Abstract
Overnutrition-induced diseases such as obesity and type-2 diabetes involve neural dysregulation of metabolic physiology. Recently, interdisciplinary research in neuroscience and immunology has linked overnutrition to a non-classical onset of inflammation in the brain, and particularly the hypothalamus. This neuroinflammation impairs central regulatory pathways of energy balance and nutrient metabolism, and leads to obesity, diabetes and cardiovascular complications. This review describes recent findings showing the roles of overnutrition-induced hypothalamic inflammation in neurodegeneration and defective adult neurogenesis, as well as impaired neural stem cell regeneration, and its relevance to obesity and related diseases. Also, commonalities in terms of neuroinflammation between neurodegenerative diseases and overnutrition-induced metabolic diseases are discussed. Targeting neuroinflammation and neurodegeneration will provide promising approaches to treating obesity and other overntrution-related diseases.
Keywords: Inflammation, neurodegeneration, neural stem cells, hypothalamus, brain, NF-κB, obesity, diabetes
Overnutrition-induced diseases and neuroinflammation
Metabolic syndrome refers to as a collection of interconnected disorders such as obesity, insulin resistance, glucose intolerance, hyperlipidemia, hypertension and a few others, and the explosion of these problems has become a global health concern. Obesity is a driver of metabolic syndrome components, and a well recognized risk factor for the development of type 2 diabetes (T2D) and related cardiovascular diseases (CVDs). Known as a chronic and pathologic outcome of excessive caloric intake and storage, obesity development is significantly attributed to overeating and insufficient physical activities; therefore, lifestyle interventions like diet and exercise are still two useful non-medical methods to control or limit obesity development. Yet, despite this awareness and practice, obesity and obesity-related complications continue to spread. One difficulty may be related to the complexity of its etiology and pathogenesis. Indeed, recent advances have disproven the principle that obesity is a simple equation of caloric intake and expenditure, but instead a rather complicated neurological process involving neurohormonal and even neurotransmitter dysregulations (see Glossary) of physiology [1–9].
Research in neuroendocrinology and immunology over the past several years reveals that overnutrition-induced neuroinflammation is an important pathologic component, leading to a range of dysfunctions in the central nervous system (CNS), in obesity and related metabolic diseases [10–14]. In addition to its negative impacts on neurohormonal signaling of hypothalamic neurons as reviewed elsewhere [10–14], overnutrition-induced inflammation contributes to neurodegeneration [15–18], and disruption of hypothalamic neural stem cells [16], and proinflammatory molecules in the nuclear factor κB (NF-κB)/IκB kinase β (IKKβ) pathway are mechanistically accountable for this neurodegenerative disorder [16]. The newly established link between obesity-related inflammation and neurodegeneration, although much still remains to be uncovered, helps enlarge the picture of obesity and related diseases. Also prospectively, a mechanistic hint seems to be emerging which underlies the close relationship between overnutrition-induced metabolic diseases and neurodegenerative diseases, such as Alzheimer’s and Parkinson’s [19–23]. Herein, we review findings in overnutrition-related neuroinflammation, and the role of neuroinflammation in neural degeneration and regeneration in the context of overnutrition-induced obesity and related metabolic diseases.
Neuroinflammation in the hypothalamus
Research during the past decades has focused on examining peripheral tissues relevant to the pathogenesis of obesity and related diseases, such as skeletal muscle, liver and fat, as they represent the metabolic sites which are predominantly responsible for nutrient utilization and storage. One significant discovery is that many metabolic dysfunctions in peripheral tissues are causally related to local inflammation [12,24–30]. Indeed, evidence derived from epidemiology, clinical medicine, and experimental research, demonstrates that obesity and related diseases are associated with chronic, low-grade inflammation in peripheral tissues and the circulation [12,24–30]. Inflammation in several peripheral tissues is mounted by the immune system as well as non-immune cells, and critically mediated by the proinflammatory IKKβ/NF-κB pathway [12,28]. Recently, chronic overnutrition was shown to induce IKKβ/NF-κB-dependent inflammation in the CNS and particularly in the hypothalamus, a change that might contribute to the development of various overnutrition-related diseases [10–13].
The concept of overnutrition-induced hypothalamic inflammation
The hypothalamus is the master regulator of energy balance, governing physiological processes including feeding, energy expenditure, body weight, and glucose metabolism [31–36]. The mediobasal hypothalamus (MBH) senses circulating metabolic signals such as leptin, insulin, gut hormones and nutrients, and commands downstream neurohormonal networks to control various aspects of metabolic physiology. In addition, hypothalamic neurons can project to the autonomic sites in the brain to modulate the sympathetic and parasympathetic nervous systems that control metabolic activities. From the perspective of pathophysiology, central neurohormonal and neurotransmitter dysregulations represent a critical neural basis for the development of metabolic diseases [1–5]. Along these lines, overnutrition-driven inflammation, also termed “metabolic inflammation” [10–13], has been found to occur in the hypothalamus in the context of obesity and related metabolic diseases [37]. This type of hypothalamic inflammation has many features that differ from classical inflammation seen in diseases such as infectious diseases and cancers, and which can exert central anorexic actions to cause cachexia and sickness syndrome (reviewed in [11]). As observed in different types of research models with chronic or acute overnutrition [38–43], overnutrition induced hypothalamic inflammation is in general manifested in modest magnitude, and often primarily in the form of molecular changes instead of morphological abnormalities, agreeing with the characteristic of “low-grade” inflammation in obesity and T2D.
