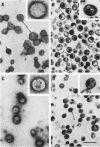
Abstract
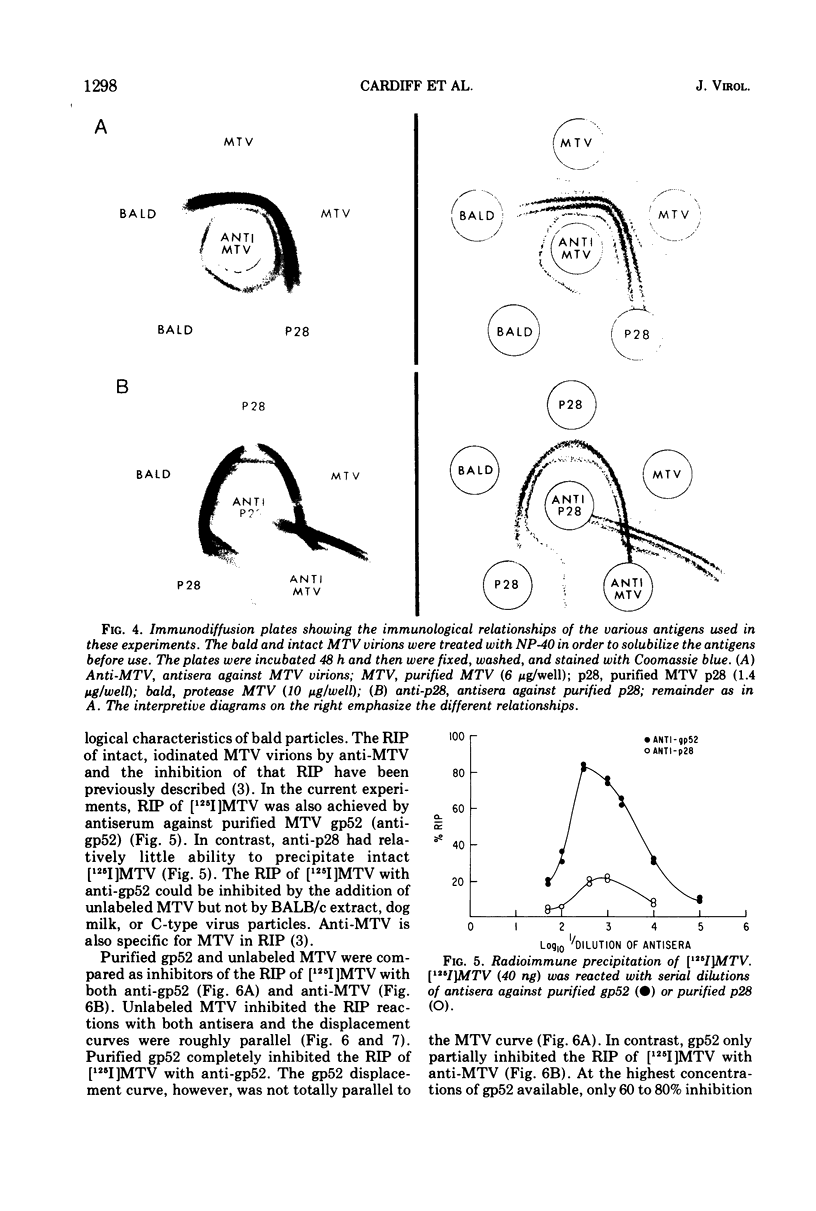
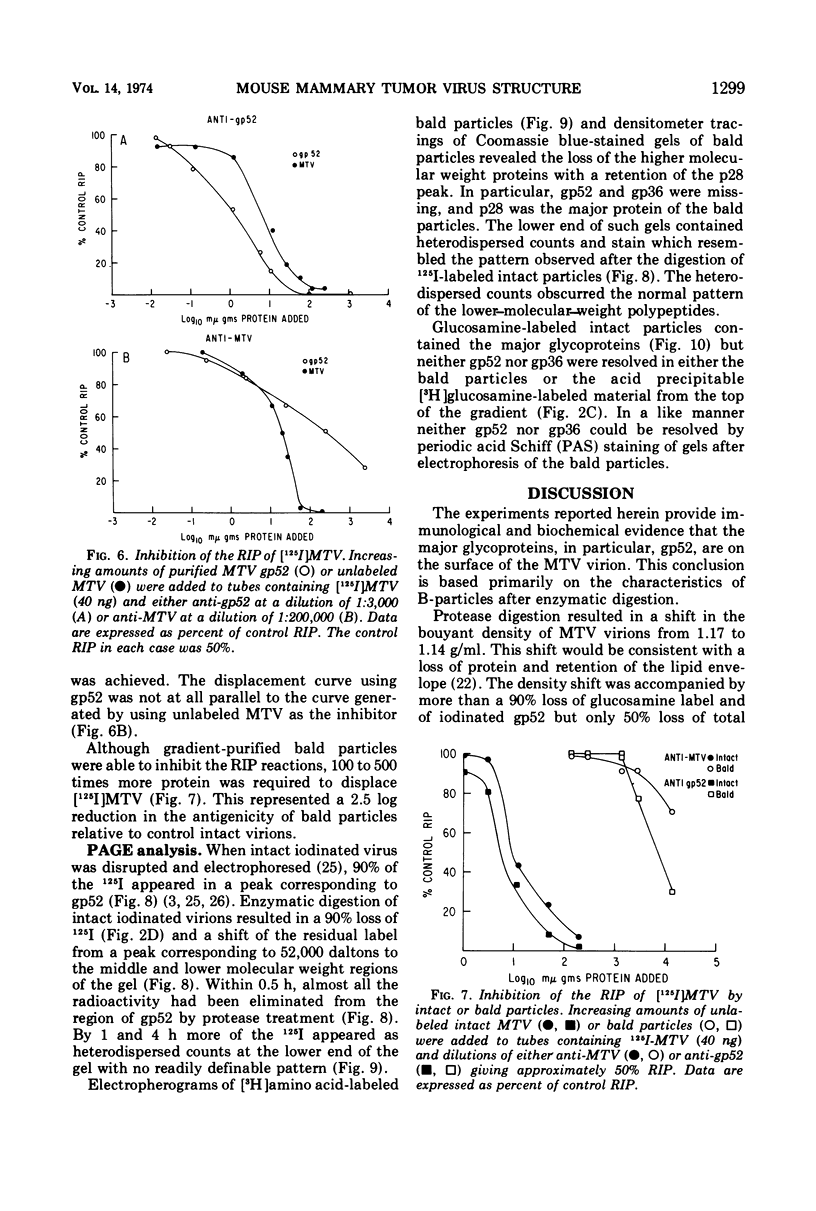
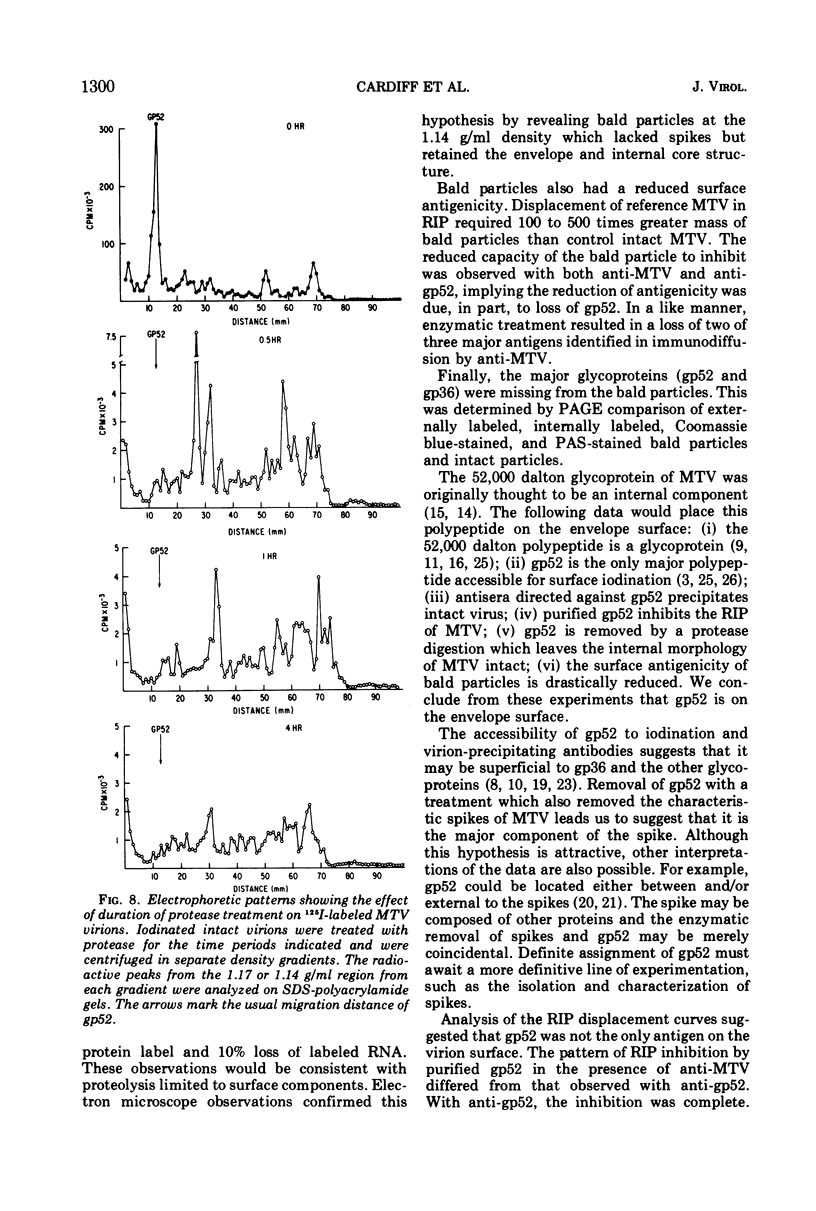
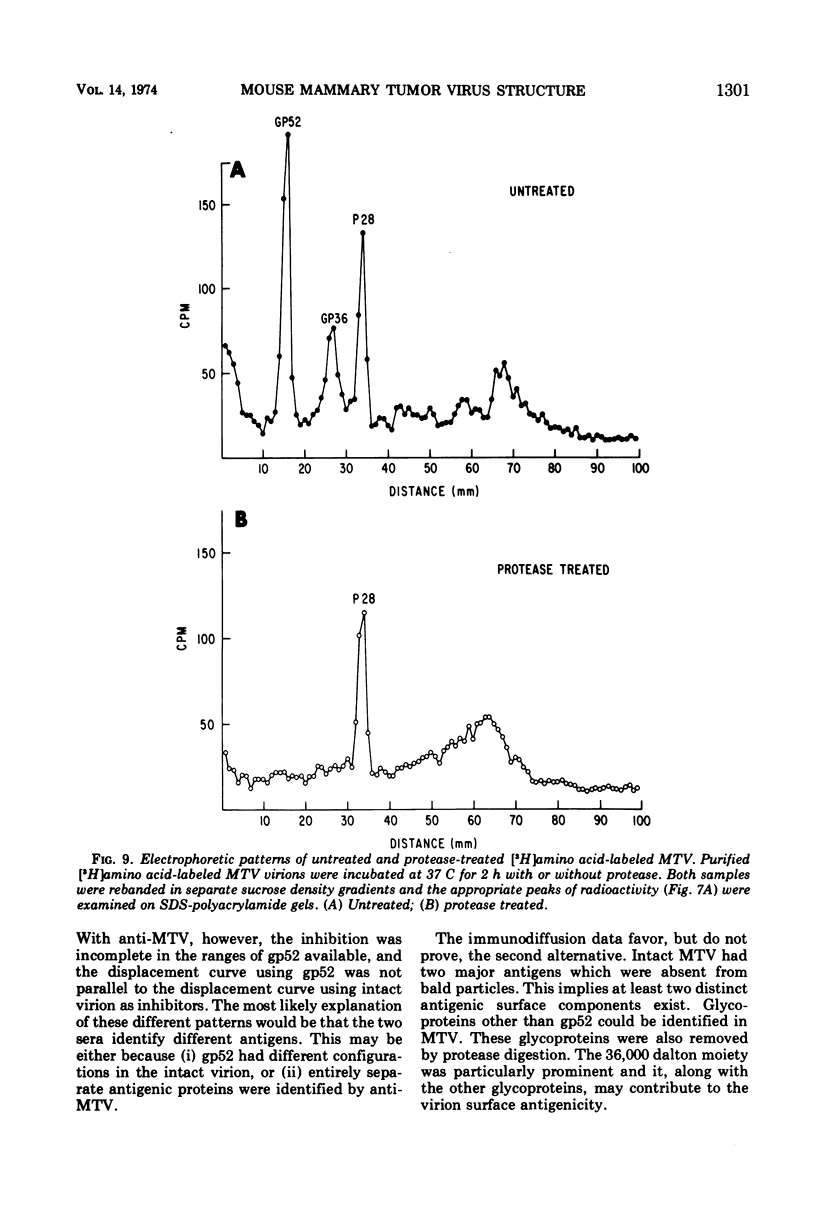
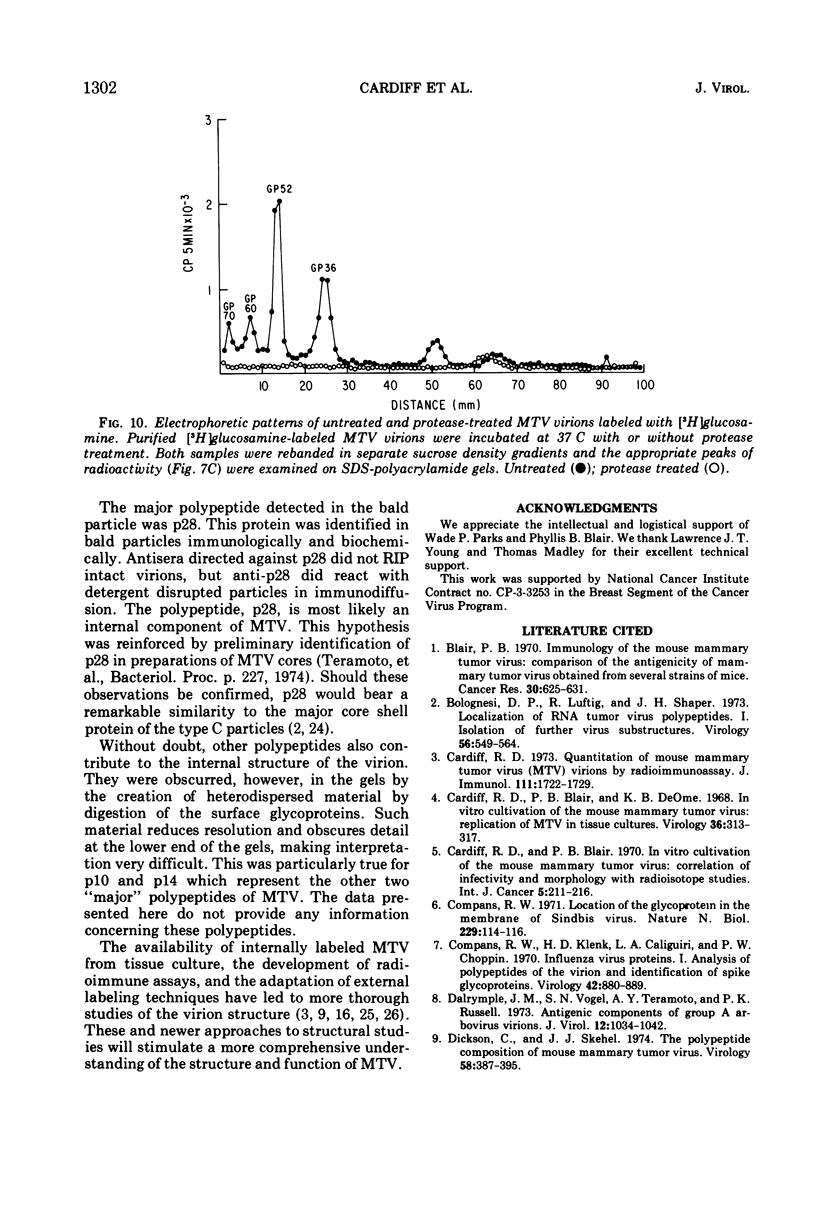
The polypeptide, antigenic, and morphological structure of the mouse mammary tumor virus was studied following protease digestion of intact virions. Intact, untreated virions (ρ = 1.17 g/ml) had characteristic envelope spikes, five major polypeptides, and were precipitated by antisera against gp52. Two of the major polypeptides, with molecular weights of 52,000 (gp52) and 36,000 (gp36), had carbohydrate moieties. Protease treatment resulted in spikeless, “bald” particles (ρ = 1.14 g/ml), which had altered surface antigenicity and which contained neither gp52 nor gp36. These data indicated that gp52 and gp36 were on the viral envelope. Bald particles retained a 28,000 dalton polypeptide (p28) which was proposed as the major internal polypeptide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair P. B. Immunology of the mouse mammary tumor virus: comparison of the antigenicity of mammary tumor virus obtained from several strains of mice. Cancer Res. 1970 Mar;30(3):625–631. [PubMed] [Google Scholar]
