in the illuminated or dark hole, so the data were combined within each genotype for subsequent analyses. After the habituation period, a reward pellet was dispensed and mice were given four seconds to eat, and the testing session began. The stimulus light was presented to the mouse for only 2 seconds and the mouse had up to 10 seconds to produce a response. If the mouse responded correctly, the stimulus light was extinguished (if still lit) and a reward pellet was dispensed. After a 5-second intertrial delay, a new trial began. If an incorrect response was made, the stimulus light was extinguished (if still lit) and the house light came on for 5 seconds. If no response was made within 10 seconds, the house light was illuminated for 5 seconds and an omission was recorded. After both incorrect responses and omissions, the 5-second period of house light illumination (timeout period) was followed by a 5-second intertrial delay (no lights). If the mouse made a response during the timeout period (timeout response) or intertrial delay (premature response) the house light would come on for a period of 5 seconds and the 5-second intertrial delay period would reset. Choice accuracy was calculated by dividing the number of correct choices by the sum of correct and incorrect choices and multiplying by 100. The percent correct, incorrect, and omitted were calculated by dividing by the total number of trials during the session and multiplying by 100. The testing session terminated after 40 minutes or 10 consecutive omissions, whichever came first. Testing occurred daily until the criterion of 8 correct trials out of 10 in the session was reached.
2.4. Statistical Analyses
Results are expressed as the mean ± SEM. One-way ANOVAs with genotype (+/+, +/−, −/−) as the independent variable were used to determine group differences in all measures except for the comparison of first and last day performance. Post hoc analyses incorporated the appropriate Bonferroni-corrected pairwise comparisions. Comparisons of performance on the first and last day of testing were conducted using two-way ANOVAs with genotype as the between-subjects variable and testing day as the within-subjects variable. T-tests were used for post hoc analysis to determine if within-genotype effects were significant when there was a significant testing day or interaction effect. The number of mice in each group was as follows: dys +/+ = 13, dys +/− = 16, and dys −/− = 16.
3. Results
3.1. No group differences in basic performance of the operant task during habituation
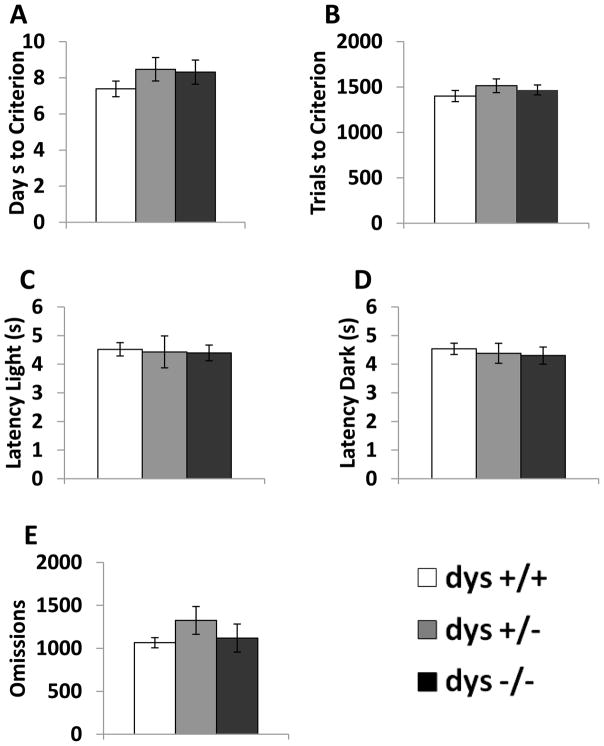
In order to determine if there were any gross differences in physical performance of the operant task, behavior was recorded during the habituation phase. There were no differences between any of the genotypes (dys+/+, +/−, −/−) in the number of days or trials required to reach the habituation criterion (Fig. 1A–B). Additionally, there were no differences in the latency to respond during either light or dark trials or in the number of omissions (Fig. 1C–E). These data indicate that reduced dysbindin-1 protein does not produce deficits in gross motor behavior that would inhibit performance in this operant task, as seen in dopamine-deficient (DD) mice [35].
Figure 1.
Response parameters for dys+/+, +/−, and −/− during the habituation phase. Average number of days of training sessions needed to reach criterion (A). Average number of trials needed to reach criterion (B). Average latency during the light (C) and dark (D) trials. Average number of omissions (E). No group differences were seen during the training phase.
