Abstract
Context
The risk of type 2 diabetes mellitus is heterogeneous among obese individuals. Factors that discriminate prediabetes or diabetes risk within this population have not been well characterized. A dysfunctional adiposity phenotype, characterized by excess visceral fat and insulin resistance, may contribute to diabetes development in those with obesity.
Objective
To investigate associations between adiposity phenotypes and risk for incident prediabetes and diabetes in a multiethnic, population-based cohort of obese adults.
Design, Setting, and Participants
Among 732 obese participants (body mass index ≥30) aged 30 to 65 years without diabetes or cardiovascular disease enrolled between 2000 and 2002 in the Dallas Heart Study, we measured body composition by dual energy x-ray absorptiometry and magnetic resonance imaging (MRI); circulating adipokines and biomarkers of insulin resistance, dyslipidemia, and inflammation; and subclinical atherosclerosis and cardiac structure and function by computed tomography and MRI.
Main Outcome Measures
Incidence of diabetes through a median 7.0 years (interquartile range, 6.6–7.6) of follow-up. In a subgroup of 512 participants with normal fasting glucose values at baseline, incidence of the composite of prediabetes or diabetes was determined.
Results
Of the 732 participants (mean age, 43 years; 65% women; 71% non-white), 84 (11.5%) developed diabetes. In multivariable analysis, higher baseline visceral fat mass (odds ratio [OR] per 1 SD [1.4 kg], 2.4; 95% CI, 1.6–3.7), fructosamine level (OR per 1 SD [1.1 μmol/L], 2.0; 95% CI, 1.4–2.7), fasting glucose level (OR per 1 SD [1.1 μmol/L], 1.9; 95% CI, 1.4–2.6), family history of diabetes (OR, 2.3; 95% CI, 1.3–4.3), systolic blood pressure (OR per 10 mm Hg, 1.3; 95% CI, 1.1–1.5), and weight gain over follow-up (OR per 1 kg, 1.06; 95% CI, 1.02–1.10) were independently associated with diabetes, with no associations observed for body mass index, total body fat, or abdominal subcutaneous fat. Among the 512 participants with normal baseline glucose values, the composite outcome of prediabetes or diabetes occurred in 39.1% and was independently associated with baseline measurements of visceral fat mass; levels of fasting glucose, insulin, and fructosamine; older age; non-white race; family history of diabetes; and weight gain over follow-up (P<.05 for each) but not with measurements of general adiposity.
Conclusion
Excess visceral fat and insulin resistance, but not general adiposity, were independently associated with incident prediabetes and type 2 diabetes mellitus in obese adults.
Amarked increase in the prevalence of overweight and obesity1 has contributed to a doubling in type 2 diabetes mellitus incidence over the past 3 decades.2 Increasing rates of diabetes among obese individuals has counterbalanced reductions in other cardiovascular disease (CVD) risk factors and is the primary factor contributing to a slowed decline in CVD event rates in the general population.3 Prediabetes, an intermediate hyperglycemia phenotype and risk factor for diabetes,4 is also associated with obesity and carries an excess risk for CVD and death.5
Although increased body mass index (BMI) is associated with diabetes at the population level,6 it does not adequately discriminate diabetes risk among obese individuals.7 Indeed, many obese persons appear resistant to the development of metabolic disease.8 Because the metabolic disease risks associated with obesity are heterogeneous, there remains an unmet clinical need for tools that differentiate obese persons who will ultimately develop prediabetes and diabetes from those who will remain metabolically healthy.
Adipose tissue dysfunction is characterized by ectopic fat deposition in the abdominal viscera and liver, inflammatory and adipokine dysregulation, and insulin resistance and may be a more important mediator of diabetes development than total fat mass in obese individuals.9–11 However, prior work has been limited by small sample sizes, homogeneous patient populations, and absence of longitudinal follow-up for diabetes incidence. Furthermore, data are lacking regarding discrimination of pre-diabetes or diabetes risk specifically in obese adults. Therefore, we investigated associations of baseline adipose tissue distribution, adipokines, lipids, and biomarkers of insulin resistance and inflammation with the risk of incident prediabetes and diabetes in a multiethnic cohort of obese adults with extensive cardiovascular, metabolic, and adipose tissue phenotyping.
METHODS
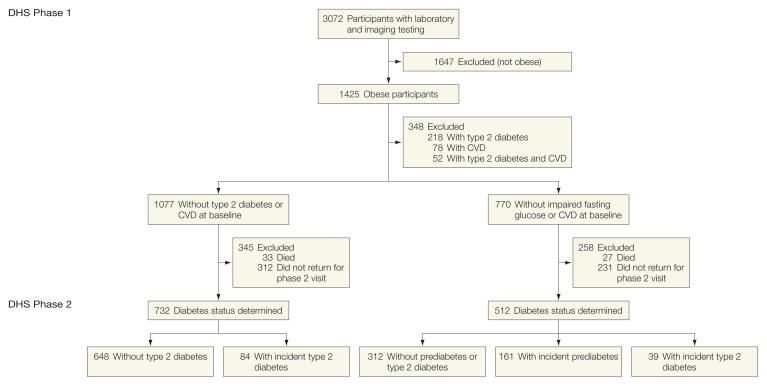
The Dallas Heart Study (DHS) is a multiethnic, probability-based, population cohort study of Dallas County adults, with deliberate oversampling of African American individuals. Detailed methods of DHS phase 1 (DHS-1) have been described previously.12 Between 2000 and 2002, 3072 participants completed DHS-1, including a detailed survey, laboratory testing, and multiple imaging studies. Among participants completing DHS-1 who were obese at enrollment (n = 1425), those with preexisting diabetes or clinical CVD (coronary heart disease [CHD], heart failure, or ischemic stroke) were excluded (n = 348), resulting in 1077 participants eligible for follow-up.
