Abstract
Two children presented with autoimmune alternating hypo- and hyperthyroidism related to the presence of blocking and stimulating thyroid antibodies. It was difficult to control their thyroid function adequately with an appropriate single drug regimen, and both children underwent total thyroidectomy with subsequent stable management with levothyroxine replacement therapy postsurgically. Although this phenomenon is well described in adults, this report is the first of such occurrence in children. The possible mechanism for the variation in the type of clinical presentation and options for management are discussed.
Keywords: blocking antibody, stimulating antibody, hashitoxicosis, ophthalmopathy, thyroidectomy
Background
Common presentations of autoimmune thyroid disease include hypothyroidism due to Hashimoto’s thyroiditis, in which the gland is destroyed by cellular immunity, and hyperthyroidism due to Graves’ disease, in which the gland is chronically stimulated by antibody. Here we consider 2 immune-mediated cases in which there was evidence of both hypo- and hyperthyroidism. The first patient presented with autoimmune hypothyroidism and then alternating hyper- and hypothyroidism and the second patient manifested with hyperthyroidism and reverted to hypo- and then again to hyperthyroidism with minimal adjustment in medications. Both thyroid-stimulating hormone (TSH)-binding inhibitor immunoglobulin (TBII) and thyroid stimulating antibodies (TSAb) are usually demonstrated in adult patients with Graves disease,1 whereas TSAb are not demonstrated in hypothyroid patients with blocking antibodies.2 Takeda et al3 suggested the possibility that both types of TSH-receptor antibodies may coexist in one patient, and his or her thyroid function may change depending on the alteration in balance between these 2 types of antibodies. Although this situation is known in adults, to our knowledge, this is the first report of both types of presentation in children.
Case 1
A girl at 5.25 years presented for evaluation of hypothyroidism. Labs had been obtained by her primary care physician because of family report of increased weight gain. At that time, laboratory data showed a TSH of >100 μU/mL and total thyroxin of 3.3 ng/mL (normal: 5.5-12.3 ng/mL; see Table 1). She was initiated on Synthroid 25 μg daily and based on thyroid function test results, the dose gradually increased to 88 μg/d. Her thyroid labs became normal within 2 months of starting therapy. Clinically, she did lose some weight and her mother noted that the child appeared to have more energy. There was no family history of thyroid disease or any known autoimmune disease. Physical exam demonstrated her height at 114.4 cm (90th percentile) and weight at 33.3 kg (>95th percentile). Physical exam was unremarkable, including no thyromegaly. She tested negative for thyroid antibodies commonly associated with Hashimoto’s thyroiditis, including antithyroid peroxidase and antithyroglobulin.
Table 1.
Case 1: Thyroid Function Test Profile and Management
| Age (Years) | TSH (μU/mL) | T4 (μg/dL) | F T4 (ng/dL) | T3 (ng/dL) | Treatment |
|---|---|---|---|---|---|
|
| |||||
| Normal = 0.3-5.0 | Normal = 5.5-12.3 | Normal = 0.6-1.6 | Normal = 30-160 | ||
| 5.25 | >100 | 3.3 | Thyroxin 25 μg/d and gradually adjusted to 88 μg/d | ||
| 5.5 | <0.03 | 15.8 | 1.99 | 224 | Off treatment |
| 6.2 | 0.86 | 1.27 | Off treatment | ||
| 6.8 | <0.01 | 3.1 | Started MTZ 0.7 mg/kg/d | ||
| 6.9 | 12.6 | 0.91 | Reduced MTZ dose to 0.4 mg/kg/d. Recommended thyridectomy | ||
Abbreviations: TSH, thyroid-stimulating hormone; MTZ, methimazole.
About 3 months into treatment, she was noted to have hyperthyroxinemia and undetectable TSH on follow-up monitoring. She had no new clinical findings suggestive of hyperthyroidism, and she was slowly weaned off thyroxine replacement. After cessation of therapy, her free thyroxine remained slightly elevated. These levels remained just out of range over the next month. She then had a technetium scan of her thyroid, which showed homogeneously increased uptake throughout the right and left lobes of the thyroid. She was initiated on methimazole (MTZ) for a short period during which time she developed hypothyroidism.
