Abstract
In this review, we first provide a historical perspective of inhibitory signaling from the discovery of inhibition through to our present understanding of the diversity and mechanisms by which GABAergic interneuron populations function in different parts of the telencephalon. This is followed by a summary of the mechanisms of inhibition in the CNS. With this as a starting point, we provide an overview describing the variations in the subtypes and origins of inhibitory interneurons within the pallial and subpallial divisions of the telencephalon, with a focus on the hippocampus, somatosensory, paleo/piriform cortex, striatum, and various amygdala nuclei. Strikingly, we observe that marked variations exist in the origin and numerical balance between GABAergic interneurons and the principal cell populations in distinct regions of the telencephalon. Finally we speculate regarding the attractiveness and challenges of establishing a unifying nomenclature to describe inhibitory neuron diversity throughout the telencephalon.
Keywords: interneurons, GABA, cortex, subpallial, ganglionic eminences
INTRODUCTION
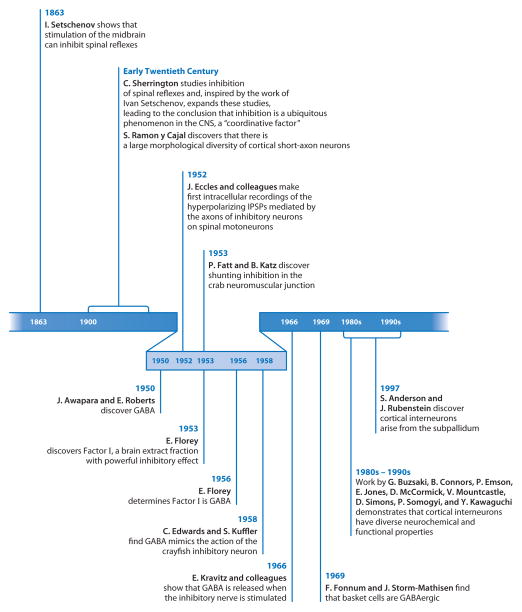
Excitation and inhibition are the two fundamental modes of signaling within the CNS. Although several neurotransmitters exist within the nervous system, glutamate and γ-aminobutyric acid (GABA), two neurotransmitters with surprisingly similar structures, mediate the majority of excitatory and inhibitory signals within much of the CNS including the telencephalon. With regards to inhibition, the discovery of GABA in 1950 (Roberts & Frankel 1950, Udenfriend 1950, Williams 1950) determined the identity of the primary inhibitory transmitter in the CNS (Figure 1). Another decade would pass, however, before work by Kravitz, Kuffler, and colleagues definitively showed that it fulfilled the criteria to act as an endogenous neurotransmitter (Kravitz et al. 1963a,b). These experiments immediately suggested that the high levels of GABA in the mammalian brain were not as then thought merely metabolites (Roberts 1963) but indicative of neuronal populations that mediate inhibition. Work over the past three decades has borne out this hypothesis and demonstrated that GABAergic neurons mediate most of the inhibition throughout the CNS.
Figure 1.
Key events in the discovery of inhibition.
With the notable exception of stellate neurons in neocortex, most excitatory cells within the telencephalon are projection neurons. In contrast, inhibitory signaling is mediated by both projection and interneuron populations, distinguished primarily by whether their axons project locally or outside the area where the cell body is located. Although such a distinction may on occasion seem semantic, there is little doubt that interneurons or “local projection neurons” subserve a unique function in gating signaling within the nervous system. Although the functional properties of a diverse set of “short-axon” cells in the cortex were unknown until recently, their identification was already noted by Ramón y Cajal more than 100 years ago (Ramón y Cajal 1891). Subsequent to the realization that these were GABAergic neurons and hence inhibitory (Dreifuss et al. 1969; Fonnum & Storm-Mathisen 1969; Hendry et al. 1984, 1986; Storm-Mathisen & Fonnum 1971), the recognition that the considerable heterogeneity in this population had functional significance took a further two decades (reviewed in Ascoli et al. 2008, Whittington & Traub 2003). During the mid-1980s a number of groups first demonstrated that, in addition to morphological diversity, interneurons throughout the telencephalon had both immunochemical (reviewed in Jones 1986) and electrophysiological (reviewed in Buzsaki 1984, Freund & Buzsaki 1996) diversity that likely reflected the diverse roles they played in both cortical and subcortical networks. In this review we examine these populations in different regions of the telencephalon from a historical and functional perspective. Our aim in doing so is not foremost to determine a unified classification for these cells, but rather to provide a context for how this diversity impacts signaling in various regions throughout the telencephalon. Although this survey of interneuron subtypes does not resolve the daunting problem of how best to compare different interneuron classes in distinct brain areas, it does serve to illustrate the remarkable computational power this population provides to neural circuitry.
DISCOVERY OF INHIBITION
Universally, nervous systems in higher organisms include two types of neurons: excitatory neurons that ensure the transmission of signals through various stages of processing and inhibitory neurons that control this transfer of information. These two, however, are intrinsically linked, as the nervous system depends on both: one cannot function without the other. Inhibition was discovered early in the investigations of the nervous system (Figure 1), when studies in both invertebrate and vertebrate preparations showed that stimulation of certain neurons (or fibers from those neurons) produced inhibition of stimulatory responses. Inhibition had a prominent place in the research of Charles Sherrington, a pioneer of neurophysiology. In fact, when Sherrington received the Nobel Prize with Edgar Douglas Adrian in 1932 for their “discoveries regarding the functions of neurons,” inhibition was a major topic of his lecture (Sherrington 1932). Sherrington spent a great deal of effort studying “reciprocal innervation,” the observation that stimuli evoking contraction in a given muscle will provoke inhibition of the antagonist muscle, leading to the view that “inhibitory” afferents conduct impulses that “at certain central loci cause, directly or indirectly, inhibition.” Inhibition also became important in his studies of brain control of spinal reflexes, having stated in his Nobel lecture that “[f]urther study of central nervous action, however, finds central inhibition too extensive and ubiquitous to make it likely that it is confined solely to the taxis of antagonistic muscles.” Sherrington cited as inspiration earlier studies including the 1863 work of Ivan Setschenov, perhaps the first to show that brain activity is linked to electric currents, demonstrating that stimulation of the midbrain could inhibit spinal reflexes. Sherrington concluded his Nobel lecture with the following:
The role of inhibition in the working of the central nervous system has proved to be more and more extensive and more and more fundamental as experiment has advanced in examining it. Reflex inhibition can no longer be regarded merely as a factor specially developed for dealing with the antagonism of opponent muscles acting at various hinge-joints. Its role as a coordinative factor comprises that, and goes beyond that. In the working of the central nervous machinery inhibition seems as ubiquitous and as frequent as is excitation itself. The whole quantitative grading of the operations of the spinal cord and brain appears to rest upon mutual interaction between the two central processes “excitation” and “inhibition,” the one no less important than the other. (Sherrington 1932)
It was not until much later, however, that the action of an inhibitory neuron was studied directly, when Eccles and his colleagues using intracellular electrodes recorded the effect of the axon of an inhibitory neuron on spinal motoneurons (Brock et al. 1952). The inseparability of excitation and inhibition is reflected in the fact that inhibitory neurons are found throughout the brain. Functional units of the mammalian CNS generally consist of both excitatory and inhibitory neurons. Indeed, in areas in which this is not the case, such as the striatum or central or medial amygdala, the inhibitory cells are the ones that can exist in the absence of excitatory neurons rather than vice versa. This no doubt reflects the fundamental instability of solely excitatory networks and serves to emphasize the ubiquitous need for inhibition in the CNS. It would be impossible to cover in one review all the inhibitory neurons of the mammalian nervous system. So, following a discussion of aspects of inhibition that apply to all CNS areas, we focus in more detail on the inhibitory neurons of the mammalian telencephalon.
INHIBITORY NEUROTRANSMITTERS
Two known neurotransmitters mediate ionotropic inhibitory responses: GABA and glycine. GABA is an inhibitory neurotransmitter in invertebrates and vertebrates and is the major inhibitory neurotransmitter in the brain (Jentsch et al. 2002). Glycine is the major inhibitory neurotransmitter in the spinal cord. Strychnine-sensitive glycine receptors (GlyRs) have also been long recognized as important mediators of synaptic inhibition in the brainstem and medulla (Betz & Laube 2006, Betz 1991). However, recent evidence suggests that glycine also serves as an inhibitory neurotransmitter in other CNS areas including in the forebrain (Betz & Laube 2006, Hernandes & Troncone 2009, Trombley & Shepherd 1994). High densities of GlyRs have been found in the striatum and the hippocampus (Zarbin et al. 1981, Araki et al. 1988, Friauf et al. 1997). Moreover, as receptors to both neurotransmitters mediate their influence predominantly by altering chloride conductances, discussing them in conjunction is appropriate.
MECHANISMS OF INHIBITION
The activation of ionotropic GABA (GABAA) and glycine receptors produces inhibition by at least two major mechanisms: hyperpolarizing and shunting. The existence of these two forms of inhibition was revealed by the very earliest investigations of synaptic inhibition. Hyperpolarizing inhibition was discovered by Eccles and colleagues (Brock et al. 1952a,b) in cat spinal motoneurons. The discovery of the inhibitory postsynaptic potential (IPSP) was the finding that finally led Eccles to abandon his electrical hypothesis for synaptic transmission. Inhibition in these cells is blocked by strychnine and simulated by the application of glycine, suggesting it is mediated by glycine receptors (Young & MacDonald 1983).
Hyperpolarizing inhibition occurs when the resting membrane potential (Em) is more positive than the equilibrium potential for Cl− (ECl). In mature neurons, [Cl−] is actively maintained at values lower than the electrochemical equilibrium for Cl− by specific transporters, resulting in an inward Cl− gradient (see Control of Intracellular Cl− and the Functions of Inhibitory Neurotransmitters, sidebar above). GABAA and glycine ionotropic receptors are ligand-gated anionic channels. The opening of the channels following association with GABA or glycine leads in mature neurons to chloride influx that hyperpolarizes the membrane. This hyperpolarization increases the difference between the membrane potential and spike threshold, thereby decreasing the effectiveness of excitatory inputs, i.e., inhibiting neuronal excitability.
However, in many instances in which the resting membrane potential is close to the chloride equilibrium potential, the activation of GABAA and glycine receptors can still produce inhibition through a mechanism known as “shunting inhibition.” In these cases, the increase in anionic conductance does not produce significant changes in membrane potential. However, the increase in membrane conductance decreases the membrane resistance, i.e., the synaptic conductance “shunts” or short-circuits the membrane thus reducing the impact of the currents generated by concurrent excitatory inputs resulting in excitatory postsynaptic potentials of smaller amplitude. Excitatory inputs are less effective because the anionic conductance transiently clamps the membrane potential to the EIPSP, resembling the effect of inward-rectifying K+ channels that clamp the membrane potential to EK. Shunting inhibition occurs when the membrane potential (Vm) is close to the chloride equilibrium potential (ECl); that is, Vm ~ ECl = EIPSP.