Disease relevance of overnutrition-induced hypothalamic inflammation
With the emerging recognition of overnutrition-mediated hypothalamic inflammation, recent research has demonstrated that is involved in the development of an increasing range of metabolic diseases, and most of these understandings were based on experimental models that targeted the IKKβ/NF-κB pathway. Hypothalamic inflammation was initially revealed to link environmental nutritional excess to overeating, with the later further sustaining the body’s condition of overnutrition, leading to chronic energy imbalance that causes overweight and obesity [37–40,42,43]. In parallel, IKKβ/NF-κB driven hypothalamic inflammation was shown to use a body weight-independent mechanism to cause diabetic changes, including glucose intolerance, hepatic insulin resistance and impaired insulin secretion [40,43–45]. More recently, it was discovered that the proinflammatory IKKβ/NF-κB pathway in the hypothalamus represents a pathogenic point that couples obesity with hypertension [46], thus further expanding the disease relevance of hypothalamic inflammation in the context of overnutrition. In addition the disease significance of IKKβ/NF-κB-related signaling molecules such as myeloid differentiation primary response gene 88 (MyD88) and the c-Jun N-terminal kinase JNK1 has also been investigated. For example, brain-specific knockout of MyD88, a downstream effector of toll-like receptor 4 (TLR-4) and inducer of the NF-κB pathway, was shown to prevent leptin resistance and dietary obesity, and this protective effect was related to hypothalamic IKKβ suppression [39]. Also, high-fat diet (HFD) feeding can activate JNK1 in the hypothalamus [47], possibly in an IKKβ/NF-κB-dependent manner. Consistently, mice with brain-specific JNK1 deletion are protected from HFD-induced energy imbalance [48] or weight gain [47,48] as well as systemic glucose and insulin disorders [47,48]. Altogether, hypothalamic inflammation in the CNS, that involves the IKKβ/NF-κB and related molecules, is now emerging as an important contributor to an increasing range of overnutrition-induced diseases.
IKKβ/NF-κB signaling in overnutrition-induced neuroinflammation
Cellular pathways converging on central NF-κB activation
It is well established that the NF-κB transcriptional program is a crucial regulator of immunity and inflammation [49–51]. Canonical NF-κB activation is induced predominately by the serine/threonine kinase IKKβ that phosphorylates and degrades IκB proteins, thus liberating NF-κB to enter the nucleus and induce transcription of many inflammatory genes. During classical immune response and inflammation, IKKβ/NF-κB activation is induced by a number of cell membrane receptors including TLRs. Recently, with the increasing recognition of TLR-mediated peripheral inflammation in obesity and T2D [52], TLRs have been shown to mediate the induction of obesity-related neuronflammation [38]. Cytokine receptors such as TNF-α receptors have also been shown to mediate neuroinflammation in the context of obesity, and loss-of-function approaches showed that TNF-α receptor knockout [45,53] or brain-directed TNF-α receptor inhibition [54] reduced dietary obesity and pre-diabetes in mice. However, cytokines in the CNS can have diverse metabolic consequences, ranging from positive energy balance in obesity, to negative energy balance in cachectic diseases such as chronic infections and cancers (reviewed in [11]). The different outcomes depend on multiple factors such as the sources and intensity of inflammatory stimuli, the affected cell types, or other yet unidentified factors (reviewed in [11,55]). In addition to TLRs and certain cytokine receptors, overnutrition-induced neuroinflammation is mediated, perhaps in a primary manner, by receptor-independent intracellular organelle stresses and disturbances involving endoplasmic reticulum (ER) [37,44] and oxidative stress [56] and defects in autophagy [43]. Recent research showed that ER stress [37,44] and autophagic defect [43] can converge on IKKβ/NF-κB to induce hypothalamic inflammation, providing a new scope in understanding the causes of overnutrition induced neuroinflammation.
Compared to dividing cells in peripheral tissues, neurons in the brain are highly sensitive to intracellular stresses [57–59], including metabolic stresses induced by chronic overnutrition [37,38,43,60–66]. The MBH in the hypothalamus is in a vulnerable anatomic position because of the partially leaky blood-brain barrier (BBB) in the MBH, and as a result, MBH neurons are exposed to excessive amounts of circulating nutrients which drive increased mitochondrial oxidation. When exposure to excess nutrients is prolonged, oxidative stress and mitochondrial dysfunction in these neurons can occur perhaps even prior to their induction in other cells [67]. While detailed experimental studies are still needed, it is known that mitochondrial dysfunction and oxidative stress lead to inflammation, and one potential mediator is intracellular NLRP3 inflammasomes [56] which can directly activate IKKβ/NF-κB [68]. At the same time, increased oxidative workload demands higher levels of ER activity such as protein synthesis, which causes ER stress, and which can also be potentiated by HFD-induced TLR4 activation [38]. Of note, hypothalamic ER stress has been shown to activate IKKβ/NF-κB in the hypothalamus [37,44]. In addition, overnutrition-induced cytosolic changes, such as dysfunctional mitochondria and ER, can affect autophagy and lead to autophagic defect. Recent research demonstrated that autophagy defect is a late-onset intracellular factor that mediates overnutrition-induced brain IKKβ/NF-κB activation [43]. Importantly, while various intracellular organelle stresses promote inflammatory reaction [37,43,44], inflammation can reciprocally render cells more prone to the induction of intracellular stresses, including ER stress [38,69–71] and autophagic defect [72–74]. From a therapeutic perspective, central inhibition of ER stress [37,44,60,75] or central improvement of autophagic function [43] can both attenuate the detrimental effects of overnutrition significantly through inhibiting NF-κB, further supporting the notion of the stress-inflammation connection in the neural mechanism of these diseases.