In DHS phase 2 (DHS-2), participants who completed DHS-1 underwent a follow-up survey, laboratory testing, and repeat imaging studies during a single visit to the University of Texas (UT) Southwestern Medical Center between September 2007 and December 2009. Among 1077 participants eligible for follow-up, 345 did not complete DHS-2, resulting in a final sample size of 732. There were no major differences in medical history, demographics, or biomarker data between eligible participants who did and did not complete DHS-2 (eTable 1, available at http://www.jama.com). Within this cohort, we also examined a subgroup with normal fasting blood glucose (FBG) values at baseline (n=512). All participants provided written informed consent, and the protocol was approved by the UT Southwestern institutional review board.
Type 2 Diabetes and Prediabetes Ascertainment
At baseline, diabetes was defined by prevalent medical treatment for diabetes, an FBG of 126 mg/dL or greater, or a nonfasting BG level 200 mg/dL or greater (to convert glucose to mmol/L, multiply by 0.0555). At follow-up, incident diabetes was defined by initiation of medical treatment for diabetes during the study interval, an FBG of 126 mg/dL or greater, a nonfasting BG of 200 mg/dL or greater, or glycated hemoglobin (HbA1c) 6.5% or greater, according to updated guidelines13 (HbA1c was not measured in DHS-1). No information was available regarding the time of diagnosis or onset of incident diabetes. Family history of diabetes was defined as any first-degree relative with diabetes.
At baseline, prediabetes was defined by the 2003 American Diabetes Association criteria for impaired fasting glucose (IFG) as an FBG of 100 to 125 mg/dL.14 At follow-up, incident prediabetes was defined as either new IFG with an FBG of 100 to 125 mg/dL or HbA1c of 5.7% to 6.4%.13 Oral glucose tolerance testing was not performed.
Variable Definitions
Obesity was defined as a BMI of 30 or greater, calculated as weight in kilograms divided by height in meters squared. Race/ethnicity, history of CVD, medication usage, and smoking status were self-reported. Definitions for hypertension, hypercholesterolemia, and low high-density lipoprotein (HDL) cholesterol have been previously described using conventional clinical definitions.15 Metabolic syndrome was defined and Framingham 10-year CHD risk estimates were calculated according to the National Cholesterol Education Program Adult Treatment Panel III report.16 The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated with the following: (fasting insulin [μIU/mL] × fasting glucose [mmol/L]) divided by 22.5.17 Physical activity was derived using self-reported frequency and type of leisure-time physical activity and a standard conversion for metabolic equivalence units (METs).18
Body Composition Measurements
Body surface area (BSA) was calculated using the method of Tikuisis et al.19 Waist circumference was measured 1 cm above the iliac crest and hip circumference at the widest circumference of the buttocks at the area of the greater trochanters. Dual-energy x-ray absorptiometry (Delphi W scanner, Hologic, and Discovery software version 12.2) was used to measure total body fat, lean mass, truncal fat, and lower body fat. Lower body fat was delineated by 2 oblique lines crossing the femoral necks and converging below the pubic symphysis and included gluteal-femoral fat.20
Visceral and subcutaneous abdominal fat mass were measured by 1.5-T MRI (Intera, Philips Medical Systems) using a prospectively designed and validated method of fat mass prediction from a single MRI slice at the L2-L3 intervertebral level.21 Single-slice measurement of subcutaneous and visceral fat mass at this intervertebral level has been shown to be highly concordant with total abdominal fat mass measured at all intervertebral levels (R2= 85%–96%).21 Liver fat was measured using 1.5-T proton magnetic resonance spectroscopy and is reported as a percentage of signal from fat to total signal from fat and water.22
Biomarker Measurements
Biomarkers reported in this study have been measured previously and the analytical methods described for levels of leptin,23 adiponectin,24 high-sensitivity C-reactive protein (hsCRP),25 and fructosamine.26 Particle concentrations of low-density lipoprotein (LDL), HDL, and very low-density lipoprotein (VLDL) subclasses were measured by LipoScience using nuclear magnetic resonance spectroscopy.27 In DHS-2, standard laboratory assays were used to measure cholesterol and glucose, and HbA1c was measured using an Ultra-2 affinity high-performance liquid chromatography assay (Trinity Biotech). The interassay coefficients of variation were 2.9%, 1.8%, and 1.1% at HbA1c levels of 5.1%, 8.6%, and 19.5%, respectively.
Cardiac and Vascular Imaging Measurements
Electron-beam computed tomography measurements of coronary artery calcium (CAC) were performed in duplicate on an Imatron 150 XP scanner, and the scores were averaged. Prevalent CAC was defined as a mean Agatston score greater than 10.28 Cardiac and aortic MRI were performed using 1.5-T MRI, and left ventricular mass and wall thickness, aortic compliance, and aortic plaque area and wall thickness were calculated according to previously published methods.29–31
Statistical Analysis
Characteristics were compared between participants with and without diabetes at follow-up using χ2 tests for dichotomous variables and Wilcoxon rank-sum tests for continuous variables. In the subgroup with normal FBG levels at baseline, comparisons among participants who remained free of prediabetes or diabetes, developed prediabetes, and developed diabetes were made using the Jonckheere-Terpstra trend test. Comparisons of diabetes incidence across sex-specific tertiles of visceral, abdominal subcutaneous, and total body fat mass were performed with the Jonckheere-Terpstra trend test; for the subgroup with normal FBG levels, a composite end point of prediabetes or diabetes was used. Analyses of incident diabetes stratified by median visceral fat mass and by HOMA-IR and fructosamine levels were also performed. Among participants with normal baseline FBG levels, stratified analyses were performed assessing unadjusted associations between visceral fat mass and the composite of incident prediabetes or diabetes across subgroups defined by sex, race, BMI, metabolic syndrome, and weight gain.
Multivariable logistic regression modeling using a backward selection strategy was performed to identify ide-pendent associations of baseline variables with incident diabetes. Candidate variables were selected for inclusion based on a P value less than .10 in unadjusted analyses, and those with an adjusted P value less than .05 were retained in the final model. In the subgroup with normal FBG levels at baseline, similar modeling was performed using the composite of prediabetes or diabetes as the outcome variable because of the small numbers of diabetes events in the subgroup. In addition to baseline variables, weight gain between study visits was tested in both models.