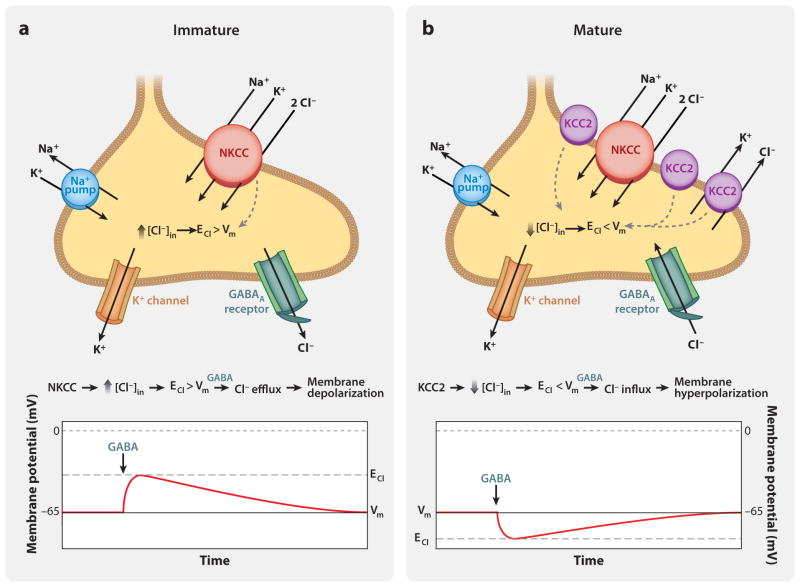
If the chloride equilibrium potential is more positive than the resting potential, the opening of the chloride channels produces depolarization. However, as long as the depolarization is well below spike threshold, the shunting effect dominates the effect on excitability, resulting in inhibition of excitability (Alger & Nicoll 1979, Andersen et al. 1980, Gulledge & Stuart 2003). However, early in development, in certain pathological situations and apparently also in certain subcellular compartments such as the axon initial segment, the chloride gradient is such that the depolarization is large and dominates over the shunting effect. Thus, GABA becomes excitatory (Ben-Ari 2002) (see Control of Intracellular Cl− and the Functions of Inhibitory Neurotransmitters, sidebar above, and Chandelier Cells May Be Excitatory, side-bar below) (see also Figure 2).
Figure 2.
(a) Immature neuron expressing the NKCC (sodium-potassium chloride cotransporter) transporter. NKCC uses the inward Na+ gradient maintained by the Na+ pump to transport Cl− into the cell. This increases the intracellular Cl− concentration, resulting in a chloride equilibrium potential (ECl) that is more positive than the membrane potential (Vm). GABA-mediated activation of GABAA receptors at Vm produces Cl− efflux, resulting in membrane depolarization. (b) Mature neuron coexpressing NKCC (sodium-potassium chloride) and KCC2 (sodium-potassium) cotransporters. KCC2 dominates over NKCC. KCC2 extrudes Cl−, lowering the intracellular Cl− concentration, resulting in a chloride equilibrium potential (ECl) that is more negative than the membrane potential (Vm). GABA-mediated activation of GABAA receptors at Vm produces Cl− influx, resulting in membrane hyperpolarization.
Fatt & Katz (1953) discovered shunting inhibition in the crab neuromuscular junction. They concluded that “the main effect of inhibitory impulses is to attenuate the ‘end-plate potentials,’ i.e., to diminish the local depolarization produced by [excitatory] motor impulses.” Shunting inhibition is believed to be a frequent inhibitory mechanism in central neurons. Recently, Mann & Paulsen (2007) and Jonas and colleagues (Vida et al. 2006) discussed the importance of this form of GABAergic inhibition in the control of spike timing of excitatory neurons and the generation of network oscillations.
Ionotropic anionic receptors may also produce inhibition by a third, and much less studied, mechanism of “depolarizing inhibition,” which has been suggested to operate in GABA-mediated presynaptic inhibition. Some neurons receive axo-axonic GABAergic inputs and contain GABAA receptors (sometimes in addition to GABAB receptors) in their terminals, where the intracellular Cl− concentration is higher than ECl (Howard et al. 2005). The activation of the GABAA receptors depolarizes the terminal and yet reduces neurotransmitter release. It has been suggested that the depolarization reduces transmitter release by blocking the invasion of action potentials into the terminals by a mixture of Na+-channel inactivation and membrane shunting (reviewed in Alvarez-Leefmans & Delpire 2009). This mechanism may thus be responsible for the GABA-mediated presynaptic inhibition of primary afferent terminals in the spinal cord. This mechanism may also explain GABA-mediated inhibition of release from secretory nerve terminals of the posterior pituitary. In this case, researchers were able to record directly from the terminal and show that GABA depolarizes the terminal via GABAA receptors and that AP invasion is blocked, thus inhibiting neurosecretion (Zhang & Jackson 1993). Zhang & Jackson (1993) suggested that the depolarization of the terminal produces inactivation of Na+ channels, thus blocking action potential invasion. However, in the case of the spinal cord, although there is evidence that GABA depolarizes primary sensory neurons, there is no evidence, to our knowledge, that this blocks spike invasion of the terminals, and even less that the depolarization produces Na+-channel inactivation. Presynaptic inhibition via GABA-mediated depolarizing inhibition has also been observed in invertebrate preparations. In a modeling study based on studies in sensory neurons innervating spider VS-3 slit sensilla, French et al. (2006) showed that either shunting or Na+ channel inactivation are sufficient to produce inhibition.
When speaking of inhibitory neurons, one usually thinks of neurons that release the neurotransmitter GABA or glycine. These neurotransmitters indeed activate ionotropic receptors that produce the changes described above leading to inhibition of excitability. However, neurotransmitters that activate metabotropic receptors can also lead to inhibition. These neurotransmitters include acetylcholine (ACh), serotonin (5HT), dopamine, neuropeptides, and even glutamate, the most prevalent excitatory neurotransmitter in the mammalian central nervous system (Lee & Sherman 2009). GABA can also activate metabotropic receptors (known as GABAB receptors). Not all the actions of metabotropic receptors are inhibitory, but one widespread example of such action in the nervous system is the activation of G-protein-activated K+ channels (GIRKs) by metabotropic receptors that activate the appropriate G proteins (Padgett & Slesinger 2010). The resulting increase in K+ conductance produces membrane hyperpolarization resulting in inhibition that can be as potent and effective as that mediated by ionotropic GABA and glycine receptors and often more long lasting. GABA action via GABAB receptors is widespread in the CNS and in some cases may be the main effect of the neurotransmitter.
INTERNEURON CLASSES WITHIN DIFFERENT REGIONS OF THE TELENCEPHALON
The telencephalon can be broadly divided into pallial and subpallial structures (Rubenstein et al. 1998). The pallium can be divided into regions based on their presumed phylogenic origin. According to this nomenclature, the cortex is comprised of archi- (hippocampus), paleo- (periamygdala, perirhinal, entorhinal, and piriform cortices), and neo- (isocortex, which includes the various areal subdivisions of the cortex, such as visual, motor, frontal, and auditory) cortical divisions. Similarly, the subpallium or pallidum can be divided into the neostriatum (caudate-putamen), globus pallidus, and the amygdala nuclei, to name only the most prominent divisions.
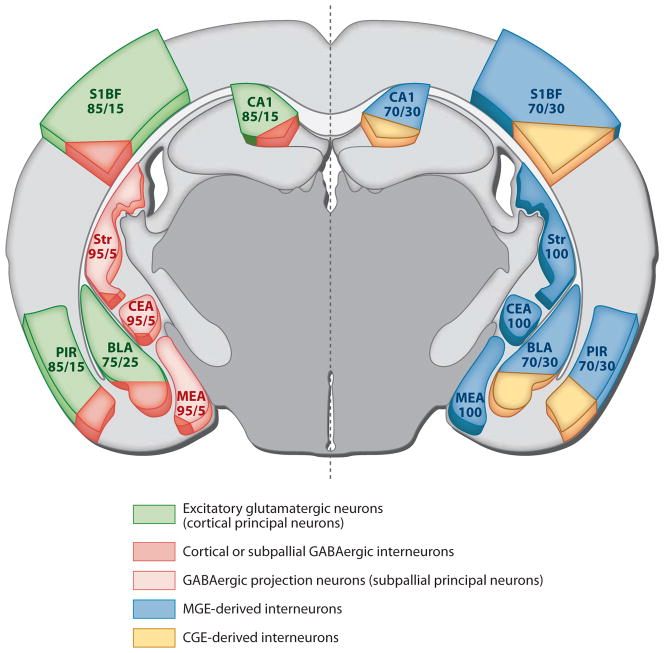
Each of these structures contains a wide cohort of specialized inhibitory projecting neurons and local inhibitory interneurons. If one defines these populations on the basis of their specific connectivity, the differences in the organization and neuronal classes within each of these regions by definition dictate that the subtypes in each of these areas are unique. Indeed, in the CA1 area of the hippocampus where this question has been most extensively addressed, the inhibitory neuronal population consists of more than 20 distinct classes (Somogyi & Klausberger 2005, Klausberger & Somogyi 2008, Klausberger 2009). Alternatively, if one uses a broad constellation of characteristics such as molecular markers (of which Ca2+-binding proteins and neuropeptides have been prominently used as a means to distinguish interneuron subtypes), morphology, and firing pattern, it is possible to compare interneurons across different telencephalic regions (cf. Benes & Berretta 2001, Tepper & Bolam 2004, Wonders & Anderson 2006, Batista-Brito & Fishell 2009). Indeed, if one excludes the special features of interneuron populations residing in each of these structures, one discovers that there are common subtypes that share a number of salient features, most prominently their site of origin within the brain (summarized in Figure 3 and Table 1).
Figure 3.
Cross section of an adult brain: (left) the relative proportion of excitatory (green) or inhibitory (light red) principal projection neurons compared with the percentage of inhibitory interneurons in each of the illustrated structures; (right) the relative proportion of inhibitory interneurons within these same structures that are thought to be derived from the medial ganglionic eminence (MGE) (blue) or the caudal ganglionic eminence (CGE) (yellow). Abbreviations: CA1, CA1 region of the hippocampus; S1BF, primary somatosensory cortex/barrel field; PIR, piriform cortex; Str, striatum; BLA, basal-lateral amygdala; CEA, central amygdala nuclei; MEA, medial amygdala nuclei.
Table 1.
| Origin | Molecular marker | Functional properties | Laminar distribution | Morphology | Diversity | |
|---|---|---|---|---|---|---|
| FS basket cells | MGE | PV | FS firing patternc. Low-input resistance and fast-membrane time constant. Mediate fast, powerful, and precise IPSPs | Layers II–VI; highest proportion in layer IV | Mostly multipolar, occasionally bitufted dendrites; dense local axon often extending to nearby columns and layers, targeting the perisomatic domain (forming “basket” terminals) of principal cells and interneurons, including other FS cells |
|
| Chandelier cells (axo-axonic cells) | MGE | PV | Firing pattern resembles FS basket cells, with higher input resistance and slower membrane time constantd | Layers II–VI. In rodents observed most often in layers II/III | Multipolar or bitufted dendrites. Preterminal axon branches form short vertical rows of boutons resembling candlesticks making synapses on the axonal initial segment of pyramidal cells | |
| Martinotti cells | MGE | SST | Often called LTS cells (low-threshold spiking). Some fire two or more spikes on slow-depolarizing humps from hyperpolarized potentials, while others have an adapting regular-spiking firing pattern. Often show rebound spike(s) on repolarization. Strongly facilitating excitatory inputs. Strong excitation by muscarinic agonists | Layers II–VI | Multipolar, bitufted, or bipolar dendrites; axon arborizes locally and then ascends to layer 1, where it usually produces a dense axonal arborization. Target distal and tuft dendrites |
|
| X-94-like SST neurons | MGE | SST | Lower input resistance and spikes of shorter duration than Martinotti cells (MCs), approaching those of FS cells. Often have a stuttering firing pattern during intermediate current injections. Capable of firing at higher frequencies than MCs but in contrast to FS neurons exhibit spike-frequency adaptation. Strongly facilitating excitatory inputs like MCs | Layers IV and Vb | Multipolar dendrites. Axon ramifies extensively in layer IV | |
| Neurogliaform cells | CGE | 5HT3aR, reelin | Late-spiking firing pattern: delayed firing preceded by a slow depolarizing ramp at threshold and low-current injections. Regular adapting firing during large-current injections. Mediate combined slow GABAA and GABAB synaptic responses. Target dendritic spines but also produce nonsynaptic “volume” GABA release | Layers I–VI | Multipolar, short highly branched dendritic and axonal arbors near the cell body | |
| LS2 | CGE | 5HT3aR, reelin | Late-spiking less robust than neurogliaform cells, no discernable delay to first spike upon firing more than one spike | Multipolar. Wider and less branched dendritic and axonal arborization than neurogliaform cells | ||
| CCK-expressing interneurons | CGE (at least for VIP+) | CCK. Two populations VIP+ and VIP−. CB1 receptorse |
BSNP or RSNP | Layers I–VI; predominantly in upper layers | At least some of these populations are basket cells. Large CCK cells are VIP−; small CCK cells are VIP+ | At least two populations VIP+ and VIP− |
| Bitufted irregular spiking | CGE | 5HT3aR, VIP, CR + | Irregular spiking at low current injections and adapting regular spiking with amplitude accommodation during larger depolarizations. High membrane resistance. | Layers I–VI; mainly layers II/III | Bipolar/bitufted dendrites, descending interlaminar axon. May preferentially target other INs | |
| Bipolar fast adapting | CGE | 5HT3aR, VIP, CR negative | Fast-adapting cells are unable to maintain continuous firing during 500 ms suprathreshold depolarizations and show a large sAHP following the depolarizing step. High input resistance like other VIP cells | Mainly layers II/III | Bipolar and sometimes tripolar or multipolar dendrites | |
| Bursting bipolar cells (bNA2) | CGE | 5HT3aR, VIP | High input resistance like other VIP cells | Mainly layers II/III | Bipolar dendritic morphology | |
| Other VIP interneurons | CGE | VIP, 5HT3aR | Mainly layers II/III | Arcade cells: multipolar or bitufted dendrites; axonal arcades, with vertical arborizations and descending collaterals. Small-basket cells |
||
| Other CGE-derived INs lacking VIP or reelin |
CGE | 5HT3aR | ||||
| IS multipolar | PoA | 5HT3aR | Multipolar |
Abbreviations: BSNP, burst spiking nonpyramidal cell; CCK, cholecystokinin; CGE, caudal ganglionic eminence; CR, calretinin; FS, fast-spiking; IN, interneuron; IS, irregular spiking; MGE, medial ganglionic eminence; PoA, preoptic area; RSNP, regular spiking nonpyramidal cell; sAHP, slow after hyperpolarization; SST, somatostatin; VIP, vasoactive intestinal peptide.