3.2. Dys−/− mice require more trials to reach criterion in the Testing Phase
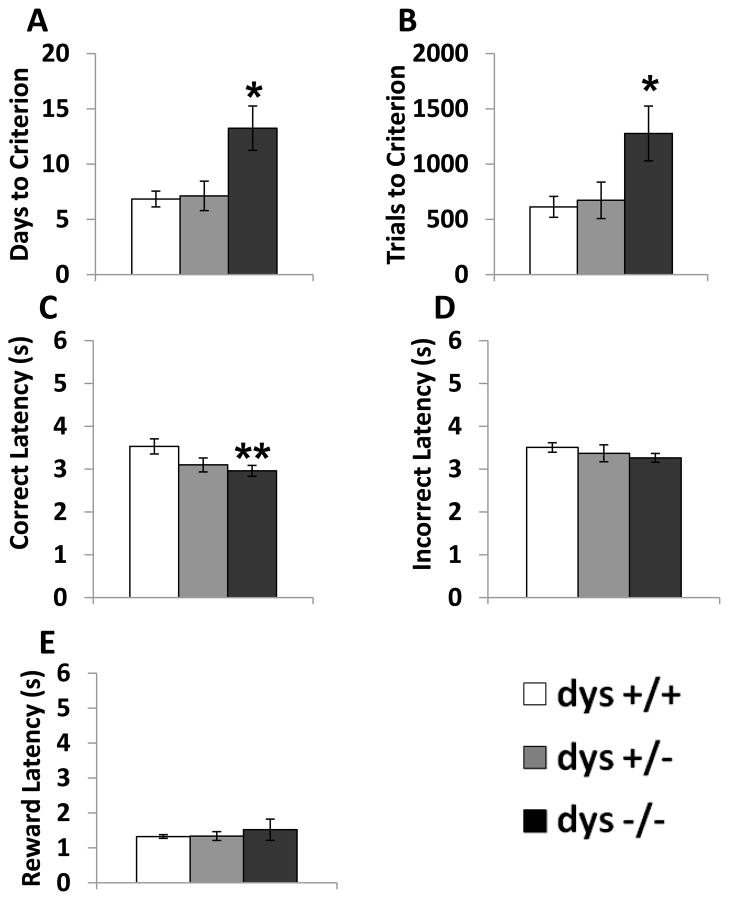
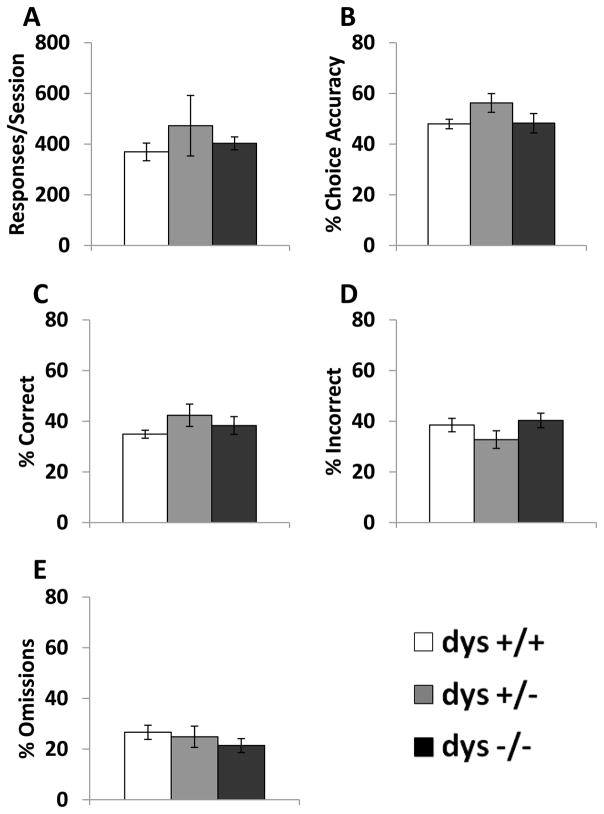
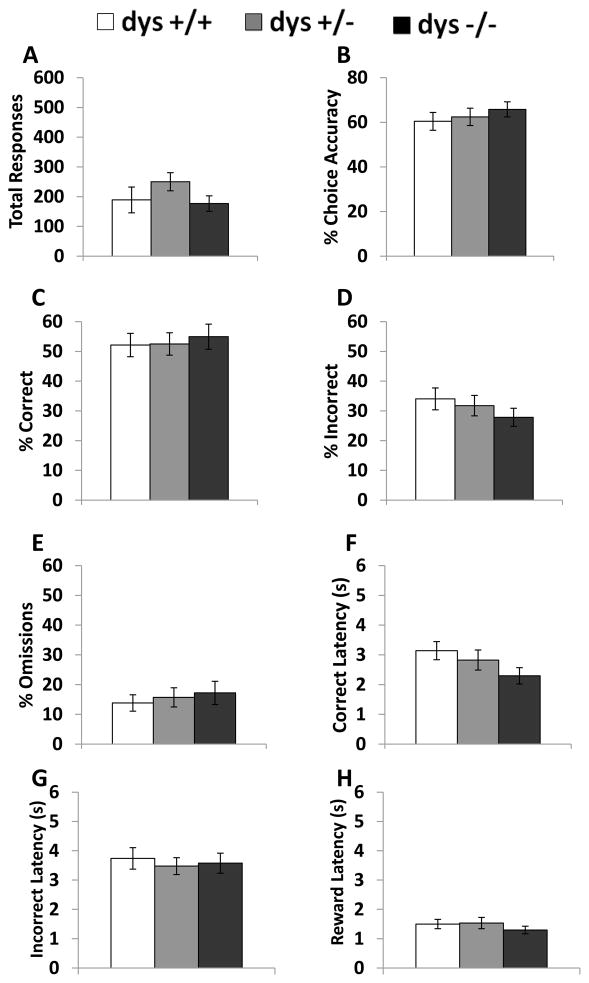
Compared to the habituation phase, where mice were rewarded for poking in either nose poke hole, during testing mice were only rewarded for responses at one of the two nose poke holes depending on which light stimulus was activated. There were significant genotype differences in the number of sessions (F(2, 42) = 5.76, p < 0.01) and trials (F(2, 42) = 4.69, p < 0.05) required to reach criterion (Fig. 2A–B). Specifically, dys−/− mice required more days and trials to reach criterion compared to both dys+/+ and dys+/− mice (p < 0.05, Bonferroni post hoc test). Interestingly, there were significant differences in response latency selectively on correct trials (F(2, 42) = 6.67, p < 0.05). Despite requiring more time to reach criterion, dys−/− mice responded faster on correct trials compared to dys+/+ mice (p < 0.01, Bonferroni post hoc test; Fig. 2C). In contrast, there were no differences in response latency on incorrect trials (F(2, 42) = 0.89, p = 0.42; Fig. 2D) or latency to retrieve reward pellets (F(2, 42) = 0.18, p = 0.84; Fig. 2E). The specificity of the effect on correct latency suggests that the difference in the speed of correct responding was not due to deficits in motor ability or reward processing. At this point, we are unaware of any other mouse models with this feature in similar operant tasks. Thus, a correct interpretation of this data awaits future investigation. Genotype did not significantly affect the total number of responses per session (F(2, 42) = 0.46, p = 0.64; Fig. 3A). Additionally, choice accuracy (F(2, 42) = 1.96, p = 0.15), % correct (F(2, 42) = 1.01, p = 0.37), % incorrect (F(2, 42) = 1.95, p = 0.16), and % omissions (F(2, 42) = 0.83, p = 0.44) were unaffected by dysbindin-1 genotype over the course of the total Testing Phase (Fig. 3B–E). Therefore, differences in accuracy of correctly-timed responses (Fig. 3) did not underlie the learning deficits seen in dys−/− mice (Fig. 2).
Figure 2.
Overall performance during the testing phase. Average number of days of testing sessions needed to reach criterion (A). Trials needed to reach criterion (B). Correct latency (C). Incorrect latency (D). Latency to retrieve reward pellet after a correct response (Reward latency) (E). Dys −/− mice required more days and trials to reach criterion compared to both dys+/− and dys+/+ mice. Additionally, dys−/− mice responded faster on correct trials than dys+/+ mice. * = p <0.05 and ** = p < 0.01.
Figure 3.