Visceral fat mass, FBG level, fructosamine level, insulin level, and HOMA-IR were log-transformed and modeled per 1-SD increment of the log-transformed variable; these SD increments were also back-transformed to provide more clinically relevant increments. For variables providing similar information (eg, VLDL particles and triglyceride levels), only the most clinically relevant measurement was tested. Cardiovascular and atherosclerosis imaging variables were not tested in the models. Adjusted absolute risk changes associated with each independent variable were estimated assuming mean levels of other covariates in the models.
For all statistical testing, a 2-sided P value less than .05 was considered statistically significant without correction for multiple comparisons. All statistical analyses were performed using SAS version 9.2 (SAS Institute).
RESULTS
Incident Diabetes
The study cohort included 732 obese participants followed up for a median period of 7 years (interquartile range [IQR], 6.6–7.6), resulting in 5207 person-years of follow-up (Figure 1). Incident diabetes developed in 84 participants (11.5%), among whom 45 (53.6%) had IFG at baseline; 12 participants were diagnosed exclusively by HbA1c criteria. At baseline, participants who subsequently developed diabetes were more likely than those who remained free of diabetes to have IFG, family history of diabetes, hypertension, and the metabolic syndrome with higher HOMA-IR, higher levels of fructosamine and triglycerides, and a higher concentration of large VLDL particles. Lower body fat mass, adiponectin levels, and large HDL and LDL particle concentrations were inversely associated with incident diabetes (Table 1 and Table 2). Follow-up characteristics of those with and without incident diabetes are shown in Table 3.
Figure 1.
Participant Selection and Follow-up
CVD indicates cardiovascular disease; DHS, Dallas Heart Study.
Table 1.
Baseline Characteristics of Obese Participants With and Without Incident Type 2 Diabetes: Demographics and Laboratory Values
| No Diabetes (n = 648) | Incident Diabetes (n = 84) | P Value | |
|---|---|---|---|
| Age, median (IQR), y | 43 (36–50) | 45 (39–53) | .02 |
|
| |||
| Male sex, No. (%) | 220 (34.0) | 38 (45.2) | .05 |
|
| |||
| Race/ethnicity, No. (%) | |||
| White | 196 (30.2) | 16 (19.0) | .04 |
|
| |||
| Black | 345 (53.2) | 50 (59.5) | .28 |
|
| |||
| Hispanic | 103 (15.9) | 17 (20.2) | .31 |
|
| |||
| Weight, median (IQR), kg | 98.3 (87.1–110.2) | 99.6 (89.2–108.9) | .51 |
|
| |||
| BMI, median (IQR)a | 34.9 (31.9–38.9) | 35.4 (33.0–39.3) | .35 |
|
| |||
| Waist circumference, median (IQR), cm | 108.5 (100.0–117.3) | 111.1 (104.0–119.5) | .04 |
|
| |||
| Waist/hip ratio, median (IQR) | 0.91 (0.85–0.97) | 0.95 (0.90–1.00) | <.001 |
|
| |||
| Impaired fasting glucose, No. (%) | 166 (25.6) | 45 (53.6) | <.001 |
|
| |||
| Family history of diabetes, No. (%) | 240 (41.5) | 50 (63.3) | <.001 |
|
| |||
| Hypertension, No. (%) | 216 (33.9) | 42 (50.6) | .003 |
|
| |||
| Systolic BP, median (IQR), mm Hg | 123 (115–134) | 131 (122–144) | <.001 |
|
| |||
| Metabolic syndrome, No. (%) | 293 (45.2) | 55 (65.5) | <.001 |
|
| |||
| Current smoking, No. (%) | 133 (20.6) | 24 (28.6) | .09 |
|
| |||
| Statin use, No. (%) | 32 (5.1) | 4 (4.9) | >.99 |
|
| |||
| Physical activity, METs × min/wkb | 99 (0–479) | 170 (0–399) | .63 |
|
| |||
| Insulin resistance, median (IQR) | |||
| Glucose, mg/dL | 93 (87–100) | 101 (92–114) | <.001 |
|
| |||
| Insulin, μU/mL | 17.5 (11.3–24) | 20.8 (14.6–30.6) | <.001 |
|
| |||
| HOMA-IR | 3.9 (2.6–5.6) | 4.8 (3.5–7.5) | <.001 |
|
| |||
| Fructosamine, μmol/L | 199 (188–210) | 211 (196–224) | <.001 |
|
| |||
| Adipokines and other, median (IQR) | |||
| Leptin, μg/L | 27.2 (13.3–41.9) | 22.5 (10.7–35.6) | .05 |
|
| |||
| Adiponectin, ng/mL | 5.9 (4.3–8.4) | 5.0 (3.4–7.8) | .04 |
|
| |||
| hsCRP, mg/L | 4.4 (2.2–9.4) | 3.6 (1.9–9.3) | .40 |
|
| |||
| Lipids, median (IQR) | |||
| Total cholesterol, mg/dL | 177 (154–203) | 181 (156–204) | .49 |
|
| |||
| HDL-C, mg/dL | 46 (39–54) | 45 (38–54) | .48 |
|
| |||
| HDL–large, μmol/Lc | 5.6 (3.6–8.0) | 4.5 (3.0–7.3) | .03 |
|
| |||
| Triglycerides, mg/dL | 99 (70–146) | 124 (90–187) | .001 |
|
| |||
| VLDL–large, nmol/Lc | 2.2 (0.8–5.6) | 4.3 (1.7–9.1) | <.001 |
|
| |||
| LDL-C, mg/dL | 108 (86–129) | 107 (83–128) | .52 |
|
| |||
| LDL–large, nmol/Lc | 423.0 (293.4–552.9) | 394.9 (239.6–498.3) | .04 |
Abbreviations: BMI, body mass index; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; METs, metabolic equivalence units; VLDL, very low-density lipoprotein.
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0555; HDL-C, LDL-C, and VLDL to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
Calculated as weight in kilograms divided by height in meters squared.
n=565 and n=74 for the no diabetes and incident diabetes groups, respectively.
Concentration of large particles.
Table 2.