Neuroinflammation inducers downstream of NF-κB
A question that arises is how neuroinflammation leads to metabolic diseases. While it is feasible to deduce that functions of inflamed neurons are generally compromised, leading to neuronal dysregulation of physiology, it is also of interest to understand the molecular events downstream of IKKβ/NF-κB that induce disease outcomes. However, this scope of research is still in early stage, and to date, only a few molecules have specifically been related to the pro-obesity and diabetic effects of neural IKKβ/NF-κB. One such molecule is suppressor of cytokine signaling-3 (SOCS3), an inhibitory signaling protein that inhibits both leptin and insulin signaling [76]. Studies have shown that overnutrition-induced IKKβ/NF-κB activation can cause upregulation of hypothalamic SOCS3 gene expression to induce hypothalamic leptin and insulin resistance [37]. Genetic mouse models have shown that SOCS3 knockout in hypothalamic neurons can improve central leptin signaling and reduce obesity [77–79], just as does central IKKβ knockout [37], and overexpression of SOCS3 in the MBH can diminish the obesity-reducing effects of neural IKKβ inhibition [37]. Protein-tyrosine phosphatase 1B (PTP1B) is another protein which similarly to SOCS3 inhibits insulin and leptin signaling, and interestingly, evidence exists implicating PTP1B in IKKβ/NF-κB inflammatory pathway. For example, TNF-α, an activator of and also gene product of IKKβ/NF-κB, can increase hypothalamic PTP1B expression [80], and neural PTP1B inhibition counteracts overnutrition-induced leptin resistance, obesity and glucose disorders [81–83]. Of interest, brain PTP1B was recently linked to Alzheimer’s disease in genetic mouse models [84], and thus may represent a connection between neurodegeneration and central mechanism of metabolic diseases. It is also noted that additional tyrosine phosphatases in the brain with functions relevant to obesity and related diseases were identified, such as T-Cell Protein Tyrosine Phosphatase (Tcptp) [85], suggesting that the molecular relationship between inflammation and tyrosine phosphatases warrants further investigation.
Neurodegeneration in obesity and related diseases
The importance of the hypothalamus in regulating body weight homeostasis was historically shown by lesion studies in animals [86]; indeed, ablation of some ventral hypothalamic regions causes overeating and obesity, while disruption of the lateral hypothalamus leads to anorexia and weight loss. However, based on the classical dogma that adult neurons do not undergo turnover, those old studies were mostly used to suggest the physiological importance of the hypothalamus, but were barely related to the etiology or pathophysiology of obesity and T2D. Recent research has shown a link between neurodegenerative mechanisms and the development of metabolic diseases such as obesity and related T2D [15–17]. First, chronic overnutrition during an 8-month period of HFD feeding induced a modest but measurable reduction in the number of pro-opiomelanocortin (POMC) neurons in adult mice [15,16]. Second, chronic overnutrition was shown to increase the apoptosis of mature neurons [15,87], newborn neurons or dividing cells [17], or neural stem cells [16] in the hypothalamus, and caloric restriction can reverse some of these defects [17]. These findings align well with the finding from studies on postnatal hypothalamic development showing that neurons in the arcuate nucleus undergo postnatal turnover even in adult ages [17], and such postnatal neurogenesis occurs under physiological or experimental conditions [17,18]. On the other hand, the disease relevance of these observations still remains to be tested; nevertheless, it was recently shown that long-term outcomes of overnutrition-induced neurodegeneration included the development of obesity and pre-diabetic changes [16]. And in conjunction with these findings, hypothalamic neurodegeneration, independent of overnutrition, was found to lead to the development of adult-onset obesity, in several genetic models [88–90]. Based on a few recent studies, hypothalamic inflammation can mediate obesity-related hypothalamic neurodegeneration [15,16,87], fitting within the context that neuroinflammation is a common background shared by obesity/T2D and neurodegenerative diseases. Molecular research in this line has further revealed that IKKβ/NF-κB pathway is critically responsible for the neurodegenerative mechanism in the development of obesity and T2D. Taken together, overnutrition-induced hypothalamic neurodegeneration via inflammation represents an emerging and intriguing research paradigm in studying the central mechanism of obesity, T2D and related diseases.