Based mainly on data in somatosensory cortex.
Brief spikes, large fast AHP, high-frequency repetitive firing with little adaptation. At threshold, these cells fire abrupt trains of action potentials after a delay or after an initial spike(s) followed by a pause.
Woodruff et al. 2009 (but see Xu & Callaway 2009).
It is controversial whether both populations or only the VIP- express CB1 receptors.
In addition to their site of origin, molecular marker expression, firing pattern, and morphology (Ascoli et al. 2008), the classical means of discerning whether interneurons are distinct include (a) whether they innervate excitatory or inhibitory neurons or both (Freund & Gulyas 1997), (b) the cellular subdomain they target for innervation (somatic, proximal or distal dendrites, or axonal) (Mittmann et al. 2004), and (c) whether their connectivity is confined within or between particular structures or nuclei (Klausberger & Somogyi 2008). Unfortunately for the vast majority of telencephalic interneurons subtypes detailed information on their connectivity and their synaptic ultrastructure is incomplete (Freund & Gulyas 1997, Meglas et al. 2001, Karube et al. 2004, Kubota et al. 2009).
Another criterion that has been employed for the classification of interneuron populations in the hippocampus is in vivo temporal dynamics. In this region (Cobb et al. 1995, Klausberger et al. 2004, Somogyi & Klausberger 2005, Kwag & Paulsen 2009, Ellender & Paulsen 2010), specific interneuron subtypes fire at particular phases of specific network oscillations. These observations provided evidence that hippocampal functional networks are not hardwired, but instead are recruited dynamically by these oscillations. If so, rather than being an innate property of given interneuron populations, the circuitry in which they are embedded may dictate the context-specific role of interneuron subtypes. Alternatively, one may speculate that specific interneuron populations have evolved to influence particular aspects of the temporal dynamics of brain rhythms regardless of context. Hence, it will be of great interest to determine if particular interneuron subtypes predict their contribution to temporal dynamics in different structures. However, this can be addressed only after in vivo recordings of interneurons in relationship to the rhythms they participate in are completed.
The task of whether interneurons can be meaningfully classified across structures is presently hampered by a lack of data (see Table 2). Even with the criteria suggested above, the extent to which different telencephalic regions have been examined is very uneven, varying from the hippocampus (most particularly the CA1 region), which has been studied extensively, to the medial amygdala, which remains much less well characterized. To focus on the region most familiar to us, we use the somatosensory (S1) cortex (also known as barrel cortex in rodents), an area that shares features with all the telencephalic regions examined, as the standard with which to compare each of these structures. Nonetheless, this is done acknowledging at the outset that if one were to consider distinct subclasses of interneurons based on their afferent/efferent connections, then even the most archetypal interneuron subtype, the fast-spiking (FS) basket cells, would be considered distinct cell types in different cortical layers (Tremblay et al. 2010).
Table 2.
Comparison of GABAergic interneuron populations across telencephalic structuresa
| Neocortex | Hippocampus | Paleocortex/Piriform Cortex | Striatum | Basolateral amygdala |
|---|---|---|---|---|
| FS basket cells | Basket PV cells | FS multipolar cells | FS cells | FS cells with diverse firing patterns resembling the diversity observed in the neocortex |
| Chandelier cells (axo-axonic cells). | Chandelier or axo-axonic cells | Chandelier cells | Chandelier cells | |
| Martinotti cells | OLM cells | Regular-spiking multipolar cells (somatostatin-expressing neurons) | LTS or pLTS SST+ interneurons | SST+ BLA interneurons |
| X-94-like SST neurons | ||||
| Neurogliaform cells (CGE derived) | Neurogliaform and Ivy cells. Most express NOS and are MGE derived | Neurogliaform cells | Neurogliaform cells | |
| CCK+ interneurons | CCK basket cells (VIP+ and VIP−/VGLUT3+) | |||
| LS2 | Horizontal cells that resemble the interneurons described in somatosensory cortex as LS2 | |||
| Bitufted irregular spiking | CR+ VIP+ interneuron targeting INs | CGE-derived VIP neurons are present in BLA but have not been characterized | ||
| Bipolar fast adapting | Bitufted cell. VIP+ bitufted neurons with a fast-adapting firing pattern | |||
| Bursting bipolar cells (bNA2) | ||||
| Projecting GABAergic neurons | Projecting GABAergic neurons: back projection cells; hippocampus to septum projecting cells; double projection; oriens- retrohippocampal projection | Medium spiny neurons | ||
| Bistratified cells | ||||
| Other CCK INs: Schaffer collateral associated cells,/LM-PP-associated cells, and the LM-R-PP-associated cells | ||||
| Trilaminar cells | ||||
| CR (VIP−) IN targeting cells | ||||
| Large calbindin |
Abbreviations: BLA, basolateral amygdala; CCK, cholecystokinin; CGE, caudal ganglionic eminence; CR, calretinin; FS, fast-spiking; IN, interneuron; LM, lacunosum-moleculare; LS, late-spiking; LTS, low-threshold spiking; MGE, medial ganglionic eminence; NOS, nitric oxide synthease; OLM, oriens-lacunosum moleculare; pLTS, persistent low-threshold spiking; PP, perforant pathway; PV, parvalbumin; R-PP, radiatum/perforant pathway; SST, somatostatin; VGLUT3, vesicular glutamate transporter; VIP, vasoactive intestinal peptide.
Somatosensory Cortex
Although some evidence exists that interneuron diversity may vary according to areal division (see Piriform Cortex section below), the repertoire within the barrel cortex is likely fairly representative of those found throughout the neocortex, albeit perhaps with slightly different ratios among subtypes. Indeed, such variances in subtype numbers have been reported in analyses comparing interneuron diversity in somatosensory (Kubota et al. 1993; Cauli et al. 1997; Gupta et al. 2000; Kawaguchi 2001; Kawaguchi & Kondo 2002; Butt et al. 2005; Miyoshi et al. 2007, 2010; Xu & Callaway 2009; Batista-Brito & Fishell 2009; Xu et al. 2010), visual (Gonchar & Burkhalter 1997, Gonchar et al. 2007, Xu et al. 2010) and prefrontal/frontal cortex (Lund & Lewis 1993, Kawaguchi & Kubota 1997, Kawaguchi & Kondo 2002, Lewis et al. 2002, Zaitsev et al. 2009). Depending on the criteria used, anywhere up to ~20 different interneuron subtypes have been described in the barrel cortex. Nonetheless, the most common subtypes can be broadly classified into six distinct categories: parvalbumin (PV)+ (a) chandelier or (b) basket cells, (c) somatostatin (SST)+ Martinotti cells, (d) vasoactive intestinal protein (VIP)+ interneurons (bipolar, bitufted and multipolar cells), (e) Reelin+ (nonsomatostatin) interneurons, and (f) other poorly defined CGE-derived interneurons that do not express VIP or reelin (reviewed in Batista-Brito & Fishell 2009, Lee et al. 2010, Rudy et al. 2010). The most stereotypical features of each of these classes as they are manifested within the somatosensory cortex is summarized in Table 1.
In total, the two most numerous populations of interneurons are the PV+ FS cells and the Martinotti cells, both of which originate embryonically within the medial ganglionic eminence (MGE) (Butt et al. 2005; Fishell 2007; Xu et al. 2004, 2008). Comprising ~40% of all GABAergic interneurons, FS cells are the most prominent subtype across all cortical layers. These cells are characterized by their narrow spike width (~0.3 ms), large and fast afterhyperpolarization, high maximal firing rate (>150 spikes/sec), and minimal firing frequency adaption during sustained depolarization (Connors & Gutnick 1990, McCormick et al. 1985) (see Table 1 for a summary). They can be divided into two broad classes, basket cells and chandelier cells, both of which express the Ca2+-binding protein PV (Celio 1986; reviewed in Kisvarday et al. 1990). Basket cells target the perisomatic domain of both excitatory and inhibitory neurons and are thought to be the strongest source of inhibition. On the other hand, chandelier cells target the axon initial segment (hence, they are often referred to as axo-axonic cells), have been observed to target only pyramidal neurons, and have recently been suggested to elicit depolarizing rather than hyperpolarizing postsynaptic responses (see Chandelier Cells May Be Excitatory, sidebar above). Despite these important differences, without visualizing their morphology the two cell types are difficult to distinguish because their firing properties are similar, although not identical (Woodruff et al. 2009; however, see also Xu & Callaway 2009).
FS basket cells are by far the most common of the interneuron subtypes and populate all layers except layer 1 (L1). Despite their relative abundance, in superficial cortical layers where they are still numerous, they are nevertheless collectively outnumbered by cells derived from the caudal ganglionic eminence (CGE) (Lee et al. 2010). Moreover, despite their common classification as a single interneuron subtype, their connectivity varies in accordance with their laminar position (Dantzker & Callaway 2000, Xu & Callaway 2009, Tremblay et al. 2010). In L5/6 this population innervates the pyramidal neurons that provide all cortical output (Kawaguchi & Kubota 1993). In L4 this population receives thalamic input, is thought to mediate thalamocortical (TC) feed-forward inhibition, and connects to the stellate cell population (Gibson et al. 1999, 2004; Cruikshank et al. 2007). In more superficial layers, basket cells provide inhibition to both L5 projecting pyramidal cells as well as commissural and associative projecting pyramidal neurons (Dantzker & Callaway 2000). They also appear to possess a delay to firing at threshold, although the physiological significance of this property remains poorly understood. FS basket cells also innervate other interneurons including other FS cells (Gibson et al. 1999, Galarreta & Hestrin 2002).
FS basket cells also vary in size; their expression of channels, receptors, and other molecules; and the dynamics of their excitatory inputs (usually depressing, except for FS cell populations in L6). Furthermore, the details of their firing patterns are variable (Chow et al. 1999; Saganich et al. 1999, 2001; Beierlein & Connors 2002; West et al. 2006; Ascoli et al. 2008) (see Table 1).