Baseline Characteristics of Obese Participants With and Without Incident Type 2 Diabetes: Body Composition and Cardiovascular Phenotypes
| No Diabetes (n = 648) | Incident Diabetes (n = 84) | P Value | |
|---|---|---|---|
| DEXA fat measures, median (IQR) | |||
| Total fat mass, kg | 35.5 (29.3–43.4) | 35.3 (28.8–42.7) | .51 |
|
| |||
| Total lean mass, kg | 57.3 (50.0–67.6) | 58.84 (52.7–70.2) | .10 |
|
| |||
| Body fat, % | 40.4 (31.6–44.5) | 39.8 (28.7–43.8) | .51 |
|
| |||
| Lower body fat mass, kg | 12.6 (9.6–16.3) | 11.2 (9.0–15.1) | .02 |
|
| |||
| Truncal fat mass, kg | 17.4 (14.8–21.4) | 17.9 (15.8–21.9) | .54 |
|
| |||
| MRI fat measures, median (IQR) | |||
| Abdominal subcutaneous fat, kg | 6.5 (5.0–8.8) | 6.9 (4.8–8.9) | .88 |
|
| |||
| Abdominal visceral fat, kg | 2.4 (1.9–3.1) | 2.9 (2.5–3.4) | <.001 |
|
| |||
| Liver fat, % | 4.8 (3.1–8.7) | 8.3 (4.6–14.4) | <.001 |
|
| |||
| Cardiac and vascular MRI measures, median (IQR) | |||
| LV mass/BSA, g/m2 | 76.6 (68.3–87.3) | 82.2 (74.2–93.1) | .003 |
|
| |||
| LV wall thickness, mm | 11.6 (10.7–12.8) | 12.4 (11.2–13.6) | <.001 |
|
| |||
| Aortic compliance, mL/mm Hg | 24.4 (17.2–32.7) | 19.7 (15.1–28.2) | .01 |
|
| |||
| Subclinical atherosclerosis | |||
| Coronary artery calcium prevalence, No. (%)a | 99 (17.7) | 13 (17.6) | .94 |
|
| |||
| Aortic plaque prevalence, No. (%)b | 165 (31.8) | 31 (47.7) | .01 |
|
| |||
| Aortic wall thickness, median (IQR), mm | 1.6 (1.5–1.8) | 1.7 (1.6–1.9) | .02 |
|
| |||
| Framingham 10-y CHD risk estimate, median (IQR), % | 1 (0–2) | 2 (0–5) | .002 |
Abbreviations: BSA, body surface area; CHD, coronary heart disease; DEXA, dual-energy x-ray absorptiometry; LV, left ventricular; METs, metabolic equivalence units; MRI, magnetic resonance imaging.
n=565 and n=74 for the no diabetes and incident diabetes groups, respectively.
n=519 and n=65 for the no diabetes and incident diabetes groups, respectively.
Table 3.
Follow-up Characteristics of Obese Participants With and Without Incident Type 2 Diabetes
| Median (IQR)
|
P Value | ||
|---|---|---|---|
| No Diabetes (n = 648) | Incident Diabetes (n = 84) | ||
| Weight, kg | 100.6 (87.2 to 113.2) | 101.4 (91.6 to 115.9) | .16 |
|
| |||
| BMIa | 35.5 (32.1 to 40.1) | 35.9 (33.3 to 40.2) | .11 |
|
| |||
| Waist circumference, cm | 106.7 (96.5 to 115.6) | 111.8 (101.6 to 121.9) | .004 |
|
| |||
| Glucose, mg/dL | 94 (88 to 101) | 133 (111 to 157) | <.001 |
|
| |||
| Hemoglobin A1c, % | 5.5 (5.3 to 5.8) | 6.6 (6.2 to 7.5) | <.001 |
|
| |||
| HDL-C, mg/dL | 48 (41 to 56) | 47 (41 to 54) | .26 |
|
| |||
| Triglycerides, mg/dL | 107 (77 to 148) | 130 (101 to 172) | <.001 |
|
| |||
| Changes from baseline | |||
| Weight change, kg | 2.1 (−3.4 to 7.9) | 4.5 (−2.2 to 8.5) | .07 |
|
| |||
| BMI change | 0.4 (−1.6 to 2.6) | 1.2 (−1.1 to 2.8) | .09 |
|
| |||
| Waist circumference change, cm | −2.3 (−8.1 to 4.1) | 0.3 (−6.3 to 5.8) | .04 |
|
| |||
| Glucose change, mg/dL | 1 (−6 to 8) | 31 (5 to 54) | <.001 |
|
| |||
| HDL-C change, mg/dL | 2 (−4 to 7) | 0 (−5 to 8) | .24 |
|
| |||
| Triglycerides change, mg/dL | 6 (−21 to 33) | 16 (−27 to 50) | .23 |
Abbreviations: BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range.
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0555; HDL-C to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
Calculated as weight in kilograms divided by height in meters squared.
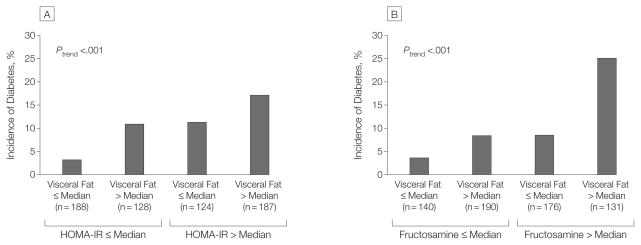
Diabetes incidence increased significantly across sex-specific tertiles of visceral fat mass (P <.001 for trend), but no association was seen for abdominal subcutaneous fat or total body fat (Table 4). Stratification by markers of insulin resistance demonstrated additive associations of visceral fat mass with both HOMA-IR (Figure 2A) and fructosamine level (Figure 2B) for incident diabetes. Baseline waist circumference, waist-to-hip ratio, and liver fat percentage were also associated with incident diabetes, but markers of general adiposity including BMI, truncal fat mass, and hsCRP level were not (Table 1 and Table 2).
Table 4.
