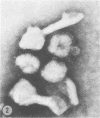
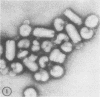
Abstract
Influenza virus particles bind rapidly to vesicular stomatitis, Sindbis, or Rauscher murine leukemia virus particles, forming mixed aggregates demonstrable by electron microscopy. The normal hemagglutinating property of influenza virus is inhibited by these viruses, providing a rapid quantitative assay. Prior treatment with neuraminidase blocks the ability of other viruses to inhibit influenza virus hemagglutination.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Huang A. S. Comparison of membrane protein glycopeptides of Sindbis virus and vesicular stomatitis virus. J Virol. 1970 Aug;6(2):176–182. doi: 10.1128/jvi.6.2.176-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A. Neuraminidase: the specific enzyme of influenza virus and Vibrio cholerae. Biochim Biophys Acta. 1957 Mar;23(3):645–646. doi: 10.1016/0006-3002(57)90389-x. [DOI] [PubMed] [Google Scholar]
- Halonen P. E., Murphy F. A., Fields B. N., Reese D. R. Hemagglutinin of rabies and some other bullet-shaped viruses. Proc Soc Exp Biol Med. 1968 Apr;127(4):1037–1042. doi: 10.3181/00379727-127-32864. [DOI] [PubMed] [Google Scholar]
- Hirst G. K. THE AGGLUTINATION OF RED CELLS BY ALLANTOIC FLUID OF CHICK EMBRYOS INFECTED WITH INFLUENZA VIRUS. Science. 1941 Jul 4;94(2427):22–23. doi: 10.1126/science.94.2427.22. [DOI] [PubMed] [Google Scholar]
- KLENK E., LEMPFRID H. Uber die Natur der Zellreceptoren für das Influenzavirus. Hoppe Seylers Z Physiol Chem. 1957;307(2-6):278–283. [PubMed] [Google Scholar]
- Klenk H. D., Caliguiri L. A., Choppin P. W. The proteins of the parainfluenza virus SV5. II. The carbohydrate content and glycoproteins of the virion. Virology. 1970 Oct;42(2):473–481. doi: 10.1016/0042-6822(70)90290-4. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycolipid content of vesicular stomatitis virus grown in baby hamster kidney cells. J Virol. 1971 Mar;7(3):416–417. doi: 10.1128/jvi.7.3.416-417.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Glycosphingolipids of plasma membranes of cultured cells and an enveloped virus (SV5) grown in these cells. Proc Natl Acad Sci U S A. 1970 May;66(1):57–64. doi: 10.1073/pnas.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Compans R. W., Choppin W. P. An electron microscopic study of the presence or absence of neuraminic acid in enveloped viruses. Virology. 1970 Dec;42(4):1158–1162. doi: 10.1016/0042-6822(70)90368-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Differences between the envelope glycoproteins and glycopeptides of avian tumor viruses released from transformed and from nontransformed cells. Virology. 1972 Nov;50(2):359–372. doi: 10.1016/0042-6822(72)90387-x. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Lenard J., Paxton J., Compans R. W. Spin-labeled electron spin resonance study of the lipid-containing membrane of influenza virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2579–2583. doi: 10.1073/pnas.68.10.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Wagner R. R. Carbohydrate composition of vesicular stomatitis virus. J Virol. 1971 Mar;7(3):412–415. doi: 10.1128/jvi.7.3.412-415.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger H. D., Schneider L. G., Kulas H. P., Diringer H. Gross chemical composition of strain Flury HEP rabies virus. Z Naturforsch C. 1973 Jan-Feb;28(1):103–104. [PubMed] [Google Scholar]
- Witter R., Frank H., Moennig V., Hunsmann G., Lange J., Schäfer W. Properties of mouse leukemia viruses. IV. Hemagglutination assay and characterization of hemagglutinating surface components. Virology. 1973 Aug;54(2):330–345. doi: 10.1016/0042-6822(73)90147-5. [DOI] [PubMed] [Google Scholar]