Disruption of neural stem cells by neuroinflammation in obesity and T2D
Adult hypothalamic neurogenesis and neural stem cells in mice
It has been known since the 1990s that adult mammalian brains contain multipotent neural stem cells able to generate different neural lineages including neurons, astrocytes and oligodendrocytes [91,92]. The biological functions of adult neural stem cells might be to mediate adult neurogenesis, a process needed by the brain to maintain its plasticity in response to intrinsic and extrinsic changes [93]. Mammalian adult neural stem cells predominantly exist in the subventricular zone of the forebrain lateral ventricle and the subgranular zone of the hippocampal dentate gyrus [94]. Not long ago, the hypothalamus of adult mice was found to have neurogenic activities in either stimulated [95] or basal [96] conditions. It was also reported that in a mouse model with genetically-induced AGRP neuron degeneration, de novo hypothalamic neurogenesis was observed to lead to new cells which differentiated into leptin-responsive AGRP neurons [18]. A recent study further demonstrated that the arcuate nucleus in adult mice undergoes physiological neuronal remodeling via neurogenesis-mediated neuronal turnover [17]. Related to these findings, a fundamental question is whether the hypothalamic neurogenesis observed in all these studies is attributed to neural stem cells in this brain region. In answering this question, a recent study showed that tanycytes in the median eminence can function as neural stem cells, and induce postnatal hypothalamic neurogenesis in newborn pups or pre-adult young mice [97]. Li et al reported that in addition to the third ventricle walls, the MBH contains a significant number of neural stem cells in adult mice, and importantly, these cells are multipotent and can differentiate into neurons, astrocytes and oligodendrocytes under both in vivo and in vitro conditions [16]. As elucidated in Figure 1, these recent stimulating findings can potentially direct hypothalamic research to a new and interesting direction.
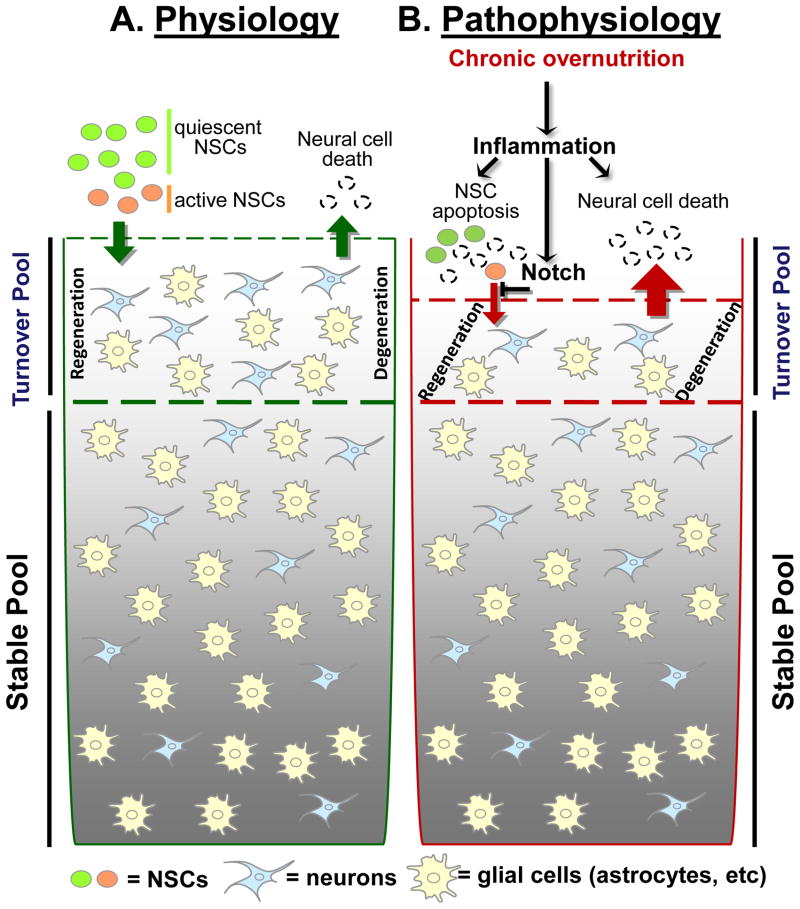
Figure 1. Inflammation-mediated neurodegeneration in overnutrition-related diseases.
A. While majority of neurons in adult mammalian brain are terminally stable, a pool of neural cells including neurons undergoes slow-speed turnover in normal physiology, and this process requires neurogenesis induced by adult neural stem cells (NSCs) including hypothalamic NSCs, a small number of multipotent cells residing in several brain regions including the mediobasal hypothalamus. Although this NSCs-directed neurogenesis in physiological conditions is rather slow and also region-specific, recent research suggests that it may have important roles in maintaining the brain’s controls during life-long physiological homeostasis.
B. Under pathophysiological conditions such as long-term overnutrition and probably aging, the resulting neuroinflammation affects the turnover pool of neural cells in adult brain. This process involves the inhibitory action of neuroinflammation on survival and differentiation of NSCs, mediated by NF-κB-directed apoptosis and Notch signaling. The neurodegenerative change under these conditions is slow and modest; however, when it affects certain neuronal types which by nature have small populations but critical functions, it can have severe disease outcomes. For example, the impairment of NSCs in the mediobasal hypothalamus can lead to a factional reduction of hypothalamic POMC neurons over a long period and mediate a late-onset development of obesity and pre-diabetes.