Incidence of Prediabetes and Type 2 Diabetes Among Obese Adults Stratified by Sex-Specific Tertiles of Visceral Fat, Abdominal Subcutaneous Fat, and Total Body Fat Mass
| Tertile 1 | Tertile 2 | Tertile 3 | P Value for Trend | |
|---|---|---|---|---|
| Incident Type 2 Diabetes in Participants Without Diabetes at Baseline | ||||
| Visceral fat, mean (range), kg | ||||
| Men | 2.3 (1.3–2.8) | 3.2 (2.8–3.5) | 4.2 (3.5–7.0) | |
|
| ||||
| Women | 1.5 (0.8–1.9) | 2.2 (1.9–2.5) | 2.9 (2.5–4.5) | |
|
| ||||
| Diabetes incidence, No./Total No. (%) | 11/211 (5.2) | 21/205 (10.2) | 38/225 (16.9) | <.001 |
|
| ||||
| Abdominal subcutaneous fat, mean (range), kg | ||||
| Men | 3.5 (1.9–4.2) | 4.9 (4.2–5.8) | 8.3 (5.8–21.3) | |
|
| ||||
| Women | 5.4 (3.4–6.4) | 7.5 (6.4–8.7) | 11.2 (8.7–18.5) | |
|
| ||||
| Diabetes incidence, No./Total No. (%) | 17/202 (8.4) | 29/222 (13.1) | 24/217 (11.1) | .40 |
|
| ||||
| Total body fat, mean (range), kg | ||||
| Men | 22.3 (12.8–26.0) | 28.3 (26.0–31.0) | 37.2 (31.0–63.3) | |
|
| ||||
| Women | 30.9 (19.1–35.3) | 39.7 (35.3–44.3) | 52.1 (44.3–82.5) | |
|
| ||||
| Diabetes incidence, No./Total No. (%) | 26/223 (11.7) | 27/240 (11.3) | 27/232 (11.6) | .99 |
|
| ||||
| Incident Prediabetes or Type 2 Diabetes in Participants With Normal Fasting Glucose Values at Baseline | ||||
| Visceral fat, mean (range), kg | ||||
| Men | 2.3 (1.3–2.8) | 3.1 (2.8–3.4) | 4.0 (3.4–6.5) | |
|
| ||||
| Women | 1.5 (0.8–1.8) | 2.1 (1.8–2.3) | 2.8 (2.3–4.5) | |
|
| ||||
| Prediabetes or diabetes incidence, No./Total No. (%) | 46/145 (31.7) | 61/151 (40.4) | 68/153 (44.4) | .02 |
|
| ||||
| Abdominal subcutaneous fat, mean (range), kg | ||||
| Men | 3.6 (1.9–4.4) | 5.1 (4.4–5.9) | 8.4 (5.9–21.3) | |
|
| ||||
| Women | 5.4 (3.4–6.4) | 7.4 (6.4–8.5) | 11.0 (8.5–18.5) | |
|
| ||||
| Prediabetes or diabetes incidence, No./Total No. (%) | 52/137 (38.0) | 65/165 (39.4) | 58/147 (39.5) | .80 |
|
| ||||
| Total body fat, mean (range), kg | ||||
| Men | 22.3 (14.6–26.1) | 28.3 (26.1–31.0) | 37.0 (31.0–56.8) | |
|
| ||||
| Women | 30.9 (21.3–35.3) | 39.5 (35.4–43.7) | 51.2 (43.7–68.6) | |
|
| ||||
| Prediabetes or diabetes incidence, No./Total No. (%) | 61/162 (37.7) | 69/168 (41.1) | 60/161 (37.3) | .94 |
Figure 2.
Incidence of Type 2 Diabetes Among Obese Individuals Stratified by Sex-Specific Median Values for Visceral Fat Mass and by HOMA-IR and Fructosamine Levels
The median cut points for visceral fat mass were 3.2 kg for men and 2.2 kg for women. A, For homeostasis model assessment of insulin resistance (HOMA-IR), the median cut point was 4 units for both men and women. B, For fructosamine, the median cut points were 204 μmol/L for men and 196 μmol/L for women.
Compared with individuals who did not develop diabetes, those with incident diabetes had higher baseline Framingham 10-year CHD risk estimates, increased aortic wall thickness and aortic plaque, and decreased aortic compliance. Left ventricular mass and wall thickness were also higher at baseline in participants who subsequently developed diabetes (P <.05 for each) (Table 2).
In multivariable analysis, baseline measurements of visceral fat mass (absolute risk increase [ARI] per 1 SD [1.4 kg], 8.8%; odds ratio [OR], 2.42; 95% CI, 1.59–3.68), fructosamine level (ARI per 1 SD [1.1 μmol/L], 6.1%; OR, 1.95; 95% CI, 1.43–2.67), FBG level (ARI per 1 SD [1.1 mg/dL], 5.7%; OR, 1.88; 95% CI, 1.38–2.56), systolic blood pressure (ARI per 10 mm Hg, 2.0%; OR, 1.26; 95% CI, 1.07–1.48), and family history of diabetes (ARI, 6.8%; OR, 2.32; 95% CI, 1.25–4.29) and weight gain over follow-up (ARI per 1 kg, 0.5%; OR, 1.06; 95% CI, 1.02–1.10) were independently associated with incident diabetes (Table 5). Findings were similar when HOMA-IR was substituted for FBG level (ARI per 1 SD [1.8 units], 4.3%; OR, 1.70; 95% CI, 1.21–2.40) and were insensitive to forcing age, sex, and race into the model or to excluding participants diagnosed exclusively by HbA1c value. The final model had a C statistic of 0.85; in comparison, the C statistic of a previously published clinical model32 including BMI, IFG, family history of diabetes, HDL cholesterol, triglycerides, and hypertension was 0.71 (P <.01 for difference).
Table 5.