The second most prevalent inhibitory cell type within the barrel cortex can be loosely classified as Martinotti cells on the basis of their ascending axon and their expression of the neuropeptide SST (Wang et al. 2004). Although it seems inevitable that all six groups described here will be further subdivided, perhaps nowhere is there a need for more reliable classifiers than with regards to this subtype (cf. Kawaguchi & Kubota 1997, Ma et al. 2006, Xu et al. 2006, McGarry et al. 2010). Classically the term Martinotti cell was used for neurons with somas located in L5/6 that send axons to L1. However, if we use the broadly defined criteria described here, the cell bodies of this population, although biased toward deeper layers, are present in all cortical layers except L1. This population is also characterized by their targeting of distal dendrites and by the fact that excitatory synapses onto these cells are strongly facilitating, a property that substantially influences their function (Oviedo & Reyes 2002, Silberberg & Markram 2007, Kapfer et al. 2007, Fanselow et al. 2008, Hull et al. 2009). Martinotti cells either have a bursting firing pattern (responding to stimulation from slightly hyperpolarized potentials with a burst of 1–3 spikes) or are characterized as adapting regular-spiking nonpyramidal cells (Kawaguchi & Kubota 1997, Gibson et al. 1999, Beierlein et al. 2003, Xu et al. 2006, Miyoshi et al. 2007).
Recently, Ma et al. (2006) characterized a population of SST+ interneurons in a mouse line called X94 that differed from classical Martinotti cells in a number of properties. These cells were located in L4 and L5b and had axonal projections that profusely innervated L4 instead of targeting L1. The cells also differed from Martinotti cells in a number of electrophysiological properties. X94 cells had a much lower input resistance, approaching that of FS cells. They had spikes of shorter duration and often produced a stuttering firing pattern. They fired at higher frequencies than did Martinotti cells, but in contrast to FS neurons, they exhibited spike frequency adaptation. However, as in the case of Martinotti cells, these SST-expressing cells received strongly facilitating excitatory synapses.
In addition, variability in SST neurons in the neocortex has been observed in their intrinsic firing properties, their expression of molecular markers, and their connectivity (Butt et al. 2005, Xu et al. 2006, Gonchar et al. 2007, Miyoshi et al. 2007, McGarry et al. 2010). For example, approximately one-third of SST interneurons in the mouse frontal, somatosensory (S1), and visual cortex (V1) contain calretinin (Xu et al. 2006). Although both SST/calretinin (CR)+ cells and SST/CR-cells exhibit similar Martinotti cell anatomical features and have similar adapting spike-firing patterns, they differ in the horizontal extension of their dendritic fields and in their number of primary processes (Xu et al. 2006). In addition, SST/CR− cells have narrower spikes with faster after-hyperpolarizing potentials. Moreover, in L2/3, where SST/CR+ cells are concentrated, the two subtypes of SST interneurons have different connectivity (Xu & Callaway 2009). Whereas SOM+/CR− Martinotti cells receive strong excitatory input from both L2/3 and L4, SOM+/CR+ Martinotti cells receive excitatory input mainly from L2/3. Further arguing for a true delineation between SOM+/CR− and SOM+/CR+ Martinotti cells is evidence suggesting that they are derived from different areas during development, with the SOM+/CR+ population mainly arising from the dorsal Nkx6-2-positive region of the MGE, while the SOM+/CR− population is derived preferentially from the more ventrally positioned portion of the MGE (Fogarty et al. 2007, Sousa et al. 2009, Xu et al. 2008). It seems likely that some of the diversity within the SST population is functionally significant.
Most of the remainder of the interneuron populations within the somatosensory cortex are derived from the caudal ganglionic eminence (CGE) and are concentrated in the more superficial layers (L1–3) where they collectively represent the largest interneuron population (60% of L1–3 and approximately 30% of all interneurons) (Miyoshi et al. 2010, Lee et al. 2010). Between six and nine electrophysiological and morphological subtypes of CGE-derived interneurons have been described (Lee et al. 2010, Miyoshi et al. 2010). They have a similar expression of the ionotropic 5-HT receptor 5HT3a, which is expressed in the cortex exclusively in these interneurons. Also similar is their fast responsiveness to serotonin and ACh (Lee et al. 2010, Vucurovic et al. 2010). Analysis of these neurons in a mouse line expressing enhanced green fluorescent protein in 5HT3aR+ cells has demonstrated that PV−, SST−, and 5HT3aR+ interneurons account for nearly 100% of all glutamate amino-decarboylase-67-expressing (GAD67, an enzyme essential for the synthesis of GABA) neurons in S1 cortex (Lee et al. 2010).
CGE-derived interneurons include all the interneurons that express the neuropeptide VIP, which account for approximately 40% of the CGE-derived population. Another ~40–50% of the CGE-derived interneurons express reelin, which is also expressed in a fraction of SST+ interneurons. Both of these subpopulations are heterogeneous. Each includes two major subtypes, each representing approximately ~5% of the entire cortical interneuron population. These include two subtypes of reelin+ late-spiking cells, the CR+ VIP+ bipolar/bitufted neurons and the CR− VIP+ neurons. The two forms of late-spiking cells, one of which is the neurogliaform cells, can be distinguished by electrophysiology and morphology. Recent work has suggested that neurogliaform cells mediate tonic inhibition (see Tonic Inhibition, sidebar below) via nonsynaptic release of GABA (referred to as volume transmission) (Olah et al. 2009). Most of the CR/VIP+ bitufted cells have been described as irregular spiking, while most the VIP CR− cells are bitufted and multipolar neurons with a strongly adapting firing pattern, and have been termed “fast-adapting” (fAD) cells. An as yet undetermined subpopulation of the VIP+ cells apparently preferentially targets other interneurons (Staiger et al. 2004, David et al. 2007, Caputi et al. 2009).
Finally, a number of reports have suggested that a population of cortico-cortical projection GABAergic neurons may exist, particularly during development. Although these populations are not well described, these findings do suggest that populations akin to those seen in the hippocampus may also exist (Jinno et al. 2007, Jinno 2009, Higo et al. 2009).
Paleocortex (Piriform Cortex)
Analysis of the GABAergic interneurons of the primary olfactory or piriform cortex, a region of paleocortex, although less studied than the neocortex, reveals similar interneuron subtypes to those found in somatosensory cortex. The piriform cortex is a three-layered paleocortex with a simpler anatomy than that of the neocortex. In recent studies, Suzuki & Bekkers (2007, 2010a,b) classified the interneurons in the anterior piriform cortex into five classes based on electrophysiological properties, morphology, and expression of molecular markers. These included some of the most common CGE-derived neocortical interneurons including neurogliaform cells and VIP+ bitufted neurons with a fast-adapting firing pattern (Lee et al. 2010, Miyoshi et al. 2010). They also observed FS basket cells and a group of “regular-spiking” multipolar somatostatin-expressing neurons resembling in morphology and electrophysiological properties neocortical Martinotti cells. Finding homologies among these five classes of interneurons in the aPC and interneuron subtypes in neocortex is not difficult. Nonetheless, some intriguing differences support the idea that interneuron classes can have specializations that allow them to adapt to the requirements of specific microcircuits.
Neurogliaform cells account for ~34% of the interneuron population in L1 of the piriform cortex and possess the delayed firing (late-spiking) characteristic of these neurons in neocortex and hippocampus. The short-term dynamics of excitatory inputs onto these cells appears to be variable. According to Suzuki & Bekkers (2010b), synapses from lateral olfactory tract (LOT) afferents onto layer Ia neurogliaform cells showed strong paired-pulse facilitation, whereas L1b, L2, and L3 intracortical inputs onto neurogliaform cells in those layers showed little or no facilitation. In the hippocampus, excitatory inputs onto neurogliaform cells are typically depressing (Price et al. 2005).
Excitatory inputs onto FS cells in the aPC also showed layer-specific short-term dynamics. Layer-specific dynamics of excitatory inputs is also observed on neocortical FS cells. Excitatory synapses on FS cells are usually depressing; however, facilitating excitatory synapses have been observed on layer 6 FS cells (Beierlein & Connors 2002, West et al. 2006). Synaptic dynamics depends on presynaptic and post-synaptic mechanisms (Reyes et al. 1998), and several still to be investigated mechanisms are likely responsible for the variability of this property among interneurons of the same subtype in different locations.
In the hippocampus, striatum and neocortex somatic targeting FS basket cells mediate powerful feed-forward inhibition of principal cells (Pouille & Scanziani 2001, 2004; Gabernet et al. 2005; Cruikshank et al. 2007). From this, one might feel justified in believing that FS interneurons are truly an interneuron subtype with a dedicated function to which they are recruited whenever the need for fast feed-forward inhibition arises. However, examination of FS cells within the piriform cortex challenges this notion. A recent study of the olfactory cortex illustrates that the function of a specific interneuron subtype is not dependent solely on its intrinsic properties. Instead, it is also a function of the circuit in which it is incorporated.
In the neocortex, TC axons synapse on both FS cells and excitatory principal neurons in thalamo-recipient layers (mainly layer 4). Activation of both excitatory and inhibitory cells establishes a simple disynaptic circuit that provides powerful, local feed-forward inhibition. The latency between the onset of the TC excitation of excitatory cells and the onset of the disynaptic feed-forward inhibition from perisomatic-targeting FS cells results in a brief time window during which the excitatory neurons can integrate TC inputs. This window is critical for the processing of sensory information in the neocortex (Miller et al. 2001, Swadlow 2002, Gabernet et al. 2005, Wilent & Contreras 2005, Cruikshank et al. 2007). A similar circuit exists in the CA1 area of the hippocampus, where basket cells mediate feed-forward inhibition of CA1 pyramidal cells. As in the neocortex, the duration of the time window within which Schaffer collateral excitatory inputs on CA1 pyramidal cells can summate to reach spike threshold is determined by the basket cell-mediated feed-forward inhibition (Pouille & Scanziani 2001). FS cells mediate powerful feed-forward inhibition in other structures, such as L2/3 of somatosensory cortex and the neostriatum (Helmstaedter et al. 2008, Tepper & Bolam 2004). In contrast, in the piriform cortex, the input layer is L1a, which receives the LOT afferents containing the axons of projecting mitral and tufted cells of the olfactory bulb. L1a lacks FS cells. Instead, LOT axons synapse on L1a interneurons that target the apical dendrites of pyramidal cells with cell bodies in deeper layers (Stokes & Isaacson 2010). LOT axons also target the dendrites of the excitatory cells. As a result, similar to what is observed in the neocortex and hippocampus, bursts of mitral and tufted cell activity mediate a short-latency feed-forward disynaptic inhibition of the pyramidal cells, except that this inhibition occurs on the distal apical dendrite, instead of the soma, of the pyramidal cells and is mediated by non-FS layer 1a INs.
On the other hand, FS cells in the piriform cortex are localized mainly in L3 where they are recruited by recurrent excitation from the pyramidal cells. As in other structures, the FS cells in the piriform cortex target the somatic domain of the pyramidal cells and their inputs are depressing. However, the excitatory inputs on the pyramidal cells are facilitating. Hence, during bursts of inputs to the piriform cortex, perisomatic-targeting FS cells are recruited late and provide “feedback” somatic inhibition of the pyramidal cells. Thus, in the olfactory cortex, inhibition shifts from the dendrite to the soma, the opposite of what is observed in the neocortex and hippocampus.
Hippocampus
Interneurons have been studied in all regions of the hippocampal formation (Ceranik et al. 1997, McBain & Fisahn 2001, Lawrence & McBain 2003, Hefft & Jonas 2005, Bartos et al. 2007, Fuentealba et al. 2010, Tricoire et al. 2010). As a result of the CA1 region’s relatively simple cellular architecture, limited afferent connectivity, and extensive examination by numerous groups, the connectivity of interneuron subtypes within this region has been characterized most extensively. Somogyi and colleagues have made a special effort to characterize interneuron diversity in the CA1 subfield and have defined at last count 21 distinct classes of GABAergic neurons in this area (Somogyi & Klausberger 2005, Klausberger & Somogyi 2008). However, the reliance on connectivity to define these populations makes extrapolation of the cell types found in this region difficult to compare with those found elsewhere. Even though certain subtypes may be specific to the hippocampus, or even more specifically to particular layers of this structure, many of the interneuron subtypes in CA1 seem to share both functional and lineal relationships (Tricoire et al. 2010) to those found in other parts of the telencephalon, most notably the neocortex (see Table 2 for comparisons).