Response rates during the testing phase. Average number of responses per session (A). % choice accuracy (correct responses/(correct responses + incorrect responses) * 100) across all sessions (B). % correct (correct responses/(correct responses + incorrect responses + omissions) * 100) across all sessions (C). % incorrect (incorrect responses/(correct responses + incorrect responses + omissions) * 100) across all sessions (D). % omissions (omissions/(correct responses + incorrect responses + omissions) * 100) across all sessions (E). There were no differences between genotypes on these measures.
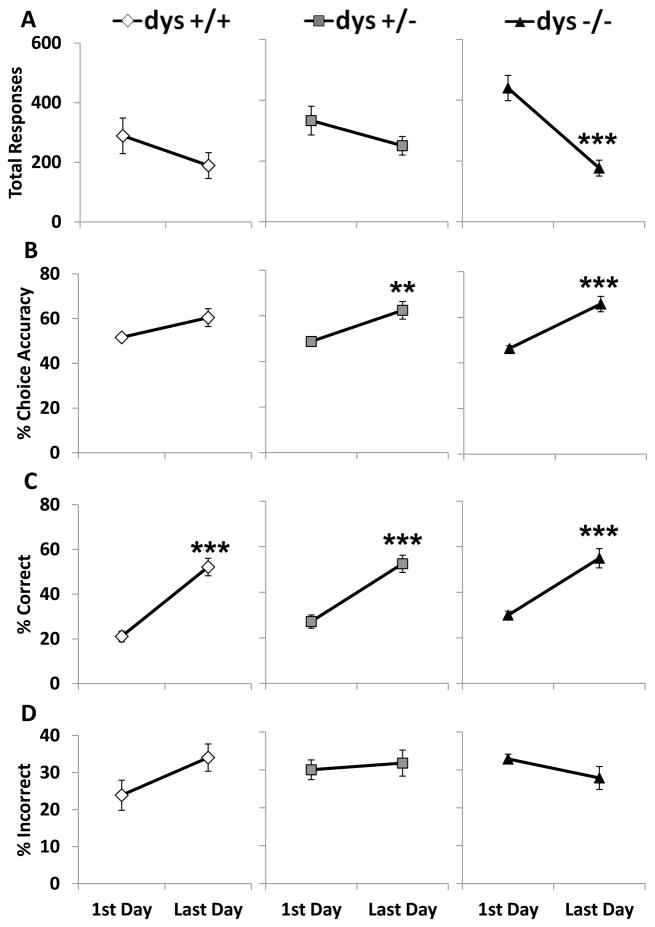
3.3. Dys−/− mice produced more responses and fewer omissions than dys+/+ mice on Day 1
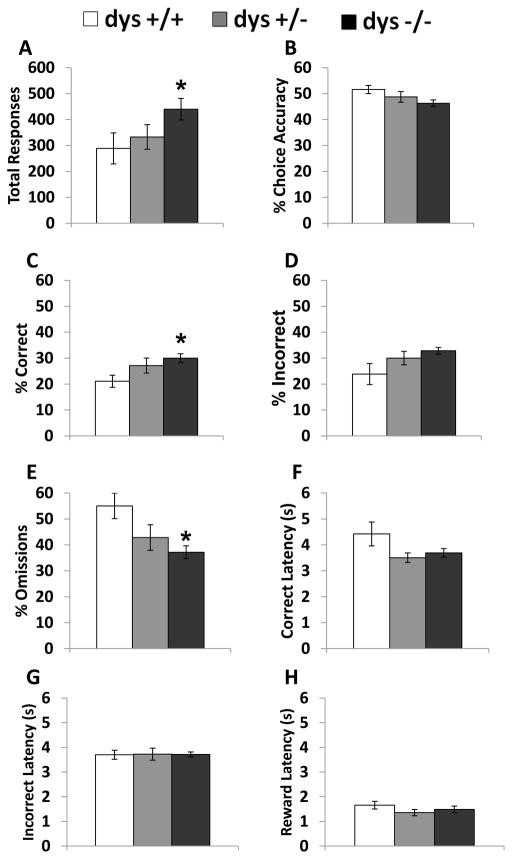
In order to probe potential causes of the impaired dys−/− performance in this task, we next analyzed behavior on the first and last day of testing for each mouse. On Day 1 of testing, all mice were unfamiliar with the discrimination testing rule used to determine reward presentation. On the last day of testing, all mice performed at a level sufficient to reach the criterion. During the first day of testing, dysbindin-1 genotype significantly affected the number of total responses (F(2, 42) = 3.69, p < 0.05). Dys−/− mice produced more responses than dys+/+ (p < 0.05; Fig. 4A) Even with a significant difference in total responses, there was no effect of genotype on choice accuracy (F(2, 42) = 1.72, p = 0.19, Fig. 4B). Interestingly, dys−/− displayed a higher percentage of correct responses (F(2, 42) = 3.29, p < 0.05) than dys +/+ mice (p < 0.05, Bonferroni post hoc test; Fig. 4C) and dys −/− exhibited a lower percentage of omissions (F(2, 42) = 4.19, p < 0.05) compared to their dys +/+ littermates (p < 0.05, Bonferroni post hoc test; Fig. 4E). Percentage of incorrect responses did not significantly differ between the groups (F(2, 42) = 2.43, p = 0.10; Fig.4D). Also, incorrect (F(2, 41) = 0.01, p = 0.99; Fig. 4G) and reward (F(2, 41) = 0.17, p = 0.85; Fig. 4H) latencies were not significantly different during the first day of testing, but there was a borderline effect on correct response latency (F(2, 41) = 3.99, p = 0.05). The dys+/− and dys−/− groups exhibited trends toward shorter correct response latencies (p = 0.06 and 0.10 respectively; Fig. 4F).
Figure 4.
Response rates and latencies during the first day of testing. Total responses (A), % choice accuracy (B), % correct (C), % incorrect (D), % omissions (E), correct latency (F), and incorrect latency (G), and reward latency (H). Dys −/− mice produced significantly more responses than dys+/+. Dys−/− mice also exhibited a higher % correct and lower % omission compared to dys+/+ mice. * = p < 0.05.