Factors Independently Associated With Incident Prediabetes and Type 2 Diabetes in Obese Adults
| Absolute Risk Increase, % | Odds Ratio (95% CI) | P Value | χ2 Value | |
|---|---|---|---|---|
| Incident Type 2 Diabetes in Participants Without Diabetes at Baseline | ||||
| Fasting blood glucose, per 1 SD (1.1 mg/dL)a | 5.7 | 1.88 (1.38–2.56) | <.001 | 16.1 |
|
| ||||
| Family history of diabetes | 6.8 | 2.32 (1.25–4.29) | .008 | 7.1 |
|
| ||||
| Systolic blood pressure, per 10 mm Hg | 2.0 | 1.26 (1.07–1.48) | .006 | 7.6 |
|
| ||||
| Visceral fat, per 1 SD (1.4 kg)a | 8.8 | 2.42 (1.59–3.68) | <.001 | 17.0 |
|
| ||||
| Fructosamine, per 1 SD (1.1 μmol/L)a | 6.1 | 1.95 (1.43–2.67) | <.001 | 17.7 |
|
| ||||
| Weight gain, per 1 kg | 0.5 | 1.06 (1.02–1.10) | .002 | 9.8 |
|
| ||||
| Incident Prediabetes or Type 2 Diabetes in Participants With Normal Fasting Glucose Values at Baseline | ||||
| Fasting blood glucose, per 1 SD (1.1 mg/dL)a | 9.4 | 1.66 (1.29–2.12) | <.001 | 16.0 |
|
| ||||
| Nonwhite race | 9.7 | 1.77 (1.08–2.91) | .02 | 5.2 |
|
| ||||
| Family history of diabetes | 8.9 | 1.60 (1.05–2.44) | .03 | 4.8 |
|
| ||||
| Age, per 10 y | 7.2 | 1.48 (1.17–1.86) | .001 | 10.9 |
|
| ||||
| Visceral fat, per 1 SD (1.4 kg)a | 7.3 | 1.48 (1.17–1.88) | .001 | 10.8 |
|
| ||||
| Fructosamine, per 1 SD (1.1 μmol/L)a | 6.5 | 1.42 (1.14–1.75) | .001 | 10.2 |
|
| ||||
| Insulin, per 1 SD (1.7 μU/mL)a | 5.7 | 1.34 (1.06–1.70) | .01 | 6.1 |
|
| ||||
| Weight gain, per 1 kg | 1.2 | 1.08 (1.05–1.10) | <.001 | 40.9 |
Incremental change equivalent to a 1-SD difference in the log-transformed continuous variable.
Participants With Normal FBG Levels at Baseline
Among 512 individuals with normal FBG levels (<100 mg/dL) at baseline, 161 (31.4%) subsequently developed prediabetes and 39 (7.6%) progressed to diabetes (Figure 1); 67 participants were diagnosed with prediabetes exclusively by HbA1c measurement. Within this subgroup, graded associations were observed with visceral fat mass, waist circumference, waist-to-hip ratio, and liver fat percentage between participants who remained normoglycemic, those who developed prediabetes, and those who progressed to diabetes (P <0.01 for trend for each) (eTable 2). Lower body fat mass and adiponectin level showed graded, inverse associations with incident prediabetes and diabetes (P ≤.01 for trend for each) (eTable 2). In contrast, general adiposity markers including BMI, abdominal subcutaneous fat mass, and hsCRP level were not associated with incident prediabetes or diabetes (eTable 2). The median change in body weight for participants who did not develop prediabetes or diabetes was 1.6 kg (IQR, −4.1 to 7.7) vs 4.5 kg (IQR, −0.5 to 10.5) for those who developed prediabetes and 7.2 kg (IQR, 3.5 to 17.4) for those who progressed to diabetes (P <.001 for trend) (eTable 2).
When participants were divided into sex-specific tertiles of visceral fat, subcutaneous abdominal fat, and total body fat, a graded association across tertiles of visceral fat was observed for the composite of prediabetes or diabetes (P =.02 for trend), but no association was seen across tertiles of subcutaneous or total body fat (Table 4). Visceral fat mass demonstrated similar associations with the composite of incident prediabetes or diabetes across subgroups defined by sex, race, obesity class, presence of metabolic syndrome, and weight gain, with no interactions detected (eFigure).
In multivariable analysis, higher visceral fat mass (ARI per 1 SD [1.4 kg], 7.3%; OR, 1.48; 95% CI, 1.17–1.88), fructosamine level (ARI per 1 SD [1.1 μmol/L], 6.5%; OR, 1.42; 95% CI, 1.14–1.75), and insulin level (ARI per 1 SD [1.7 μU/mL], 5.7%; OR, 1.34; 95% CI, 1.06–1.70) were independently associated with the composite of incident pre-diabetes or diabetes among participants with normal FBG levels at baseline (Table 5). Other significant associations were seen for age (ARI per 10 years, 7.2%; OR, 1.48; 95% CI, 1.17–1.86), nonwhite race (ARI, 9.7%; OR, 1.77; 95% CI, 1.08–2.91), family history of diabetes (ARI, 8.9%; OR, 1.60; 95% CI, 1.05–2.44), FBG level (ARI per 1 SD [1.1 mg/dL], 9.4%; OR, 1.66; 95% CI, 1.29–2.12), and weight gain (ARI per 1 kg, 1.2%; OR, 1.08; 95% CI, 1.05–1.10). Similar findings were seen when HOMA-IR was substituted for FBG and insulin levels (ARI per 1 SD [1.8 units], 9.2%; OR, 1.64; 95% CI, 1.30–2.07), when participants diagnosed exclusively by HbA1c criteria were excluded, or when participants prescribed weight-modifying diabetic medications (insulin, thiazolidin-ediones, or metformin) during follow-up were excluded. The final model for the composite of prediabetes or diabetes incidence in this subgroup had a C statistic of 0.79.
COMMENT
Among obese individuals without prevalent CVD, a dysfunctional adiposity phenotype, characterized by excess visceral fat and biomarkers of insulin resistance, was independently associated with the development of prediabetes and diabetes. Even among individuals with normal FBG levels at baseline, graded associations were observed between those who subsequently developed prediabetes and those who developed frank diabetes, suggesting a spectrum of ectopic visceral fat deposition and insulin resistance among obese persons. In contrast, we show that markers of general adiposity that are associated with diabetes in the general population, such as BMI, total body fat, and abdominal subcutaneous fat, were not associated with prediabetes or diabetes incidence in this obese population. These findings suggest that clinically measurable markers of adipose tissue distribution and insulin resistance may be useful in prediabetes and diabetes risk discrimination among obese individuals and support the notion of obesity as a heterogeneous disorder with distinct adiposity subphenotypes.