The role of hypothalamic neurogenesis and neural stem cells in metabolic disease
The consistent observation of hypothalamic neurogenesis in adult mice leads to another key question, that is, if hypothalamic neurogenesis is relevant to metabolic diseases. Indeed, dietary obesity and leptin deficiency-induced obesity are both associated with reduced arcuate neurogenesis, with the arcuate nucleus containing fewer new neurons but more old neurons [17]. In the study by Li et al [16], authors showed that chronic HFD feeding markedly impaired the survival and neurogenesis of adult neural stem cells in the MBH, leading to a fractional (~10%) loss of POMC neurons in the MBH. To explore a potential causal role of impaired hypothalamic neurogenesis in metabolic diseases, this study further revealed that mice, that were genetically engineered to have depletion of neural stem cells in the MBH, chronically developed metabolic disorders including overeating, glucose disorder, insulin resistance and obesity [16]. Therefore, hypothalamic neurodegeneration in obesity can be a result of neuronal loss and reduced neural regeneration, which arise from impaired neural stem cells (Figure 1). Of note, this neurodegeneration requires long duration of overnutrition [16], which agrees with the slow-speed feature in the pathogenesis of neurodegenerative diseases. Also, it seems that only certain types of neurons are susceptible to this injury, and this mechanism could result in only a modest loss in neurons, which may not be sufficient to affect many classical neurological functions; however, the modest change of certain neurons, such as POMC neurons, which have small populations by nature, but have important metabolic regulatory functions, can be significant and cause the consequences of metabolic diseases.
As mentioned above, Li et al demonstrated that IKKβ/NF-κB-mediated inflammation has an important role in obesity-associated hypothalamic neurodegeneration [16]. Earlier research showed that NF-κB-mediated inflammation, via activation of TLR4 or MyD88 pathway [98] or IL-1 receptor [99], can impair hippocampal neurogenesis in the disease context of memory loss or mood disorders. Along these lines, Li et al found that chronic overnutrition led to IKKβ/NF-κB over-activation in hypothalamic neural stem cells in adult mice. Mechanistically, obesity-related neurodegeneration was attributed to excessive production of IKKβ/NF-κB-dependent cytokines such as TNF-α and IL-1β from microglial cells which sustained an inflammatory state through the paracrine actions of these cytokines. Microglia-specific IKKβ ablation showed that breaking the inflammatory crosstalk between microglia and neural stem cell can help the survival and neurogenesis of hypothalamic neural stem cells [16]. In this research, the authors further discovered that IKKβ/NF-κB activation employed the apoptotic program to impair the survival of hypothalamic neural stem cells, and the Notch signaling pathway to inhibit the neuronal differentiation of these cells [16]. Taken together, IKKβ/NF-κB-mediated neural inflammation not only affects the neurohormonal signaling of hypothalamic neurons in regulation of body and metabolic physiology, it also hinders neurogenesis leading to neurodegeneration and the development of metabolic diseases. A schematic in Figure 2 is used to summarize the neuroinflammation-induced mechanisms of signaling defects as well as neurodegeneration which are both important for the development of overnutrition-induced disease.
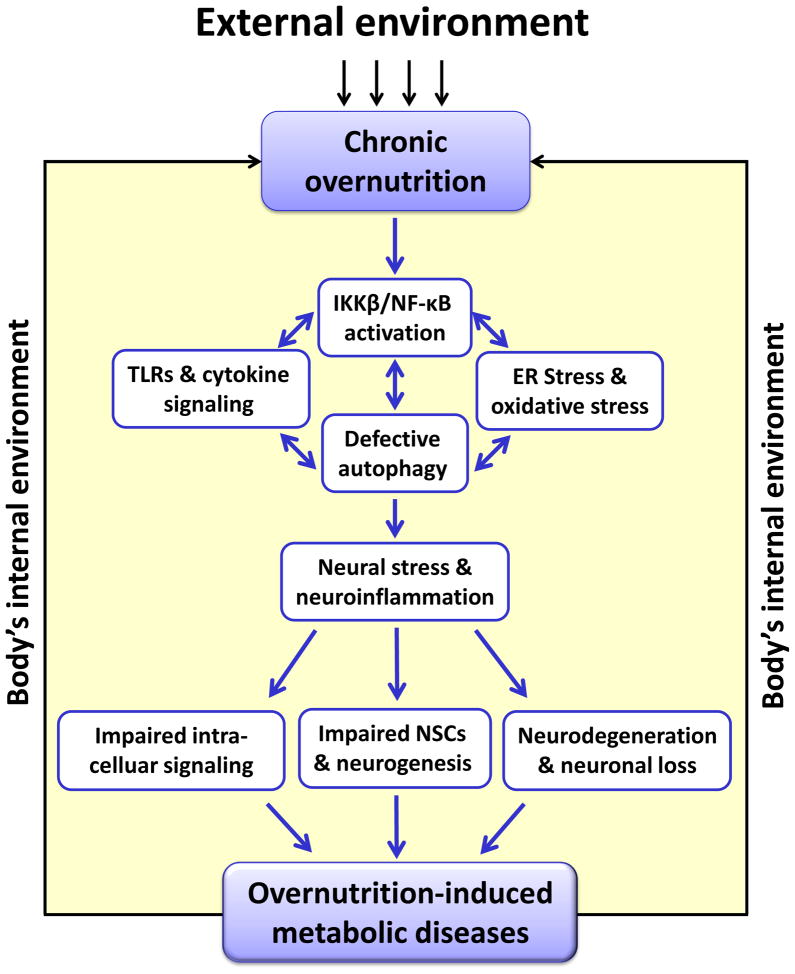
Figure 2. Role of neuroinflammation in overnutrition-induced diseases.