3.4. Strain differences are not present on the last day of testing
In order to determine the effects of training on the behavioral parameters of this operant task, we analyzed the testing results from each mouse’s final day of testing. This was the testing day on which each mouse reached criterion. These data are presented in Figure 5. Here, we found no differences between the genotypes on total response (F(2, 42) = 2.24, p = 0.12), % choice accuracy (F(2, 42) = 0.44, p = 0.65), % correct (F(2, 42) = 0.13, p = 0.88), % incorrect (F(2, 42) = 0.74, p = 0.49), % omissions (F(2, 42) = 0.20, p = 0.82), correct latency (F(2, 42) = 2.12, p = 0.13), incorrect latency (F(2, 42) = 0.15, p = 0.86), or reward latency (F(2, 42) = 0.81, p = 0.45).
Figure 5.
Response rates and latencies during the last day of testing. Total responses (A), % choice accuracy (B), % correct (C), % incorrect (D), % omissions (E), correct latency (F), and incorrect latency (G), and reward latency (H). There were no group differences observed on the last day of testing in any of the measures.
3.5. Comparison between first and last day of testing
We next directly compared behavior on the first and last day of testing to determine if there were any within-subject changes that could account for the performance disparity. There was a significant genotype X testing day interaction on total responses (F(2, 42) = 6.16, p < 0.001). Dys +/+ and +/− did not significantly change the number of total responses from the first day of testing to the last day (p = 0.15 and 0.10, respectively; Bonferroni post hoc test; Fig. 6A). In contrast, dys −/− drastically reduced the number of total responses over this time period (p < 0.001, Bonferroni post hoc test; Fig. 6A). Choice accuracy improved from the first to last day (F(1, 42) = 36.52, p < 0.001). The improvement in choice accuracy was significant in the dys +/− and dys −/− groups (p < 0.01 and 0.001, respectively; Bonferroni post hoc test) while the increase in choice accuracy did not quite reach the level of significance in the dys +/+ mice (p = 0.07, Bonferroni post hoc test; Fig. 6B). The percentage of correct responses increased during testing (F(1, 42) = 77.59, p < 0.001). The increases in correct percentage were similar between the three groups (p < 0.001 for all groups, Bonferroni post hoc test; Fig. 6C). The incorrect response percentage did not change much over time as there was no overall testing day effect (F(1, 42) = 0.48, p = 0.49), genotype effect (F(2, 42) = 1.87, p = 0.17), or genotype X testing day interaction (F(2, 42) = 1.62, p = 0.21; Fig. 6D).