The easiest populations to compare with those found in the cortex are the PV+ basket and chandelier (axo-axonic) cells. The location of their output synapses, perisomatic and the axon initial segment, respectively, intrinsic biophysical properties, properties of input and output synapses, the expression of the Ca2+-binding protein PV, as well as the fact that they are all MGE-derived suggest that these interneuron subtypes are closely homologous to those found in the cortex.
Despite their expression of PV, oriens-lacunosum moleculare cells (known as OLM cells) also appear to be related to populations seen in the cortex. Their biophysical properties, long axon targeting distal apical dendrites, and marker expression (somatostatin) suggest they are homologous to the neocortical Martinotti cells. Additional populations that have close homologs within the hippocampus and the cortex are CR− and/or VIP+ interneuron populations that preferentially innervate other inhibitory interneuronal populations (Staiger et al. 2004, David et al. 2007, Caputi et al. 2009).
Even though neurogliaform cells would also appear to exist in both the hippocampus and cortex, the relationship between those within the cortex and hippocampus seems more complicated. Neurogliaform cells in the two structures are very similar in morphology and they share a number of unique functional properties (Fuentealba et al. 2008, 2010; Tricoire et al. 2010). Furthermore, there is a neurogliaform population in the hippocampus that like those in neocortex arises from the CGE. However, the majority of neurogliaform cells in the hippocampus express nitricoxidase synthetase- (nNOS−) and are MGE-derived (Tricoire et al. 2010). Moreover, another MGE-derived (Tricoire et al. 2010) nNOS+ hippocampal population of interneurons termed Ivy cells has been identified. Although their somas are positioned in the pyramidal rather than the lacunosum-moleculare layer, the Ivy cells strongly resemble the nNOS+ neurogliaform cells (Fuentealba et al. 2008, Szabadics & Soltesz 2009). Interestingly, the nNOS+ Ivy interneuron population appears to be numerically the largest interneuron population within the hippocampus. Whether there are any MGE-derived cortical homologs of the nNOS+ neurogliaform and Ivy interneurons within the neocortex remains to be determined.
Another population of interneurons that exists in both the neocortex and the hippocampus expresses the neuropeptide cholecystokinin (CCK) (Nunzi et al. 1985). In the CA1 area, at least five classes of CCK interneurons have been identified (Klausberger et al. 2004), including two types of CCK+ basket cells (with and without VIP) (Somogyi et al. 2004), the Schaffer collateral-associated cells, lacunosum-moleculare/perforant pathway (LM-PP)-associated cells (Vida et al. 1998), and the lacunosum-moleculare/radiatum/perforant pathway (LM-R-PP)-associated cells (Cossart et al. 1998, Vida et al. 1998). At least two types of CCK interneurons have been described in the neocortex, those expressing VIP and those that do not (Wang et al. 2002, Gonchar et al. 2007, Xu et al. 2010). The latter type closely resembles the CCK basket cells in the hippocampus on the basis of both morphology and multiple functional properties including modulation by cannabinoid (CB1) receptors and many other neuromodulators (Freund & Katona 2007). However, much less is known about their connectivity. Moreover, immunostaining within the cortex has shown a sparse CCK+ population, raising the question of whether CCK basket cells are much more abundant in hippocampus. Indeed, CCK mRNA expression was identified in a recent microarray analysis of developing neocortical interneurons (Batista-Brito et al. 2008). While the origin of the CCK+ cortical interneuron population is at present unknown, the coexpression of 5HT3aR in a substantial population of CCK+ interneurons in the hippocampus (Somogyi et al. 2004) and neocortex (Lee et al. 2010, Vucurovic et al. 2010) argues that this population may be CGE derived. This suggestion fits well with the fact that there is as yet no identified marker available for a portion of the CGE-derived cortical interneurons.
The hippocampus, like the neocortex, also contains projection GABAergic neurons. In CA1, projection GABAergic neurons include subiculum-projecting trilaminar cells; back-projecting CA1 cells that provide widespread innervation to CA1, CA3, and the dentate hilus (Sik et al. 1994, 1995); and the hippocampal-septal cells (Freund & Buzsaki 1996).
Among the populations of hippocampal interneurons for which there does not appear to be a parallel population in the neocortex, the cells called bistratified stand out. These interneurons first described in the CA1 area by Somogyi and colleagues (Buhl et al. 1994, Pawelzik et al. 1999, Maccaferri et al. 2000) have axons targeting the dendrites of CA1 pyramidal cells in stratum oriens and stratum radiatum (hence the term bistratified). They are coaligned with the Schaffer collateral input onto those dendrites and receive both Schaffer collateral input and recurrent input from CA1 pyramidal cells. They are PV+ and have a FS firing pattern (Pawelzik et al. 2002, Fujiwara-Tsukamoto et al. 2010). Bistratified cells make synapses with small dendritic shafts of the pyramidal cells, and they fire at the trough of the theta oscillation, similar to oriens-lacunosum molecular cells. However, in contrast to oriens-lacunosum molecular cells, which are silenced during ripple episodes, bistratified cells fired strongly during these events (Klausberger et al. 2004).
Striatal Interneurons
The basal ganglia is comprised of several brain structures, most notably the striatum and the globus pallidus. In both these structures, the only projecting neuronal population, the medium spiny neurons (MSNs) of the striatum, and the principal projection cells of the globus pallidus are inhibitory GABAergic neurons (Difiglia et al. 1982, Wilson 2007). Of the various basal ganglia, only the interneurons within the striatum have been extensively studied (Kawaguchi 1997, Tepper & Bolam 2004). The striatum contains several subtypes of interneurons, including GABAergic and cholinergic neurons (the sole excitatory striatal population). Given the diversity of interneurons that have been described in the neocortex and hippocampus, it is surprising how few subtypes have been observed to date within the basal ganglia and even there the focus of investigators has been almost entirely limited to the striatum. Over 15 years ago, Kawaguchi (1993) described three major interneuron sub-types within the striatum and these collectively are thought to comprise less than 3% of all the neurons within this structure (Tepper et al. 2004, Tepper & Bolam 2004) (see Figure 3 for a comparison between structures). These are the large aspiny cholinergic interneurons, so called tonically active neurons (TAN), two types of GABAergic interneurons, PV+ FS basket cells and low-threshold spiking (LTS or pLTS, persistent low-threshold spiking owing to their long calcium-mediated plateau) SST+ interneurons. This later population is heterogeneous and includes NPY+ and NOS+ sub-populations. Beyond this, the only additional population that has been reported is a small population of CR+ neurons that are provisionally thought to possess LTS-like physiological features, suggesting that, although they are SST-negative, they may be related to the SST+ populations. Finally, a few references, most notably from Ramón y Cajal (1911), have suggested that a small population of neurogliaform cells reside within the striatum (Fox & Rafols 1971, DiFiglia et al. 1976, Chang et al. 1982).
The interneuron subtypes within the striatum are quite similar to, albeit apparently less diverse than, subtypes within the neocortex and hippocampus. However, the principal neuronal population in the striatum is GABAergic (the medium spiny neurons), and as such, in contrast to interneuron populations in the pallium, striatal interneurons (with the exception of those targeting the ACh interneuron population) target inhibitory GABAergic neurons. Interestingly, the interneuron marker expression within the striatum is consistent with most, if not all, interneurons within this structure being MGE-derived (Marin et al. 2000). If the striatum does not receive CGE-derived interneuron populations, this could explain the apparently lower level of diversity of interneurons within this structure.
In terms of the connectivity and function of the interneurons within the striatum, whereas the cholinergic populations are thought to get input primarily from the thalamus, the FS and LTS populations are thought to receive afferents mainly from the neocortex (although to some extent all these cell types receive afferent inputs from both structures). Functionally, these latter two interneuron subtypes form synapses on MSNs, thus providing feed-forward inhibition (Vuillet et al. 1989, Sidibe & Smith 1999, Gertler et al. 2008). In addition to their extremely small numbers, the FS interneurons give sparse input to the MSNs, as compared with the extensive excitatory input provided to MSNs by afferent pyramidal neurons (Koos & Tepper 1999, Kreitzer 2009, Gittis et al. 2010). However, like in the neocortex, these populations possess FS properties, PV expression, and high release probability. They also produce strongly temporally coordinated inhibition. Indeed, although a typical MSN receives on the order of ten times more excitatory cortical inputs (100–125 compared with 15–25 synapses from FS cells), the FS population is thought to be extremely effective at reducing MSN excitability (Koos et al. 2004, reviewed in Kreitzer 2009). By contrast, the LTS population, which also provides weak input to MSN neurons, likely mediates much of its impact on striatal function through neuromodulation mediated through release of NPY or NOS (Chesselet & Graybiel 1986, Kubota et al. 1993). Indeed, a primary function of LTS cells may be related to the control of blood flow (Aoki & Pickel 1990).
Neuromodulation by dopamine and ACh plays an extremely important role in regulating the function of the striatum, as evidenced by Parkinson’s and Huntington’s diseases where imbalances in neuromodulation are strongly associated with the dysfunction seen in these syndromes (Shen et al. 2008, reviewed in Pisani et al. 2001). Indeed, studies examining striatal function belie the simplistic notion that specific neuron’s contribution to signaling can be reduced to providing net excitation or inhibition. Hence, the ability of striatal interneurons to regulate complex interactions between all cell types in this structure including other interneurons, combined with their secretion of neuro-modulators suggest that they are central to the control and timing of striatal activity.
The Amygdala Nuclei
In many regards, the amygdala nuclei represent a hybrid of cortical and subcortical structures (reviewed in Sah et al. 2003). Whereas lateral amygdala nuclei such as the lateral and basal nuclei (collectively termed BLA) have a cortical-like architecture, the medial nuclei such as the central nucleus (which can be subdivided into the lateral and medial divisions) and the medial amygdala (MeA) all more closely resemble the GABAergic basal ganglia. Linking these portions of the amygdala are the intercalated cell masses, which largely consist of GABAergic populations that appear more specialized and have recently been suggested to have arisen from a unique origin near the pallial-subpallial boundary (Stenman et al. 2003, Waclaw et al. 2010; J.G. Corbin, personal communication). Functionally, the amygdala can be divided into the more laterally positioned nuclei including the BLA and central nucleus, which are more involved in fear circuits, and the MeA, which processes innate behaviors such as aggression and mating and is closely linked to the hypothalamus (MacDonald 1984, reviewed in Ehrlich et al. 2009). Nonetheless, all structures within the amygdala are characterized by the presence of at least some inhibitory interneuron populations, which resemble those found in other portions of the telencephalon but may differ in their electrophysiological characteristics, connectivity, and site of origin.