- Bolognesi D. P., Luftig R., Shaper J. H. Localization of RNA tumor virus polypeptides. I. Isolation of further virus substructures. Virology. 1973 Dec;56(2):549–564. doi: 10.1016/0042-6822(73)90057-3. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Blair P. B. In vitro cultivation of the mouse mammary tumor virus: correlation of infectivity and morphology with radioisotope studies. Int J Cancer. 1970 Mar 15;5(2):211–216. doi: 10.1002/ijc.2910050207. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Blair P. R., DeOme K. B. In vitro cultivation of the mouse mammary tumor virus: replication of MTV in tissue culture. Virology. 1968 Oct;36(2):313–317. doi: 10.1016/0042-6822(68)90152-9. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D. Quantitation of mouse mammary tumor virus (MTV) virions by radioimmunoassay. J Immunol. 1973 Dec;111(6):1722–1729. [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Location of the glycoprotein in the membrane of Sindbis virus. Nat New Biol. 1971 Jan 27;229(4):114–116. doi: 10.1038/newbio229114a0. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Vogel S. N., Teramoto A. Y., Russell P. K. Antigenic components of group A arbovirus virions. J Virol. 1973 Nov;12(5):1034–1042. doi: 10.1128/jvi.12.5.1034-1042.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Skehel J. J. The polypeptide composition of mouse mammary tumor virus. Virology. 1974 Apr;58(2):387–395. doi: 10.1016/0042-6822(74)90074-9. [DOI] [PubMed] [Google Scholar]
- Fritz R. B. Enzymatic radioiodination of the envelope proteins of avian myeloblastosis virus. J Virol. 1974 Jan;13(1):42–45. doi: 10.1128/jvi.13.1.42-45.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Skehel J. J., Crumpton M. J. Purification of virus glycoproteins by affinity chromatography using Lens culinaris phytohaemagglutinin. FEBS Lett. 1973 Jan 15;29(2):185–188. doi: 10.1016/0014-5793(73)80557-5. [DOI] [PubMed] [Google Scholar]
- McGrath C. M. Replication of mammary tumor virus in tumor cell cultures: dependence on hormone-induced cellular organization. J Natl Cancer Inst. 1971 Aug;47(2):455–467. [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Old L. J., Sarkar N. H., Moore D. H. Common properties of the oncogenic RNA viruses (oncornaviruses). Virology. 