Figure 6.
Comparison of response rates between the first day and last day of testing. Total responses (A), % choice accuracy (B), % correct (C), and % incorrect (D). Dys −/− mice significantly reduced their total responses from the first day to the last. Both dys+/− and dys−/− significantly increased their % choice accuracy. All three groups showed increases in % correct. None of the groups had a significant change in the % incorrect. * = p < 0.05, ** = p < 0.01, and *** = p < 0.001.
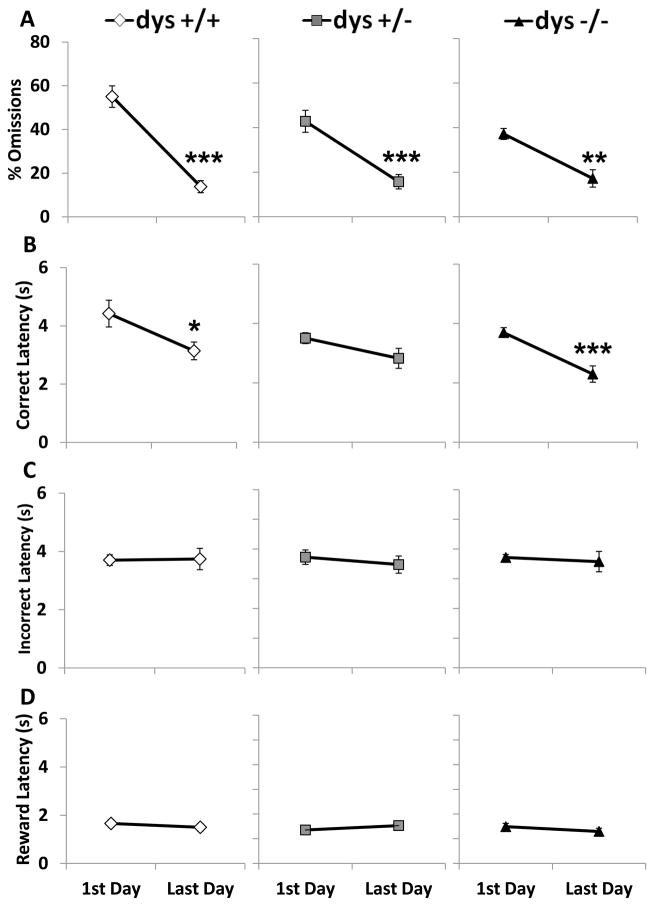
As performance improved over time, the omission percentage decreased for all three groups (F(1, 42) = 65.21, p < 0.001; Fig. 7A). Correct response latency also decreased overall (F(1, 42) = 20.45, p < 0.001) with significant decreases in the dys+/+ and dys−/− groups (p < 0.05 and 0.001, respectively; Bonferroni post hoc test; Fig. 7B). None of the groups altered their incorrect (F(1, 42) = 0.23, p = 0.63; Fig. 7C) or reward (F(1, 42) = 0.03, p = 0.86; Fig.7D) latencies over the course of the Testing Phase.
Figure 7.
Comparison of % omissions and latencies between the first day and last day of testing. % omissions (A), correct latency (B), incorrect latency (C), and reward latency (D). All groups decreased % omissions while dys+/+ and dys−/− decreased their correct latency over time. There were no significant changes in either incorrect latency or reward latency. * = p < 0.05, ** = p < 0.01, and *** = p < 0.001.