Adiposity Phenotypes and the Transition to Diabetes
Prior cross-sectional studies have reported a strong correlation between visceral fat and insulin resistance in obese white11 and African American populations.33 However, studies of incident diabetes have been limited to ethnically homogeneous and primarily non-obese populations.32,34 Our findings confirm observations from the Framing-ham Heart Study32 (mean BMI, 27) that hypertension, hyperglycemia, and family history of diabetes were independently associated with incident diabetes. Additionally, we found that visceral adiposity, increased liver fat, decreased lower body fat, insulin resistance, elevated triglycerides, and low adiponectin levels were associated with incident prediabetes and diabetes in obese individuals while markers of general adiposity were not.
To our knowledge, only a single prospective study (performed in non-obese Japanese American individuals) has examined the association of abdominal fat distribution with incident diabetes.10 In that study, visceral adipose tissue area, characterized by computed tomography, was independently associated with diabetes while markers of general adiposity demonstrated weaker and inconsistent associations. Our results confirm that visceral, but not general, adiposity was independently associated with incident diabetes in a diverse population of obese individuals with a high proportion of women and African American participants while extending this knowledge to both incident prediabetes and diabetes. Importantly, although women and African American individuals have less visceral fat than men and white individuals, respectively,35 we observed similar associations of visceral fat with prediabetes and diabetes incidence across subgroups defined by sex and race.
Fasting glucose is known to be an insensitive measure of insulin resistance in obese persons.13 Notably, we found that even among obese individuals with normal FBG levels at enrollment, those who subsequently developed prediabetes or diabetes had baseline evidence of insulin resistance (higher HOMA-IR) and impaired intermediate-term glycemic control (higher fructosamine level), with moderate elevations in HOMA-IR and fructosamine among those who developed prediabetes and more marked elevations in those who developed diabetes. These findings suggest that prediabetes may represent a true intermediate phenotype between metabolically healthy obesity and diabetes.
The mechanisms behind the transition from functional to dysfunctional adiposity are not well understood. Subcutaneous adipose tissue acts as a functional site of fat storage; accumulation of fat leads to hyperplastic expansion of the subcutaneous compartment and ensuing obesity. However, the amount of subcutaneous fat might not differ between insulin-sensitive and insulin-resistant individuals.36 In fact, subcutaneous fat mass transplantation into rodents has beneficial metabolic effects, suggesting that the expandability of subcutaneous fat may be a critical factor in maintaining healthy obesity.37 A deficient expansion of the subcutaneous fat depot may promote ectopic fat deposition with excessive free fatty acid and adipokine release leading to lipotoxicity and insulin resistance in muscle, liver, and pancreatic β cells. This may be especially apparent in obese persons in whom functional fat storage is overwhelmed by excess energy input. In these individuals, ectopic fat deposition in the viscera and liver may indicate deficient fat storage capacity in subcutaneous adipose tissue.38
Understanding Metabolically Healthy Obesity
Our study may have implications for understanding differences between metabolically healthy and pathologic obesity.39 The current findings suggest that a more metabolically healthy obesity phenotype is associated with decreased fat deposition in the abdominal viscera, increased lower body subcutaneous fat storage, insulin sensitivity, increased adiponectin, and a favorable lipoprotein profile characterized by larger HDL and LDL particles. Importantly, we observed that BMI, total body fat, and abdominal subcutaneous fat mass did not differ between the 2 groups, suggesting that resistance to diabetes in these individuals may be explained by the ability to shunt excess fat away from visceral and other ectopic sites and preferentially deposit it in the lower body subcutaneous compartment. Indeed, participants who remained free from prediabetes and diabetes in our study had more lower body subcutaneous fat than those who developed metabolic disease. This key finding supports prior cross-sectional data20 suggesting that lower body subcutaneous fat may protect against adiposity-associated metabolic disease. However, the biological factors that determine whether an individual obese person will favor visceral vs expandable subcutaneous storage are unknown and remain an essential area for further research.
Adiposity Phenotypes and Cardiovascular Risk
Although participants with clinically evident CVD were excluded from our study, we observed a more adverse cardiovascular risk profile and evidence of greater subclinical CVD at baseline among obese individuals who subsequently developed prediabetes or diabetes. Participants who developed pre-diabetes or diabetes had only slightly higher 10-year estimated CHD risk at baseline, yet we observed a higher baseline prevalence of CAC, aortic plaque, and left ventricular hypertrophy and greater aortic wall thickness and lower aortic compliance among those who subsequently developed metabolic disease. These findings raise the possibility that in addition to effects on metabolic parameters, visceral fat deposition and insulin resistance may contribute directly, indirectly, or both to subclinical CVD and adverse cardiac and vascular remodeling prior to the clinical manifestations of metabolic disease.
Strengths and Limitations
Strengths of the current study include a diverse sample of adults applicable to the general obese population, extensive and detailed phenotyping using advanced imaging and laboratory techniques, and longitudinal follow-up in a prospective cohort. Limitations include the absence of glucose tolerance testing in the DHS and lack of HbA1c measurements in DHS-1. In addition, the number of diabetes events was modest and information was not available with regard to time of prediabetes or diabetes onset. Findings are not necessarily generalizable to individuals older than 65 years or those of Asian descent/ethnicity.
Clinical Implications
In a multiethnic, population-based sample of obese adults, a dysfunctional adiposity phenotype, characterized by excess visceral fat and insulin resistance, identified obese individuals at risk for prediabetes and diabetes, whereas markers of general adiposity did not. Identification of high-risk obese individuals in the clinical setting is an important but elusive goal. Because the metabolic consequences of obesity are not predictable based on simple anthropometric measurements,40 new tools are needed to identify appropriate candidates for intensive life style modification and therapeutic interventions. In addition, therapies for obesity such as bar-iatric surgery or pharmacologic treatment may be tailored to individuals at greatest risk of developing diabetes.