Under conditions of chronic overnutrition, the mediobasal hypothalamus (MBH) is affected by chronic overnutrition, a prolonged and persistent nutritional changes in the body which primarily arises from environmental and social-behavioral factors such as Western diet, sedentary lifestyle and disrupted diurnal order of life. These lead to IKKβ/NF-κB-directed inflammatory response and several intracellular organelle stresses in the MBH. Many of these cellular and molecular components promote each other, resulting in overnutrition-related neuroinflammation. Such neuroinflammation impairs intracellular hormonal signaling of regulatory neurons and disrupts neurogenesis through depletion of NSCs. The progression of overnutrition-related diseases such as obesity and diabetes, characterized by hyperlipidemia and hyperglycemia, secondarily leads to pathophysiological overnutrition in the body’s internal environment and exacerbates this mechanism. In summary, neuroinflammation employs multiple pathways including hormonal signaling dysfunction and neurodegeration to link overnutrition to overnutrition-related diseases such as obesity, diabetes and related complications. Abbreviations: TLRs: toll-like receptors; NSCs: neural stem cells.
Links between neuroinflammation seen in obesity/T2D and neurodegenerative diseases
Epidemiological and clinical studies suggest that obesity, T2D and their related lifestyle (e.g. physical inactivity) are highly associated with Alzheimer’s diseases and Parkinson’s disease [20–23]. Conversely, therapeutic interventions of metabolic diseases have often been shown to protect against neurodegenerative disorders as well [100,101], suggesting that obesity and diabetes contribute to the development of neural degeneration and neurodegenerative diseases. The potential causal relationship between obesity/T2D and neurodegenerative diseases has also been suggested by several experimental animal models. For example, overnutrition can directly promote dopaminergic neurodegeneration in a mouse model of Parkinson disease [102]. Brain insulin signaling changes in diabetes were reported to cause neuronal oxidative stress and mitochondrial dysfunction and to promote Huntington disease [103]. PTEN-induced putative kinase-1 (PINK1), a genetic locus responsible for familial Parkinson disease via neuronal apoptosis [104], was demonstrated to undergo altered regulation in obesity and T2D [105]. Also, obesity and T2D driven neurodegenerative diseases depend on neuroinflammation, and an important inflammatory mediator is the IKKβ/NF-κB pathway, which controls cell survival and apoptosis. For example, interleukin-6, a cytokine which is over-produced in obesity and diabetes, was shown to mediate degeneration of forebrain GABAergic inter-neurons, and this neurodegenerative effect was attributed to neuronal NF-κB activation and the subsequent induction of neurotoxic inflammatory products [106]. Also, while obesity and T2D are associated with metabolic overload and neuronal insulin resistance, both of these changes render neurons vulnerable to cell death through neural stress and inflammation [107,108]. Altogether, neuroinflammation-induced neurodegeneration may act as a common basis for not only metabolic diseases like obesity and T2D but also neurodegenerative diseases, and future research in this direction will broaden our understandings of both categories of diseases.
Concluding remarks
Research during the past decade has demonstrated that obesity and its co-morbidities are not only disorders of peripheral tissues but changes of neurological process resulting in neural dysregulation and altered metabolic physiology. Recently, interdisciplinary research in neuroscience and immunology has linked overnutrition to IKKβ/NF-κB-directed inflammation in the brain, and particularly the hypothalamus. This neuroinflammation was shown to impair the neurohormonal as well as autonomic regulations of energy balance and nutrient metabolism, leading to obesity, diabetes and related cardiovascular diseases. As depicted in Figure 2, obesity-related neuroinflammation is induced in the brain by multiple processes, and while some might still be unknown, it has been appreciated that intracellular disturbances and stresses including ER stress, oxidative stress and autophagic defects are important mediators. In the context of obesity and co-morbidities, neuroinflammation-induced pathologic changes are also multifold, including loss of regulatory neurons, and impaired neural regeneration resulting from depletion and defect of neural stem cells. Collectively, all these pathologic changes contribute to a battery of central dysregulations that underlie the induction of overnutrition-induced diseases. The neurodegenerative mechanism of these diseases represents the most recent research advance, and the involvement of adult neural stem cell-directed neural regeneration is particularly attractive. On the other hand, as this is an emerging area of research, many unsolved questions remain and the clinical significance and possible avenues of targeting neuroinflammation and neurodegeneration to treat obesity and co-morbidities warrants our attention.
OUTSTANDING QUESTIONS BOX.
What are the dynamic, interactive upstream and downstream network pathways of overnutrition-induced neuroinflammation?
What are the mechanistic aspects of the glia-neuron interaction in overnutrition-induced neuroinflammation?
How important is the role of adult neural stem cells in the hypothalamus, in metabolic physiology and diseases?
How does neuroinflammation disrupt neural stem cells generation?
Could neural stem cells be therapeutic targets for treating neuroinflammation and thus relevant diseases?
Is neuroinflammation casually responsible for the existing relationships between classical neurodegenerative diseases and overnutrition-induced metabolic diseases?