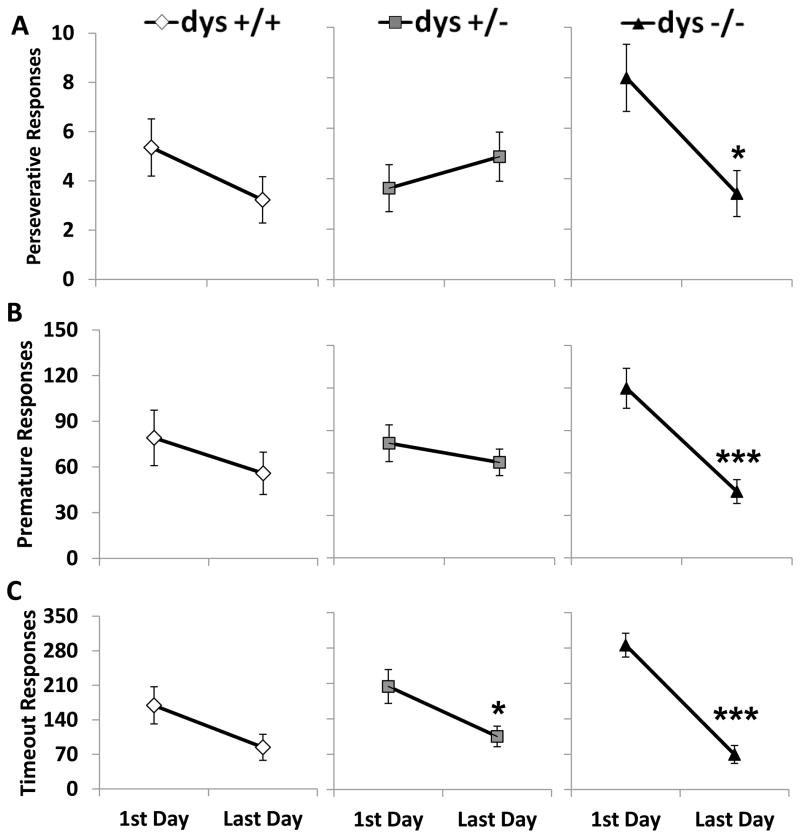
3.6. Dys−/− mice reduce the number of compulsive and impulsive responses between the first and last day
As mentioned previously, dys−/− mice produce more total responses on the first day of testing compared to the other genotypes, but on the last day of testing the difference was no longer present. The high level of total responses produced by dys−/− mice on the first day of testing appears to be due to increased compulsive and impulsive responses. In fact, the number of perseverative (F(2, 42) = 4.82, p < 0.05), premature (F(2, 42) = 4.86, p < 0.05), and timeout (F(2, 42) = 5.21, p < 0.05) responses showed significant genotype X testing day interactions. These interactions revealed a significant decline in both perseverative (p < 0.05, Bonferroni post hoc test; Fig 8A) and premature (p < 0.001, Bonferroni post hoc test; Fig 8B) responses only in the dys−/− group; while the decrease in timeout responses was driven by the dys+/− (p < 0.05) and dys−/− (p < 0.001; Fig 8C)groups.
Figure 8.
Comparison of impulsive and compulsive responses between the first and last day of testing. Perseverative responses (A), premature responses (B), and timeout responses (C). Only dys−/− mice significantly decreased the number of perseverative and premature responses. Both dys+/− and dys−/− decreased the number of timeout responses made over time. Dys+/+ mice did not significantly alter any of these incorrectly timed responses * = p < 0.05 and *** = p < 0.001.
4. Discussion
In the current study, dys−/− mice required more testing sessions to reach criterion in a reward-based operant task compared to dys+/+ controls and dys+/− heterozygotes. Dys−/− mice also produced more premature, perseverative, and timeout responses at the beginning of testing and significantly decreased these behaviors between the first and last testing sessions.
Dys+/− mice also showed a progressive decrease in the number of timeout responses suggesting that there may be a gene dose effect on impulsive behavior. Dys−/− mice exhibited impaired acquisition of the operant task utilized in this set of experiments. This finding is in contrast to the improved acquisition of a working memory discrete paired-trial T-maze task shown by dys−/− mice [21]. The discrepancy may be due to the increased attentional load of the current task, the different cognitive domain studied, and/or the different perceptual demands required for correct responses (visual in the present study vs. spatial in the T-maze task). The discrete paired-trial T-maze task, previously used with dys−/− mice [21], is a specific working memory task and its acquisition is strongly dependent on the medial PFC [29]. During the acquisition of this T-maze paradigm, mice are only required to learn a non-match-to position rule and remember information from the sample trials for a short period of time (4 sec) and over the course of a limited number of trials (10 paired-trials a day). Therefore, attentional processes might play a minor role in the overall performance of this particular task. In contrast, the current operant task requires sustained attention during a larger number of trials, over an extended period of time, in order for mice to correctly estimate interval timing and reach criterion.
The higher level of impulsive and compulsive responses produced by dys−/− mice during the early testing sessions suggests that abnormal interval timing is responsible for their performance deficits. Accurate interval timing is thought to be mediated by intact pathways connecting the PFC and striatum [36], regions where dys−/− mice display altered neuronal signaling [21, 26]. For example, inactivation of the PFC in rats increases premature responding in an operant reaction time task [37]. GABAergic interneurons taken from the PFC of dys−/− mice display reduced excitability [26] and layer II/III pyramidal cells in the medial prefrontal cortex are hyperexcitable [21]. Additionally, reduced dysbindin-1 expression is associated with glutamatergic hypofunction in the PFC of mice [16, 22]. These alterations in PFC circuitry in dys−/− inhibit the production of a coherent PFC output. Specifically, intact GABAergic interneuron signaling in the PFC has been implicated in the maintenance of neuronal synchronization [38–40] critical for performance in PFC-mediated cognitive tasks. Abhorrent signaling in the striatum may also contribute to the cognitive deficits exhibited by dys −/− mice. Previous work has implicated the region in the modulation of cognitive function [41]. Recent work has linked overexpression of the striatal dopamine D2 receptor to cognitive dysfunction and altered PFC function in mice [28–29, 42]. These studies implicate striatal signaling in the maintenance of normal PFC function. Additionally, the disruption of synchronized oscillations in the PFC is associated with schizophrenia and other psychiatric disorders in humans [39], suggesting a possible circuit mechanism by which reduced dysbindin-1 could contribute to the cognitive deficits present in schizophrenia.