Our understanding of the contribution of interneurons within each of these set of nuclei is varied; the BLA is the most studied and MeA the least. Within the BLA, immunological and electrophysiological analyses indicate that the diversity of interneurons closely resemble that seen in the neocortex or hippocampus with PV−, SST−, VIP−, CCK−, CR−, and NOS-expressing populations all having been reported (Mascagni & McDonald 2003). As in the cortex, interneurons make up a percentage of the total neuronal population of the BLA (only approximately 25%) (McDonald & Augustine 1993) (see Figure 3 for comparisons between structures). Similarly, as in the cortex, approximately 50% of all interneurons within these regions appear to be PV positive. This population is primarily comprised of FS basket cells (although chandelier neurons have also been reported in this region) (McDonald 1982); however, a variety of physiological firing patterns and some variation in morphology has been used to suggest that some heterogeneity exists within this population (Woodruff & Sah 2007a,b). This heterogeneity is similar to that observed for FS cells in the neocortex (Ascoli et al. 2008, Tremblay et al. 2010). In addition, the function of this population has been speculated to involve both feed-forward and feedback regulation of signaling (Woodruff et al. 2006). Nonetheless, many of these variations have also been observed within the neocortex and hippocampus; hence, these cells may appropriately be classified as belonging to the FS basket cell subtype. A further similarity with the cortex is that interneurons within the BLA (and to a lesser extent the MeA) are under the strong control of a variety of neuromodulators. The PV and VIP populations receive pronounced serotonin modulation, and at least the latter of these also possesses CB1-receptors as well as significant cannabinoid modulation (Morales et al. 2004). The similarities of the interneurons within the BLA and cortex in part reflect their common origin. As in the cortex, both our work and that of others have implicated the MGE as the source of both PV and SST+ BLA interneurons (Nery et al. 2002, Xu et al. 2008, Hirata et al. 2009). In addition, consistent with the presence of 5HT3aR+ interneurons in this region (Mascagni & McDonald 2007, Muller et al. 2007), recent unpublished genetic fate-mapping efforts (G. Miyoshi and J. Corbin, personal communication) have reported that CGE-derived interneurons populate the BLA (as well as the CeA).
Central and Medial Amygdala Nuclei
As noted above, the central nuclei of the amygdala is striatal-like in organization and provides the major output for fear (although unlike the majority of cells in the medial amygdala, these cells are glutamatergic). Despite being overwhelmingly comprised of GABAergic neurons, it does not appear to contain a major interneuron population. Like the striatum, the central and medial amygdala nuclei possess only sparse PV, VIP, and CCK populations and a somewhat larger somatostatin-expressing cohort particularly in the medial amygdala (McDonald 1989, Pare & Smith 1993, McDonald et al. 1995, Kemppainen & Pitkänen 2000, Równiak et al. 2008). The intrinsic physiological properties and morphologies of cells within these subdivisions have yet to be thoroughly analyzed. However, consistent with the MGE origin of populations within this region (Xu et al. 2008), both FS and LTS burst spiking (with hyperpolarizing rebound burst properties) have been reported. These observations coupled with the general structure of these nuclei indicate that the central and medial amygdala will ultimately prove reasonably similar in its interneuron subtypes to those seen in the striatum (Figure 3).
Least well studied are the interneuron populations associated with innate behaviors and located within the medial divisions of the amygdala. Recent fate-mapping experiments indicate that this region receives cells that developmentally express Shh or related genes, including Gli1 and Nkx2.1 (Carney et al. 2010). In addition, it has been reported that this region possesses large numbers of cells that express the 5HT3a receptor (Mascagni & McDonald 2007). Although the interneuron subtypes and their origins have yet to be sorted out thoroughly, this structure appears to receive its interneuron repertoire from the same set of eminences that supply the rest of the telencephalon (Figure 3).
PERSPECTIVE
This survey of the cellular substrates and mechanisms mediating inhibition within the telencephalon, although far from complete, provides a sense of the breadth of cell types and mechanisms that mediate inhibition. It also highlights commonalities in inhibitory function within this brain region. Although a growing cohort of scientists have called for a standardization of nomenclature for interneuron subtypes (Ascoli et al. 2008), the challenges to this effort remain daunting. Despite the undeniable appeal of providing a common vernacular for specific cell types, the variance in anatomy both across and within structures dictates that such identifiers are uncomfortably restricting. An alternative approach is to be less precise but capture some of the salient features of particular subsets. For instance, referring to FS, perisomatic-targeting basket cells as a common category based on their shared site of origin within the MGE, coupled with their anatomical and physiological similarities, is tacitly accepted. This is true despite the fact that depending on structure they primarily target either excitatory (cortex, hippocampus, BLA) or inhibitory projection neurons (striatum, CeA, MeA) or receive depressing (most neocortical layers and hippocampus) versus facilitating (layer 6) excitatory synapses as a result of their afferent connectivity. On balance, we would argue that using an interneuron developmental origin and molecular signature provides a guide to the identification of commonalities that provides a compelling argument for acceptance of certain classes as orthologs, if not homologs. There is no doubt that we are at the cusp of a deluge of new findings that will greatly enhance our understanding of the connectome and transcriptome of all classes of interneurons. The foremost goal in this effort is to organize the wealth of information addressing the similarities and differences in inhibitory neural signaling across the telencephalon, and there seems little doubt that the points of view of both the “lumpers” (those individuals who focus on the commonalities between different interneuron subtypes) and “splitters” (those who focus on the differences) will aid in this effort. In the words of Maurice Sendak, “Let the wild rumpus begin.”
CONTROL OF INTRACELLULAR Cl− AND THE FUNCTIONS OF INHIBITORY NEUROTRANSMITTERS.
In adult CNS neurons, the ionotropic actions of GABA and glycine are generally inhibitory. However, it is believed they both have excitatory actions during embryonic development and early postnatal ages. This is due to elevated intracellular concentrations of Cl− and hence a positive chloride equilibrium potential of the postsynaptic cell in young neurons, resulting in chloride efflux upon receptor activation and, hence, membrane depolarization (Ben-Ari 2002, Plotkin et al. 1997, Reichling et al. 1994). These excitatory actions of GABA and glycine are believed to be important for proliferation, migration, synaptogenesis, neuronal differentiation, and neuronal network stability (Kirsch & Betz 1998, Wester et al. 2008). The shift from excitatory to inhibitory at later stages is due to a shift in the chloride equilibrium potential to more negative values as a result of the expression of the K+/Cl− cotransporter KCC2 (Figure 2). This transporter produces active chloride extrusion, thus reducing intracellular Cl− concentrations (Alvarez-Leefmans 1990, Alvarez-Leefmans & Delpire 2009). As a result of this shift in the Cl− equilibrium potential, receptor activation produces hyperpolarization. Na+K+/Cl− (NKCCs) cotransporters dominate in immature neurons of cortex and hippocampus, whereas KCC2 expression is induced only after birth (compare Figure 2, left and right).
Cation-chloride cotransporters (CCCs) constitute a family of transporters that include Na-Cl (NCCs), Na/K-Cl (NKCCs), and K+-Cl− (KCCs) cotransporters, which differ in tissue and cellular distribution and have a variety of physiological roles (reviewed in Delpire & Mount 2002; Payne et al. 2003; Alvarez-Leefmans 1990, Alvarez-Leefmans & Delpire 2009). Under physiological conditions, NCCs and NKCCs provide the main route for Cl− uptake, whereas KCCs are responsible for Cl− extrusion. NKCC and KCC are of particular interest because they are critically involved in Cl− homeostasis in the brain.
CHANDELIER CELLS MAY BE EXCITATORY.
It was traditionally believed that Chandelier cells might exert a particularly powerful inhibitory control of spike generation by virtue of the location of their synapses to the axon initial segment (AIS), the site of spike generation. A recent report questioned this classic conceptualization of chandelier cells as inhibitory, showing that chandelier cell activity can actually drive spikes in target pyramidal cells in layer 2/3 of the human and rodent neocortex (Szabadics et al. 2006). The basis of this phenomenon was suggested to be due to a relative absence of the chloride transporter KCC2 at the AIS of pyramidal neurons. As a result, chloride concentration is elevated in this subcellular compartment, and the reversal potential of the GABA-mediated postsynaptic potential produced by chandelier cell activity becomes depolarizing and its action excitatory (see also Control of Intracellular Cl− and the Functions of Inhibitory Neurotransmitters, sidebar above, as well as Figure 2). Additional data indicate that a high internal chloride concentration at the AIS is produced by chloride influx by the chloride transporter NKCC1 (Khirug et al. 2008). However, this view of chandelier cells remains controversial (Glickfeld et al. 2009).
TONIC INHIBITION.
Recently, evidence of a new form of GABAA receptor-mediated inhibition has been reported and termed “tonic” inhibition to distinguished it from the classical synaptic GABAA receptor-mediated “phasic” (transient) inhibition (Otis et al. 1991, Salin & Prince 1996). This tonic inhibition is mediated by extrasynaptic or perisynaptic GABAA receptors that are activated by bulk (“volume”) release of GABA to produce a long lasting or “persistent” contribution to the resting membrane potential and membrane leak (Soltesz et al. 1990, Brickley et al. 1996, Mody 2001, Nusser & Mody 2001, Stell & Mody 2002; reviewed in Semyanov et al. 2004, Farrant & Nusser 2005, Glykys & Mody 2007, Belelli et al. 2009). GABAA receptor-mediated tonic inhibition can be revealed by the depolarization of the membrane and the increase in membrane resistance following application of GABAA receptor inhibitors.
The GABA mediating this signaling was thought to exclusively originate through spillover from the synaptic cleft; however, Tamas and colleagues (Oláh et al. 2009) recently suggested that, in the cortex, neurogliaform cells may make a particularly strong contribution to tonic inhibition by producing “volume” transmission of GABA. Originally studied in cerebellar granule cells and the hippocampus, where it has important roles in modulating neuronal and network excitability (Mitchell & Silver 2003, Rothman et al. 2009), tonic inhibition has now been observed in many other structures including the neocortex, thalamus, hypothalamus, striatum, and spinal cord (Belelli et al. 2009) and is considered to provide a significant contribution to several physiological and pathological processes.
Following synaptic GABA release, the concentration of GABA in the extracellular fluid is much lower than that reached in the synaptic cleft. Extrasynaptic GABAA receptors are able to respond to low concentrations of GABA because the subunit composition of these channels bestow on them a higher affinity for GABA. Extrasynaptic receptors also tend to desensitize less than synaptic receptors. As a result of these properties, activation of these channels tends to produce more persistent actions than are produced by synaptic receptors (Saxena & Macdonald 1994, 1996; Haas & Macdonald 1999; Bianchi & Macdonald 2002; Brown et al. 2002).
Acknowledgments
We are grateful to Michael Long, Jens Hjerling-Leffler, Theofanis Karayannis, and Soohyun Lee for suggestions and comments on the manuscript and Drs. David Anderson and Joshua Corbin for help with understanding the amygdala. Both Drs. Fishell and Rudy are generously supported by grant funding from the NIH. Dr. Fishell is specifically supported by the National Institute of Health grants (5RO1MH068469-08 and 2R01MH071679-09), National Institute of Neurological Disorders and Stroke grant (5R01NS039007-1), Simons Foundation and New York Stem Cell Science State grant (NGSG-130), and Dr. Rudy by National Institute of Neurological Disorders and Stroke grants (5R01NS045217-08, 2R01NS030989-18, and 3R01NS045217-06A1S1) and the Alzheimer’s Association.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding or financial holding that might be perceived as affecting the objectivity of this review.
Contributor Information
Gord Fishell, Email: fishell@saturn.med.nyu.edu.
Bernardo Rudy, Email: rudyb01@med.nyu.edu.