Acknowledgments
The author sincerely thanks Cai lab members for their research relevant to this writing. Cai’s research relevant to this writing has been supported by NIH R01 DK078750 and R01 AG031774, and American Diabetes Association Basic Research Award #1-12-BS-20 (all to D. Cai). D Cai is an Irma T. Hirschl Scholar.
GLOSSARY
- Neurodegeneration
A general term indicating the progressive loss of structure or function of neurons, including death of neurons and can include ER stress, protein misfolding and degradation, defects in autophagy, mitochondrial dysfunction, apoptosis, among others
- Neuroinflammation
A collection of molecular and cellular changes in the brain in response to inflammatory stimuli ranging from externally-induced brain injuries such as infections, trauma and stroke, to the body’s physiological changes such as metabolic abnormalities and aging
- Hypothalamic inflammation
Molecular and cellular changes in the hypothalamus in response to inflammatory stimuli ranging from externally-induced local injuries such as infections, trauma and stroke to the body’s physiological changes such as metabolic abnormalities and aging
- Metabolic inflammation
Chronic and low-grade inflammatory changes in overnutrition-induced metabolic diseases (such as obesity and T2D), which are displayed primarily in the form of inflammatory signaling and molecular products, without evident onset of histological abnormalities or symptoms that are typically seen in infection- or cancer-induced inflammation
- Neurohormonal dysregulation
Abnormal regulation arising from altered synthesis, release, signaling or actions of neurohormones, which refer to hormonal substances that are released by neurons in the brain and in particular the hypothalamus
- Neurotransmitter dysfunction
Abnormal regulation arising from altered synthesis, release, signaling or actions of neurotransmitters, which refer to chemicals that transmit signals from a neuron to a target cell across a synapse, and release of, which usually follows arrival of, an action potential, at the synapse
- Neurogenesis
A process of generating neurons and other terminal neural cells by neural stem cells and/or progenitor cells, which is active typically during prenatal brain development and was revealed in recent research to limitedly continue in several brain regions during the adulthood
- Overnutrition
Body’s persistent and prolonged exposure to excessive amount of calorie-rich nutrients which are often presented in the form of excessive lipids and carbohydrates
- Pro-opiomelanocortin (POMC) neurons
A group of hypothalamic neurons that synthesize and cleave POMC leading to the release of peptidyl hormone α-MSH, which plays an important role in regulating appetite, energy expenditure, body weight and other metabolic parameters
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belgardt BF, Bruning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- 2.Myers MG, et al. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 3.Myers MG, Jr, et al. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elmquist JK, Flier JS. Neuroscience. The fat-brain axis enters a new dimension. Science. 2004;304:63–64. doi: 10.1126/science.1096746. [DOI] [PubMed] [Google Scholar]
- 5.Rahmouni K, et al. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 6.Elmquist JK, et al. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 7.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi CX, et al. A role for astrocytes in the central control of metabolism. Neuroendocrinology. 2011;93:143–149. doi: 10.1159/000324888. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur J Neurosci. 2009;30:1688–1696. doi: 10.1111/j.1460-9568.2009.06963.x. [DOI] [PubMed] [Google Scholar]
- 10.Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging (Albany NY) 2012;4:98–115. doi: 10.18632/aging.100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai D, Liu T. Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann N Y Acad Sci. 2011;1243:E1–39. doi: 10.1111/j.1749-6632.2011.06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai D. NFkappaB-mediated metabolic inflammation in peripheral tissues versus central nervous system. Cell Cycle. 2009;8:2542–2548. doi: 10.4161/cc.8.16.9386. [DOI] [PubMed] [Google Scholar]
- 13.Cai D. One step from prediabetes to diabetes: hypothalamic inflammation? Endocrinology. 2012;153:1010–1013. doi: 10.1210/en.2011-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 2010;151:4109–4115. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, et al. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNay DE, et al. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest. 2012;122:142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 2010;30:723–730. doi: 10.1523/JNEUROSCI.2479-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballard C, et al. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 20.Lees AJ, et al. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 21.Beydoun MA, et al. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis. Obes Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu FP, et al. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 24.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 25.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoelson SE, Goldfine AB. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat Med. 2009;15:373–374. doi: 10.1038/nm0409-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenk S, et al. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 29.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 31.Marino JS, et al. Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab. 2011;22:275–285. doi: 10.1016/j.tem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandoval D, et al. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol. 2008;70:513–535. doi: 10.1146/annurev.physiol.70.120806.095256. [DOI] [PubMed] [Google Scholar]
- 33.Coll AP, et al. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton GJ, et al. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 35.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 36.Lam TK, et al. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milanski M, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinridders A, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posey KA, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 42.Oh I, et al. Central administration of interleukin-4 exacerbates hypothalamic inflammation and weight gain during high-fat feeding. Am J Physiol Endocrinol Metab. 2010;299:E47–E53. doi: 10.1152/ajpendo.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J Biol Chem. 2011;286:32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purkayastha S, et al. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci U S A. 2011;108:2939–2944. doi: 10.1073/pnas.1006875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arruda AP, et al. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology. 2011;152:1314–1326. doi: 10.1210/en.2010-0659. [DOI] [PubMed] [Google Scholar]