The inclusion of adipose distribution assessment in our multivariable model yielded robust discrimination of diabetes incidence (C statistic, 0.85), outperforming a clinical model developed previously in a white, nonobese population.32 Further research is needed to determine whether assessment of adipose tissue distribution and function using imaging tools, circulating biomarkers, or both can improve clinical risk prediction in obese individuals. Moreover, the present findings also suggest that the development of novel therapies that modify adipose tissue distribution may improve metabolic and cardiovascular outcomes in obese individuals. The association between weight gain and incident prediabetes and diabetes in our cohort suggests that preventing weight gain, even among those already obese, may favorably affect metabolic health independent of baseline adipose tissue distribution.
Supplementary Material
Acknowledgments
Funding/Support: Grant support for the Dallas Heart Study was provided by the Donald W. Reynolds Foundation and by US Public Health Service General Clinical Research Center grant M01-RR00633, with additional support for the present study provided by grants UL1DE019584 and PL1DK081182 from the National Institutes of Health (NIH). Dr Neeland is supported by award T32HL007360 from the National Heart, Lung, and Blood Institute (NHLBI). Dr Powell-Wiley is funded by the Division of Intramural Research of the NHLBI of the NIH. Biomarker measurements were supported by investigator-initiated grants from Alere and Roche Diagnostics.
Role of the Sponsor: Alere, Roche Diagnostics, and the NIH had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Online-Only Material: The eTables and eFigure are available at http://www.jama.com.
Author Contributions: Dr de Lemos had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Neeland, Vega, Farzaneh-Far, McGuire, de Lemos.
Acquisition of data: Neeland, Vega, McGuire, de Lemos.
Analysis and interpretation of data: Neeland, Turer, Ayers, Powell-Wiley, Vega, Farzaneh-Far, Grundy, Khera, McGuire, de Lemos.
Drafting of the manuscript: Neeland, de Lemos.
Critical revision of the manuscript for important intellectual content: Neeland, Turer, Ayers, Powell-Wiley, Vega, Farzaneh-Far, Grundy, Khera, McGuire, de Lemos.
Statistical analysis: Neeland, Ayers, Farzaneh-Far.
Obtained funding: Grundy, McGuire, de Lemos. Administrative, technical, or material support: Neeland, Grundy, Khera, de Lemos.
Study supervision: Khera, McGuire, de Lemos.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr McGuire reported having received consulting income from F. Hoffmann LaRoche, Genentech, sanofiaventis, Daiichi Sankyo, Novo Nordisk, and Tethys Bioscience. Dr de Lemos reported having received grant support from Roche Diagnostics, Abbott Diagnostics, and Alere; consulting income from Tethys Bioscience, AstraZeneca, and Daiichi Sankyo; and lecture honoraria from Bristol-Myers Squibb/sanofiaventis. No other disclosures were reported.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D’Agostino RB., Sr Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation. 2006;113 (25):2914–2918. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 3.Wijeysundera HC, Machado M, Farahati F, et al. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994–2005. JAMA. 2010;303(18):1841–1847. doi: 10.1001/jama.2010.580. [DOI] [PubMed] [Google Scholar]
- 4.Garber AJ, Handelsman Y, Einhorn D, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia: when do the risks of diabetes begin? a consensus statement from the American College of Endocrinology and the American Association of Clinical Endocrinologists. Endocr Pract. 2008;14(7):933–946. doi: 10.4158/EP.14.7.933. [DOI] [PubMed] [Google Scholar]
- 5.Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation. 2007;116(2):151–157. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G European Group for the Study of Insulin Resistance (EGIR) Insulin resistance and hyper-secretion in obesity. J Clin Invest. 1997;100(5):1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168(15):1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96(11):E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23(4):465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 11.Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study. Obesity (Silver Spring) 2010;18(11):2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Victor RG, Haley RW, Willett DL, et al. Dallas Heart Study Investigators. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93(12):1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 15.Deo R, Khera A, McGuire DK, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44(9):1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 19.Tikuisis P, Meunier P, Jubenville CE. Human body surface area: measurement and prediction using three dimensional body scans. Eur J Appl Physiol. 2001;85(3–4):264–271. doi: 10.1007/s004210100484. [DOI] [PubMed] [Google Scholar]
- 20.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91(11):4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 21.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65(2):403–408. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- 22.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276(5 pt 1):E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 23.Abdullah SM, Khera A, Leonard D, et al. Sex differences in the association between leptin and CRP: results from the Dallas Heart Study. Atherosclerosis. 2007;195(2):404–410. doi: 10.1016/j.atherosclerosis.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Turer AT, Khera A, Ayers CR, et al. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54(10):2515–2524. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khera A, Vega GL, Das SR, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94(9):3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdullah SM, Khera A, Das SR, et al. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of n-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (the Dallas Heart Study) Am J Cardiol. 2005;96(9):1284–1289. doi: 10.1016/j.amjcard.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 27.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Jain T, Peshock R, McGuire DK, et al. Dallas Heart Study Investigators. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44(5):1011–1017. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 29.Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46(1):124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 30.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304(22):2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohatgi A, Ayers CR, Khera A, et al. The association between peptidoglycan recognition protein-1 and coronary and peripheral atherosclerosis: observations from the Dallas Heart Study. Atherosclerosis. 2009;203(2):569–575. doi: 10.1016/j.atherosclerosis.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167(10):1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt MI, Duncan BB, Bang H, et al. Atherosclerosis Risk in Communities Investigators. Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28(8):2013–2018. doi: 10.2337/diacare.28.8.2013. [DOI] [PubMed] [Google Scholar]
- 35.Cornier MA, Després JP, Davis N, et al. American Heart Association Obesity Committee of the Council on Nutrition; Physical Activity and Metabolism; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing, Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease, and Stroke Council. Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124(18):1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 36.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002;282 (3):E657–E663. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 37.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans. 2008;36(pt 5):935–940. doi: 10.1042/BST0360935. [DOI] [PubMed] [Google Scholar]
- 39.Blüher M. The distinction of metabolically “healthy” from “unhealthy” obese individuals. Curr Opin Lipidol. 2010;21(1):38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- 40.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25 (7):1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.