Intact dopaminergic neurotransmission is critical for the performance of reward-based visual discrimination [35]. Dysbindin-1 is intimately involved in the regulation of dopaminergic neurotransmission. Cortical neurons with downregulated dysbindin-1 in culture [43] or taken from dys −/− mice have increased neuronal surface expression of functionally active D2 receptors and exhibit altered sensitivity to D2 agonist/antagonists [21, 26]. D2 receptor signaling is involved in the regulation of interval timing as overexpression of D2 receptors in the striatum of mice produces interval timing deficits in another operant task [28]. Interestingly, striatal fast-spiking GABA interneurons in dys−/− mice exhibit abnormal firing patterns, suggesting that alterations in the striatum may also underlie the performance deficits in dys−/− mice [26]
Dysbindin-1 has also been shown to play a critical role in neurodevelopmental processes implicated in schizophrenia [4, 23, 44] and the convergence between dysbindin-1 function and development of DA/D2 receptor signaling pathways could contribute to the cognitive deficits in schizophrenia. Relevant to the current findings, patients with schizophrenia are less proficient in estimating timing intervals [45]. Increased D2 receptor density in humans, which is also present in dys−/− mice, has been reported in drug-naïve patients with schizophrenia [46]
Interestingly, over the course of testing, dys −/− decreased their impulsive and compulsive responses while dys+/+ mice did not show any significant changes in these categories. When the dys−/− mice reach criterion, their impulsive and compulsive response totals were no different from dys+/+ mice. Therefore, the response pattern exhibited by dys−/− early in testing is not static and can improve with extensive training. Follow-up studies may be able to determine if there are concomitant changes in either PFC or striatal signaling that directly contribute to the changes in behavioral performance.
To our knowledge, this particular operant task paradigm has not yet been used in mice. However, there is body of literature on putative mouse models of schizophrenia-associated cognitive deficits using other operant paradigms based on visual discriminations [47]. For example, models of impaired glutamatergic function produce complicated effects on touchscreen-based visual discrimination. NR2A subunit knockout mice and GLAST-deficient mice exhibit specific impairment in the initial visual discrimination task [48–49] while GluA1 subunit knockout mice learn the discrimination task faster than WT mice and subchronic PCP has no effect on learning [50–51]
In conclusion, the present study adds to a growing literature showing learning and memory deficits in dysbindin-1 deficient mice on a C57BL/6 genetic background [20–22]. These mice display alterations in PFC circuitry including glutamatergic and DA/D2 regulation that could be the basis of the deficits in cognitive performance [24, 47]. Specifically, dys−/− mice take longer to acquire a reward-based operant task. Their decreased performance coincides with high levels of impulsive and compulsive behaviors during early sessions that significantly decrease as they learn the task. While we cannot conclude that the pattern of impulsive and compulsive responses explains the delayed learning, our findings suggest that interval timing deficits may be a critical part of the abnormal cognition displayed by dys−/− mice and that these deficits can be improved with increased training and experience.
Highlights.
Dysbindin-1 null mice exhibit deficits in cognitive performance.
Dysbindin-1 null mice show impaired acquisition of a reward-based, operant task.
Impaired performance is due to relatively high numbers of impulsive and compulsive responses compared to littermate controls.
Impulsivity and compulsivity in the dysbindin-1 null mice improves with training.
Acknowledgments
We thank Qingjun Tian and Jingshan Chen for technical assistance and Audrey Bebensee, Randy Xun, and Omoye Akhile for critical review of the manuscript. This research was supported by the National Institute of Mental Health Intramural Research Program, by the NIMH Julius Axelrod Memorial Fellowship Training Award, and by the Marie Curie FP7 Reintegration Grant N°268247.