- 46.Purkayastha S, et al. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belgardt BF, et al. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci U S A. 2010;107:6028–6033. doi: 10.1073/pnas.1001796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabio G, et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 51.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 52.Konner AC, Bruning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22:16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Romanatto T, et al. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem. 2009;284:36213–36222. doi: 10.1074/jbc.M109.030874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Milanski M, et al. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012;61:1455–1462. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thaler JP, et al. Hypothalamic inflammation and energy homeostasis: resolving the paradox. Front Neuroendocrinol. 2010;31:79–84. doi: 10.1016/j.yfrne.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 57.Melov S. Modeling mitochondrial function in aging neurons. Trends Neurosci. 2004;27:601–606. doi: 10.1016/j.tins.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Sorrells SF, et al. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 60.Ozcan L, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 61.Camacho A, et al. Ablation of PGC1 beta prevents mTOR dependent endoplasmic reticulum stress response: PGC1 beta coordinates endoplasmic reticulum stress response. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nerurkar PV, et al. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J Neuroinflammation. 2011;8:64. doi: 10.1186/1742-2094-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charradi K, et al. Grape seed and skin extract prevents high-fat diet-induced brain lipotoxicity in rat. Neurochem Res. 2012;37:2004–2013. doi: 10.1007/s11064-012-0821-2. [DOI] [PubMed] [Google Scholar]
- 64.White CL, et al. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrison CD, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114:1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Son SM, et al. Accumulation of autophagosomes contributes to enhanced amyloidogenic APP processing under insulin-resistant conditions. Autophagy. 2012:8. doi: 10.4161/auto.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh RB, et al. Metabolic syndrome: a brain disease. Can J Physiol Pharmacol. 2012;90:1171–1183. doi: 10.1139/y2012-122. [DOI] [PubMed] [Google Scholar]
- 68.Strowig T, et al. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 69.Salminen A, et al. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galizzi G, et al. Different early ER-stress responses in the CLN8(mnd) mouse model of neuronal ceroid lipofuscinosis. Neurosci Lett. 2011;488:258–262. doi: 10.1016/j.neulet.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 71.Valenzuela V, et al. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell Death Dis. 2012;3:e272. doi: 10.1038/cddis.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alirezaei M, et al. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS One. 2008;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meissner F, et al. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proc Natl Acad Sci U S A. 2010;107:13046–13050. doi: 10.1073/pnas.1002396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alirezaei M, et al. Autophagy, inflammation and neurodegenerative disease. Eur J Neurosci. 2011;33:197–204. doi: 10.1111/j.1460-9568.2010.07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Won JC, et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 76.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17:365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 77.Reed AS, et al. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010;59:894–906. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kievit P, et al. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Mori H, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 80.Zabolotny JM, et al. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Banno R, et al. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Picardi PK, et al. Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology. 2008;149:3870–3880. doi: 10.1210/en.2007-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bence KK, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 84.Liao G, et al. Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in Npc1−/− mouse brain. Brain Res. 2009;1270:140–151. doi: 10.1016/j.brainres.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loh K, et al. Elevated Hypothalamic TCPTP in Obesity Contributes to Cellular Leptin Resistance. Cell Metab. 2011;14:684–699. doi: 10.1016/j.cmet.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 87.Moraes JC, et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4:e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Susaki E, et al. Increased E4 activity in mice leads to ubiquitin-containing aggregates and degeneration of hypothalamic neurons resulting in obesity. J Biol Chem. 2010;285:15538–15547. doi: 10.1074/jbc.M110.105841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ryu KY, et al. Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene. Proc Natl Acad Sci U S A. 2008;105:4016–4021. doi: 10.1073/pnas.0800096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramesh BJ, et al. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J Neurochem. 2008;106:107–120. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- 91.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 92.Cameron HA, McKay R. Stem cells and neurogenesis in the adult brain. Curr Opin Neurobiol. 1998;8:677–680. doi: 10.1016/s0959-4388(98)80099-8. [DOI] [PubMed] [Google Scholar]
- 93.Emsley JG, et al. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 94.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 95.Kokoeva MV, et al. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 96.Kokoeva MV, et al. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 97.Lee DA, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rolls A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 99.Koo JW, et al. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schapira AH. Mitochondrial diseases. Lancet. 2012;379:1825–1834. doi: 10.1016/S0140-6736(11)61305-6. [DOI] [PubMed] [Google Scholar]
- 101.Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bousquet M, et al. High-fat diet exacerbates MPTP-induced dopaminergic degeneration in mice. Neurobiol Dis. 2012;45:529–538. doi: 10.1016/j.nbd.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 103.Sadagurski M, et al. IRS2 increases mitochondrial dysfunction and oxidative stress in a mouse model of Huntington disease. J Clin Invest. 2011;121:4070–4081. doi: 10.1172/JCI46305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Petit A, et al. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;280:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- 105.Scheele C, et al. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration? FASEB J. 2007;21:3653–3665. doi: 10.1096/fj.07-8520com. [DOI] [PubMed] [Google Scholar]
- 106.Dugan LL, et al. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruce-Keller AJ, et al. Obesity and vulnerability of the CNS. Biochim Biophys Acta. 2009;1792:395–400. doi: 10.1016/j.bbadis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de la Monte SM, et al. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10:1049–1060. [PMC free article] [PubMed] [Google Scholar]