LITERATURE CITED
- Alger BE, Nicoll RA. GABA-mediated biphasic inhibitory responses in hippocampus. Nature. 1979;281:315–17. doi: 10.1038/281315a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ. Intracellular Cl− regulation and synaptic inhibition in vertebrate and invertebrate neurons. In: Alvarez-Leefmans FJ, Russell JM, editors. Chloride Channels and Carriers in Nerve, Muscle and Glial Cells. New York: Plenum; 1990. pp. 109–58. [Google Scholar]
- Alvarez-Leefmans FJ, Delpire E. Thermodynamics and kinetics of chloride transport in neurons: an outline. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporters and Channels in the Nervous System. New York: Academic; 2009. pp. 81–108. [Google Scholar]
- Andersen P, Dingledine R, Gjerstad L, Langmoen IA, Laursen AM. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980;305:279–96. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Pickel VM. Neuropeptide Y in cortex and striatum. Ultrastructural distribution and coexistence with classical neurotransmitters and neuropeptides. Ann NY Acad Sci. 1990;611:186–205. doi: 10.1111/j.1749-6632.1990.tb48931.x. [DOI] [PubMed] [Google Scholar]
- Araki T, Yamano M, Murakami T, Wanaka A, Betz H, Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988;25:613–24. doi: 10.1016/0306-4522(88)90263-1. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Machold R, Klein C, Fishell G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex. 2008;18:2306–17. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Connors BW. Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex. J Neurophysiol. 2002;88:1924–32. doi: 10.1152/jn.2002.88.4.1924. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–63. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci. 1991;14:458–61. doi: 10.1016/0166-2236(91)90045-v. [DOI] [PubMed] [Google Scholar]
- Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem. 2006;97:1600–10. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Slow phases of GABAA receptor desensitization: structural determinants and possible relevance for synaptic function. J Physiol. 2002;544:3–18. doi: 10.1113/jphysiol.2002.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–59. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock LG, Coombs JS, Eccles JC. The nature of the monosynaptic excitatory and inhibitory processes in the spinal cord. Proc R Soc Lond B Biol Sci. 1952a;140:170–76. [PubMed] [Google Scholar]
- Brock LG, Coombs JS, Eccles JC. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952b;117:431–60. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δGABAA receptors. Br J Pharmacol. 2002;136:965–74. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–28. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Feed-forward inhibition in the hippocampal formation. Prog Neurobiol. 1984;22:131–53. doi: 10.1016/0301-0082(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Caputi A, Rozov A, Blatow M, Monyer H. Two calretinin-positive GABAergic cell types in layer 2/3 of the mouse neocortex provide different forms of inhibition. Cereb Cortex. 2009;19:1345–59. doi: 10.1093/cercor/bhn175. [DOI] [PubMed] [Google Scholar]
- Carney RS, Mangin JM, Hayes L, Mansfield K, Sousa VH, et al. Sonic hedgehog expressing and responding cells generate neuronal diversity in the medial amygdala. Neural Dev. 2010;5:14. doi: 10.1186/1749-8104-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, et al. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986;231:995–97. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- Ceranik K, Bender R, Geiger JR, Monyer H, Jonas P, et al. A novel type of GABAergic interneuron connecting the input and the output regions of the hippocampus. J Neurosci. 1997;17:5380–94. doi: 10.1523/JNEUROSCI.17-14-05380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HT, Wilson CJ, Kitai ST. A Golgi study of rat neostriatal neurons: light microscopic analysis. J Comput Neurol. 1982;208:107–26. doi: 10.1002/cne.902080202. [DOI] [PubMed] [Google Scholar]
- Chow A, Erisir A, Farb C, Nadal MS, Ozaita A, et al. K+ channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J Neurosci. 1999;19:9332–45. doi: 10.1523/JNEUROSCI.19-21-09332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, Graybiel AM. Striatal neurons expressing somatostatin-like immunoreactivity: evidence for a peptidergic interneuronal system in the cat. Neuroscience. 1986;17:547–71. doi: 10.1016/0306-4522(86)90030-8. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci. 1998;1:470–78. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feed-forward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–68. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci. 2000;3:701–7. doi: 10.1038/76656. [DOI] [PubMed] [Google Scholar]
- Dávid C, Schleicher A, Zuschratter W, Staiger JF. The innervation of parvalbumin-containing interneurons by VIP-immunopositive interneurons in the primary somatosensory cortex of the adult rat. Eur J Neurosci. 2007;25:2329–40. doi: 10.1111/j.1460-9568.2007.05496.x. [DOI] [PubMed] [Google Scholar]
- Delpire E, Mount DB. Human and murine phenotypes associated with defects in cation-chloride co-transport. Annu Rev Physiol. 2002;64:803–43. doi: 10.1146/annurev.physiol.64.081501.155847. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Pasik P, Pasik T. A Golgi study of neuronal types in the neostriatum of monkeys. Brain Res. 1976;114:245–56. doi: 10.1016/0006-8993(76)90669-7. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Pasik P, Pasik T. A Golgi and ultrastructural study of the monkey globus pallidus. J Comp Neurol. 1982;212:53–75. doi: 10.1002/cne.902120105. [DOI] [PubMed] [Google Scholar]
- Dreifuss JJ, Kelly JS, Krnjević K. Cortical inhibition and gamma-aminobutyric acid. Exp Brain Res. 1969;9:137–54. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- Ellender TJ, Paulsen O. The many tunes of perisomatic targeting interneurons in the hippocampal network. Front Cell Neurosci. 2010;4:26. doi: 10.3389/fncel.2010.00026. ii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–71. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–52. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. The effect of inhibitory nerve impulses on a crustacean muscle fibre. J Physiol. 1953;121:374–89. doi: 10.1113/jphysiol.1953.sp004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G. Perspectives on the developmental origins of cortical interneuron diversity. Novartis Found Symp. 2007;288:21–35. discuss. 35–44, 96–98. [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marín O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–46. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Storm-Mathisen J. GABA synthesis in rat hippocampus correlated to the distribution of inhibitory neurons. Acta Physiol Scand. 1969;76:35A–36A. [PubMed] [Google Scholar]
- Fox CA, Rafols JA. Observations on the oligodendroglia in the primate striatum. Are they Ramón y Cajal’s “dwarf ” or “neurogliaform” neurons? J Hirnforsch. 1971;13:331–40. [PubMed] [Google Scholar]
- French AS, Panek I, Torkkeli PH. Shunting vs inactivation: simulation of GABAergic inhibition in spider mechanoreceptors suggests that either is sufficient. Neurosci Res. 2006;55:189–96. doi: 10.1016/j.neures.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyás AI. Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol. 1997;75:479–87. [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Friauf E, Hammerschmidt B, Kirsch J. Development of adult-type inhibitory glycine receptors in the central auditory system of rats. J Comp Neurol. 1997;385:117–34. doi: 10.1002/(sici)1096-9861(19970818)385:1<117::aid-cne7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Begum R, Capogna M, Jinno S, Márton LF, et al. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–29. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Klausberger T, Karayannis T, Suen WY, Huck J, et al. Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J Neurosci. 2010;30:1595–609. doi: 10.1523/JNEUROSCI.4199-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Imanishi M, Ninomiya T, Tsukada M, et al. Prototypic seizure activity driven by mature hippocampal fast-spiking interneurons. J Neurosci. 2010;30:13679–89. doi: 10.1523/JNEUROSCI.1523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–27. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci USA. 2002;99:12438–43. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–24. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol. 2004;93:467–80. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci. 2010;30:2223–34. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12:21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–70. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–58. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat. 2007;1:3. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37(2):299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–78. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol. 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–28. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Staiger JF, Sakmann B, Feldmeyer D. Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex. J Neurosci. 2008;28:8273–84. doi: 10.1523/JNEUROSCI.5701-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, DeFelipe J, Schmechel D, Brandon C, Emson PC. Neuropeptide-containing neurons of the cerebral cortex are also GABAergic. Proc Natl Acad Sci USA. 1984;81:6526–30. doi: 10.1073/pnas.81.20.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, DeFelipe J, Schmechel D, Brandon C, Emson PC. Neuropeptide-containing neurons of the cerebral cortex are also GABAergic. Science. 1986;231:995–97. doi: 10.1073/pnas.81.20.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandes MS, Troncone LR. Glycine as a neurotransmitter in the forebrain: a short review. J Neural Transm. 2009;116:1551–60. doi: 10.1007/s00702-009-0326-6. [DOI] [PubMed] [Google Scholar]
- Higo S, Akashi K, Sakimura K, Tamamaki N. Subtypes of GABAergic neurons project axons in the neocortex. Front Neuroanat. 2009;3:25. doi: 10.3389/neuro.05.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, Corbin JG. Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nat Neurosci. 2009;12:141–49. doi: 10.1038/nn.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–16. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Hull C, Adesnik H, Scanziani M. Neocortical disynaptic inhibition requires somatodendritic integration in interneurons. J Neurosci. 2009;29:8991–95. doi: 10.1523/JNEUROSCI.5717-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–68. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, et al. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci. 2007;27:8790–804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S. Structural organization of long-range GABAergic projection system of the hippocampus. Front Neuroanat. 2009;3:13. doi: 10.3389/neuro.05.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. Neurotransmitters in the cerebral cortex. J Neurosurg. 1986;65:135–53. doi: 10.3171/jns.1986.65.2.0135. [DOI] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–53. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karube F, Kubota Y, Kawaguchi Y. Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes. J Neurosci. 2004;24:2853–65. doi: 10.1523/JNEUROSCI.4814-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–96. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–86. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–23. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Distinct firing patterns of neuronal subtypes in cortical synchronized activities. J Neurosci. 2001;21:7261–72. doi: 10.1523/JNEUROSCI.21-18-07261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–87. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunore-activity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comput Neurol. 2000;426:441–67. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci. 2008;28:4635–39. doi: 10.1523/JNEUROSCI.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998;392:717–20. doi: 10.1038/33694. [DOI] [PubMed] [Google Scholar]
- Kisvárday ZF, Gulyas A, Beroukas D, North JB, Chubb IW, Somogyi P. Synapses, axonal and dendritic patterns of GABA-immunoreactive neurons in human cerebral cortex. Brain. 1990;113:793–812. doi: 10.1093/brain/113.3.793. [DOI] [PubMed] [Google Scholar]
- Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009;30:947–57. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Márton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. Erratum. 2006. Nat. Neurosci. 9:979. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–72. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–22. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz EA, Kuffler SW, Potter DD. Gamma-aminobutric acid and other blocking compounds in crustacea. III Their relative concentrations in separated motor and inhibitory axons. J Neurophysiol. 1963a;26:739–51. doi: 10.1152/jn.1963.26.5.739. [DOI] [PubMed] [Google Scholar]
- Kravitz EA, Kuffler SW, Potter DD, Vangelder NM. Gamma-aminobutyric acid and other blocking compounds in crustacea. II Periperal nervous system. J Neurophysiol. 1963b;26:729–38. doi: 10.1152/jn.1963.26.5.729. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–47. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hatada SN, Kawaguchi Y. Important factors for the three-dimensional reconstruction of neuronal structures from serial ultrathin sections. Front Neural Circuits. 2009;3:4. doi: 10.3389/neuro.04.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Mikawa S, Kawaguchi Y. Neostriatal GABAergic interneurones contain NOS, calretinin or parvalbumin. Neuroreport. 1993;5:205–8. doi: 10.1097/00001756-199312000-00004. [DOI] [PubMed] [Google Scholar]
- Kwag J, Paulsen O. The timing of external input controls the sign of plasticity at local synapses. Nat Neurosci. 2009;12:1219–21. doi: 10.1038/nn.2388. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation—feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–40. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Glutamatergic inhibition in sensory neocortex. Cereb Cortex. 2009;19:2281–89. doi: 10.1093/cercor/bhn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Burgos GG. Specificity in the functional architecture of primate prefrontal cortex. J Neurocytol. 2002;31:265–76. doi: 10.1023/a:1024174026286. [DOI] [PubMed] [Google Scholar]