Footnotes
The authors declare that they have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276:24232–41. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- 2.Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181:791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O’Brien EP, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–9. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, et al. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry. 2010;15:115, 204–15. doi: 10.1038/mp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, et al. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet. 2006;15:1563–8. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]
- 6.Fallgatter AJ, Herrmann MJ, Hohoff C, Ehlis AC, Jarczok TA, Freitag CM, et al. DTNBP1 (dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology. 2006;31:2002–10. doi: 10.1038/sj.npp.1301003. [DOI] [PubMed] [Google Scholar]
- 7.Wolf C, Jackson MC, Kissling C, Thome J, Linden DE. Dysbindin-1 genotype effects on emotional working memory. Mol Psychiatry. 2011;16:145–55. doi: 10.1038/mp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–48. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab SG, Knapp M, Mondabon S, Hallmayer J, Borrmann-Hassenbach M, Albus M, et al. Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet. 2003;72:185–90. doi: 10.1086/345463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Den Bogaert A, Schumacher J, Schulze TG, Otte AC, Ohlraun S, Kovalenko S, et al. The DTNBP1 (dysbindin) gene contributes to schizophrenia, depending on family history of the disease. Am J Hum Genet. 2003;73:1438–43. doi: 10.1086/379928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–34. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 12.Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE. Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res. 2008;98:105–10. doi: 10.1016/j.schres.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–63. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG, et al. Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet. 2009;18:3851–63. doi: 10.1093/hmg/ddp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–55. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 16.Jentsch JD, Trantham-Davidson H, Jairl C, Tinsley M, Cannon TD, Lavin A. Dysbindin modulates prefrontal cortical glutamatergic circuits and working memory function in mice. Neuropsychopharmacology. 2009;34:2601–8. doi: 10.1038/npp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takao K, Toyama K, Nakanishi K, Hattori S, Takamura H, Takeda M, et al. Impaired long-term memory retention and working memory in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Mol Brain. 2008;1:11. doi: 10.1186/1756-6606-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7:170–9. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Este L, Casini A, Puglisi-Allegra S, Cabib S, Renda TG. Comparative immunohistochemical study of the dopaminergic systems in two inbred mouse strains (C57BL/6J and DBA/2J) J Chem Neuroanat. 2007;33:67–74. doi: 10.1016/j.jchemneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Cox MM, Tucker AM, Tang J, Talbot K, Richer DC, Yeh L, et al. Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav. 2009;8:390–7. doi: 10.1111/j.1601-183X.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN, et al. Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry. 2012;17:85–98. doi: 10.1038/mp.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsgodt KH, Robleto K, Trantham-Davidson H, Jairl C, Cannon TD, Lavin A, et al. Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance. Biol Psychiatry. 2011;69:28–34. doi: 10.1016/j.biopsych.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickman DK, Davis GW. The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 2009;326:1127–30. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papaleo F, Weinberger DR. Dysbindin and Schizophrenia: it’s dopamine and glutamate all over again. Biological psychiatry. 2011;69:2–4. doi: 10.1016/j.biopsych.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumamoto N, Matsuzaki S, Inoue K, Hattori T, Shimizu S, Hashimoto R, et al. Hyperactivation of midbrain dopaminergic system in schizophrenia could be attributed to the down-regulation of dysbindin. Biochem Biophys Res Commun. 2006;345:904–9. doi: 10.1016/j.bbrc.2006.04.163. [DOI] [PubMed] [Google Scholar]
- 26.Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, et al. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci U S A. 2009;106:19593–8. doi: 10.1073/pnas.0904289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13:2699–708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- 28.Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–9. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–15. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Nagai T, Kitahara Y, Shiraki A, Hikita T, Taya S, Kaibuchi K, et al. Dysfunction of dopamine release in the prefrontal cortex of dysbindin deficient sandy mice: an in vivo microdialysis study. Neurosci Lett. 2010;470:134–8. doi: 10.1016/j.neulet.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 31.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–90. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Dalton GL, Ma LM, Phillips AG, Floresco SB. Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning. Psychopharmacology (Berl) 2011;216:525–35. doi: 10.1007/s00213-011-2246-z. [DOI] [PubMed] [Google Scholar]
- 33.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Kato S, Kuramochi M, Kobayashi K, Fukabori R, Okada K, Uchigashima M, et al. Selective neural pathway targeting reveals key roles of thalamostriatal projection in the control of visual discrimination. J Neurosci. 2011;31:17169–79. doi: 10.1523/JNEUROSCI.4005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson S, Rainwater AJ, Hnasko TS, Palmiter RD. Viral restoration of dopamine signaling to the dorsal striatum restores instrumental conditioning to dopamine-deficient mice. Psychopharmacology (Berl) 2007;191:567–78. doi: 10.1007/s00213-006-0579-9. [DOI] [PubMed] [Google Scholar]
- 36.Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–31. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 39.Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–11. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–82. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–87. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 42.Li YC, Kellendonk C, Simpson EH, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:12107–12. doi: 10.1073/pnas.1109718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iizuka Y, Sei Y, Weinberger DR, Straub RE. Evidence that the BLOC-1 protein dysbindin modulates dopamine D2 receptor internalization and signaling but not D1 internalization. J Neurosci. 2007;27:12390–5. doi: 10.1523/JNEUROSCI.1689-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito H, Morishita R, Shinoda T, Iwamoto I, Sudo K, Okamoto K, et al. Dysbindin-1, a schizophrenia-related molecule, is involved in the regulation of neuronal dendritic development. Mol Psychiatry. 2010;15:969. doi: 10.1038/mp.2010.93. [DOI] [PubMed] [Google Scholar]
- 45.Elvevåg B, McCormack T, Gilbert A, Brown GD, Weinberger DR, Goldberg TE. Duration judgements in patients with schizophrenia. Psychol Med. 2003;33:1249–61. doi: 10.1017/s0033291703008122. [DOI] [PubMed] [Google Scholar]
- 46.Wong DF, Wagner HN, Tune LE, Dannals RF, Pearlson GD, Links JM, et al. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986;234:1558–63. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- 47.Papaleo F, Lipska BK, Weinberger DR. Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology. 2012;62:1204–20. doi: 10.1016/j.neuropharm.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 48.Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–4. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–89. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brigman JL, Ihne J, Saksida LM, Bussey TJ, Holmes A. Effects of Subchronic Phencyclidine (PCP) Treatment on Social Behaviors, and Operant Discrimination and Reversal Learning in C57BL/6J Mice. Front Behav Neurosci. 2009;3:2. doi: 10.3389/neuro.08.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A, et al. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62:1263–72. doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]