1970 Dec;42(4):1152–1157. doi: 10.1016/0042-6822(70)90367-3. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Scolnick E. M., Oroszlan S., Gilden R. V. Immunochemical characterization of two major polypeptides from murine mammary tumor virus. J Virol. 1974 Jun;13(6):1200–1210. doi: 10.1128/jvi.13.6.1200-1210.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Nowinski R. C., Moore D. H. Characteristics of the structural components of the mouse mammary tumor virus. I. Morphological and biochemical studies. Virology. 1971 Oct;46(1):1–20. doi: 10.1016/0042-6822(71)90002-x. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield J. B. Envelope of mouse mammary tumor virus studied by freeze-etching and freeze-fracture techniques. J Virol. 1973 Sep;12(3):616–624. doi: 10.1128/jvi.12.3.616-624.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu T., Dmochowski L. Studies on the acid mucopolysaccharide coat of viruses and transformed cells. Cancer. 1973 Jan;31(1):165–174. doi: 10.1002/1097-0142(197301)31:1<165::aid-cncr2820310123>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Stanley P., Haslam E. A. The polypeptides of influenza virus. V. Localization of polypeptides in the virion by iodination techniques. Virology. 1971 Dec;46(3):764–773. doi: 10.1016/0042-6822(71)90078-x. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Hurley N. E., Davis N. L., Rueckert R. R., Fleissner E. Structural studies of avian myeloblastosis virus: comparison of polypeptides in virion and core component by dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1974 Feb;13(2):513–528. doi: 10.1128/jvi.13.2.513-528.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Puentes M. J., Young L. J., Cardiff R. D. Structure of the mouse mammary tumor virus: polypeptides and glycoproteins. J Virol. 1974 Feb;13(2):411–418. doi: 10.1128/jvi.13.2.411-418.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L., Kaplan H. S. Structural characteristics of some murine RNA tumor viruses studied by lactoperoxidase iodination. Proc Natl Acad Sci U S A. 1973 Jan;70(1):36–40. doi: 10.1073/pnas.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettner A., Duly P. E. Principles of competitive binding assays (saturation analyses). II. Sequential saturation. Clin Chem. 1974;20(1):5–14. [PubMed] [Google Scholar]