- Lund JS, Lewis DA. Local circuit neurons of developing and mature macaque prefrontal cortex: Golgi and immunocytochemical characteristics. J Comput Neurol. 1993;328:282–312. doi: 10.1002/cne.903280209. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neo-cortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–82. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol. 2000;524(Pt. 1):91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–49. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20(16):6063–76. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–84. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. A novel subpopulation of 5-HT type 3A receptor subunit immunoreactive interneurons in the rat basolateral amygdala. Neuroscience. 2007;144:1015–24. doi: 10.1016/j.neuroscience.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comput Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neuronal organization of the lateral and basolateral amygdaloid nuclei in the rat. J Comput Neurol. 1984;222:589–606. doi: 10.1002/cne.902220410. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52:281–94. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Augustine JR. Neuropeptide Y and somatostatin-like immunoreactivity in neurons of the monkey amygdala. Neuroscience. 1995;66:959–82. doi: 10.1016/0306-4522(94)00629-j. [DOI] [PubMed] [Google Scholar]
- McGarry LM, Packer AM, Fino E, Nikolenko V, Sippy T, Yuste R. Quantitative classification of somatostatin-positive neocortical interneurons identifies three interneuron subtypes. Front Neural Circuits. 2010;14(4):12. doi: 10.3389/fncir.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megías M, Emri Z, Freund TF, Gulyás AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:5275–40. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Mihály A. Histochemistry and functional significance of putative neocortical transmitter substances: a review. Z Mikrosk Anat Forsch. 1982;96:916–36. [PubMed] [Google Scholar]
- Miller KD, Pinto DJ, Simons DJ. Processing in layer 4 of the neocortical circuit: new insights from visual and somatosensory cortex. Curr Opin Neurobiol. 2001;11:488–97. doi: 10.1016/s0959-4388(00)00239-7. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–45. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mittmann W, Chadderton P, Häusser M. Neuronal microcircuits: frequency-dependent flow of inhibition. Curr Biol. 2004;14:R837–39. doi: 10.1016/j.cub.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–98. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–94. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–13. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Morales M, Wang SD, Diaz-Ruiz O, Jho DH. Cannabinoid CB1 receptor and serotonin 3 receptor subunit A (5-HT3A) are co-expressed in GABA neurons in the rat telencephalon. J Comput Neurol. 2004;468:205–16. doi: 10.1002/cne.10968. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comput Neurol. 2007;505:314–35. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–87. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Alger BE. Presynaptic inhibition: transmitter and ionic mechanisms. Int Rev Neurobiol. 1979;21:217–58. doi: 10.1016/s0074-7742(08)60639-x. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Gorio A, Milan F, Freund TF, Somogyi P, Smith AD. Cholecystokinin-immunoreactive cells form symmetrical synaptic contacts with pyramidal and nonpyramidal neurons in the hippocampus. J Comput Neurol. 1985;237:485–505. doi: 10.1002/cne.902370406. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–28. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Oláh S, Füle M, Komlósi G, Varga C, Báldi R, Barzó P, Tamás G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–81. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Staley KJ, Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–50. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- Oviedo H, Reyes AD. Boosting of neuronal firing evoked with asynchronous and synchronous inputs to the dendrite. Nat Neurosci. 2002;5:261–66. doi: 10.1038/nn807. [DOI] [PubMed] [Google Scholar]
- Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol. 2010;58:123–47. doi: 10.1016/S1054-3589(10)58006-2. [DOI] [PubMed] [Google Scholar]
- Paré D, Smith Y. Distribution of GABA immunoreactivity in the amygdaloid complex of the cat. Neuroscience. 1993;57:1061–76. doi: 10.1016/0306-4522(93)90049-l. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Bannister AP, Deuchars J, Ilia M, Thomson AM. Modulation of bistratified cell IPSPs and basket cell IPSPs by pentobarbitone sodium, diazepam and Zn2+: dual recordings in slices of adult rat hippocampus. Eur J Neurosci. 1999;11:3552–64. doi: 10.1046/j.1460-9568.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Hughes DI, Thomson AM. Physiological and morphological diversity of immunocyto-chemically defined parvalbumin- and cholecystokinin-positive interneurones in CA1 of the adult rat hippocampus. J Comput Neurol. 2002;443:346–67. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Picconi B, Tolu M, Giacomini P, Scarnati E. Role of tonically-active neurons in the control of striatal function: cellular mechanisms and behavioral correlates. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:211–30. doi: 10.1016/s0278-5846(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol. 1997;33:781–95. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–63. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–23. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, et al. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci. 2005;25:6775–86. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Sur la structure de l’ écorce cérébrale de quelques mammifères. Cellule. 1891;7:123–76. [Google Scholar]
- Ramón y Cajal S. In: Histologie du Système Nerveux de l’Homme et des Vertèbrès. Azoulay L, translator. Vol. 2. Paris: Maloine; 1911. pp. 504–18. [Google Scholar]
- Reichling DB, Kyrozis A, Wang J, MacDermott AB. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol. 1994;476:411–21. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–85. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Roberts E. Gamma-aminobutyric acid (gamma-ABA)-vitamin B6 relationships in the brain. Am J Clin Nutr. 1963;12:291–307. doi: 10.1093/ajcn/12.4.291. [DOI] [PubMed] [Google Scholar]
- Roberts E, Frankel S. γ-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem. 1950;187:55–63. [PubMed] [Google Scholar]
- Rothman JS, Cathala L, Steuber V, Silver RA. Synaptic depression enables neuronal gain control. Nature. 2009;457:1015–18. doi: 10.1038/nature07604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Równiak M, Robak A, Bogus-Nowakowska K, Kolenkiewicz M, Bossowska A, et al. Somatostatin-like immunoreactivity in the amygdala of the pig. Folia Histochem Cytobiol. 2008;46:229–38. doi: 10.2478/v10042-008-0035-2. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annu Rev Neurosci. 1998;21:445–77. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. J Dev Neurobiol. 2010;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saganich MJ, Vega-Saenz de Miera E, Nadal MS, Baker H, Coetzee WA, Rudy B. Cloning of components of a novel subthreshold-activating K+ channel with a unique pattern of expression in the cerebral cortex. J Neurosci. 1999;19:10789–802. doi: 10.1523/JNEUROSCI.19-24-10789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001;21:4609–24. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Salin PA, Prince DA. Spontaneous GABAA receptor-mediated inhibitory currents in adult rat so-matosensory cortex. J Neurophysiol. 1996;75:1573–88. doi: 10.1152/jn.1996.75.4.1573. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994;14:7077–86. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49:567–79. [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–69. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–51. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C. Inhibition as a coordinative factor. 1932 http://nobelprize.org/nobel_prizes/medicine/laureates/1932/sherrington-lecture.html.
- Sidibé M, Smith Y. Thalamic inputs to striatal interneurons in monkeys: synaptic organization and co-localization of calcium binding proteins. Neuroscience. 1999;89:1189–208. doi: 10.1016/s0306-4522(98)00367-4. [DOI] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–24. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–65. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–46. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Roberts JD, Takagi H, Richards JG, Mohler H, Somogyi P. Synaptic and nonsynaptic localization of benzodiazepine/GABAA receptor/cl- channel complex using monoclonal antibodies in the dorsal lateral geniculate nucleus of the cat. Eur J Neurosci. 1990;2:414–29. doi: 10.1111/j.1460-9568.1990.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, et al. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19:552–69. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):1–10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger JF, Masanneck C, Schleicher A, Zuschratter W. Calbindin-containing interneurons are a target for VIP-immunoreactive synapses in rat primary somatosensory cortex. J Comput Neurol. 2004;468:179–89. doi: 10.1002/cne.10953. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Yu RT, Evans RM, Campbell K. Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development. 2003;130:1113–22. doi: 10.1242/dev.00328. [DOI] [PubMed] [Google Scholar]
- Stokes CC, Isaacson JS. From dendrite to soma: dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron. 2010;67:452–65. doi: 10.1016/j.neuron.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm-Mathisen J, Fonnum F. Quantitative histochemistry of glutamate decarboxylase in the rat hippocampal region. J Neurochem. 1971;18:1105–11. doi: 10.1111/j.1471-4159.1971.tb12039.x. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Inhibitory interneurons in the piriform cortex. Clin Exp Pharmacol Physiol. 2007;34:1064–69. doi: 10.1111/j.1440-1681.2007.04723.x. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. J Comput Neurol. 2010a;518:1670–87. doi: 10.1002/cne.22295. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Distinctive classes of GABAergic interneurons provide layer-specific phasic inhibition in the anterior piriform cortex. Cereb Cortex. 2010b;20:2971–84. doi: 10.1093/cercor/bhq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Thalamocortical control of feed-forward inhibition in awake somatosensory ‘barrel’ cortex. Philos Trans R Soc Lond B. 2002;357:1717–27. doi: 10.1098/rstb.2002.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Soltesz I. Functional specificity of mossy fiber innervation of GABAergic cells in the hippocampus. J Neurosci. 2009;29:4239–51. doi: 10.1523/JNEUROSCI.5390-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnár G, Oláh S, Barzó P, Tamás G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–35. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koós T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–69. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–92. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Clark BD, Rudy B. Layer-specific organization within the fast-spiking interneuron population of mouse barrel cortex. Soc Neurosci Abstr 2010 [Google Scholar]
- Tricoire L, Pelkey KA, Daw MI, Sousa VH, Miyoshi G, et al. Common origins of hippocampal ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci. 2010;30:2165–176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ, Shepherd GM. Glycine exerts potent inhibitory actions on mammalian olfactory bulb neurons. J Neurophysiol. 1994;71:761–67. doi: 10.1152/jn.1994.71.2.761. [DOI] [PubMed] [Google Scholar]
- Udenfriend S. Indentification of γ-aminobutyric acid in brain by the isotope derivative method. J Biol Chem. 1950;187:65–69. [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol. 1998;506(Pt. 3):755–73. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Bartos M, Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49:107–17. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, et al. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20:2333–47. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillet J, Kerkerian L, Salin P, Nieoullon A. Ultrastructural features of NPY-containing neurons in the rat striatum. Brain Res. 1989;477:241–51. doi: 10.1016/0006-8993(89)91412-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gupta A, Toledo-Rodriguez M, Wu CZ, Markram H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex. 2002;12:395–410. doi: 10.1093/cercor/12.4.395. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester MR, Teasley DC, Byers SL, Saha MS. Expression patterns of glycine transporters (xGlyT1, xGlyT2, and xVIAAT) in Xenopus laevis during early development. Gene Expr Patterns. 2008;8:261–70. doi: 10.1016/j.gep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- West DC, Mercer A, Kirchhecker S, Morris OT, Thomson AM. Layer 6 corticothalamic pyramidal cells preferentially innervate interneurons and generate facilitating EPSPs. Cereb Cortex. 2006;16:200–11. doi: 10.1093/cercor/bhi098. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–82. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci. 2005;8:1364–70. doi: 10.1038/nn1545. [DOI] [PubMed] [Google Scholar]
- Williams JN., Jr The effects of folic acid and aminopterin upon enzyme systems in vitro. J Biol Chem. 1950;187:47–54. [PubMed] [Google Scholar]
- Wilson CJ. GABAergic inhibition in the neostriatum. Prog Brain Res. 2007;160:91–110. doi: 10.1016/S0079-6123(06)60006-X. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007a;27:553–63. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol. 2007b;98:2956–61. doi: 10.1152/jn.00739.2007. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Monyer H, Sah P. GABAergic excitation in the basolateral amygdala. J Neurosci. 2006;26:11881–87. doi: 10.1523/JNEUROSCI.3389-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;2426:2612–22. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–40. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comput Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Xu X, Callaway EM. Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci. 2009;29:70–85. doi: 10.1523/JNEUROSCI.4104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comput Neurol. 2006;499:144–60. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comput Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Ehrman LA, Pierani A, Campbell K. Developmental origin of the neuronal subtypes that comprise the amygdalar fear circuit in the mouse. J Neurosci. 2010;30:6944–53. doi: 10.1523/JNEUROSCI.5772-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AB, MacDonald RL. Glycine as a spinal cord neurotransmitter. In: Davidoff RA, editor. Handbook of the Spinal Cord: Pharmacology. I. New York: Marcel Dekker; 1983. pp. 1–44. [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, et al. Interneuron diversity in layers 2–3 of monkey prefrontal cortex. Cereb Cortex. 2009;19:1597–615. doi: 10.1093/cercor/bhn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbin MA, Wamsley JK, Kuhar MJ. Glycine receptor: light microscopic autoradiographic localization with [3H]strychnine. J Neurosci. 1981;1:532–47. doi: 10.1523/JNEUROSCI.01-05-00532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Jackson MB. GABA-activated chloride channels in secretory nerve endings. Science. 1993;259:531–34. doi: 10.1126/science.8380942. [DOI] [PubMed] [Google Scholar]