Because of the unexpected switch from hypothyroidism to hyperthyroidism and back to hypothyroidism, she underwent thyrotropin receptor antibody testing. Lab results revealed elevated thyroid-stimulating immunoglobulins (TSI) at 224 IU (normal adult <125 IU). The thyroid-binding inhibitory immunoglobulin (TBII) test also came back as elevated at 33 IU (normal = 0-14 IU). Her thyroglobulin antibodies were <0.3 U/mL (normal = 0-0.2 U/mL) and thyroid peroxidase antibody was 0.8 U/mL (normal = 0.-2.0 U/mL).
Her TSH and free T4 levels became normal in the next 2 months in the absence of any treatment. However, she then developed bouts of tachycardia and faintness. Repeat labs demonstrated free T4 of 3.1 ng/dL and TSH <.01 μU/mL. She was treated with MTZ 10 mg per os twice a day for the next 2 months, which was stopped when she became hypothyroid again (Figure 1).
Figure 1.
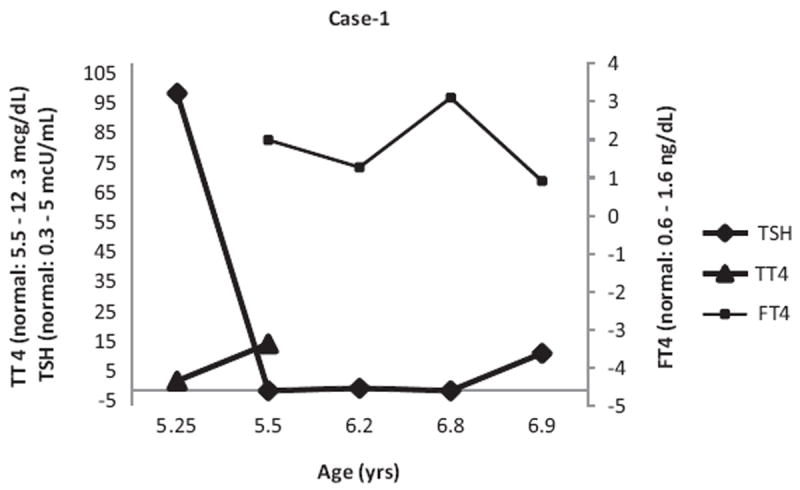
TSH, total T4, Free T4 values in Case 1
Subsequently, she had another bout of normal thyroid function followed by hyperthyroidism, detected both biochemically and symptomatically. Because of the need for frequent monitoring as well as anxiety of her parents about these episodes, thyroidectomy was performed. Thyroid labs and clinical status normalized on thyroid hormone replacement thereafter.
Case 2
An 8-year-old girl was evaluated for hyperthyroidism due to weight loss over the previous 6 months, increased hunger, and excessive tiredness. On evaluation in the clinic, she was fidgety, had thyromegaly of about 5 times the normal adult size (100 g), had resting tachycardia (110 beats/min), had no exophthalmos, and was prepubertal. Her thyroid function tests (TFT) at referral by the primary care physician showed TSH = 0.01 μIU/mL (normal = 0.5-4.3 μIU/mL) and free T4 (FT4) = 4.8 ng/dL (normal = 0.9-1.6 ng/dL; see Table 2). Repeat TFT at initial evaluation confirmed hyperthyroidism (TSH = 0.04 μU/mL, free T4 = 4.79 ng/dL). TSI qualitative = 183% (normal: <130%), antithyroglobulin antibody = 6753 U/mL (normal = 0-40 U/mL) and antithyroid peroxidase antibody = 3065 U/mL (normal = 0-40 U/mL) were elevated. She was initiated on MTZ 0.7 mg/kg/d and propranolol. Propranolol was discontinued about 2 weeks later when the sleeping pulse rate was <80 per minute. She was compliant with her medications.
Table 2.
Case 2: Thyroid Function Test Profile and Management
| Age (Years) | TSH (μU/mL) | Free T4 (ng/dL) | T3 (ng/dL) | Treatment |
|---|---|---|---|---|
|
| ||||
| Normal = 0.3-5.0 | Normal = 0.9-1.6 | Normal = 127-221 | ||
| 8.00 | 0.01 | 4.8 | MTZ 0.74 mg/kg/d. Propranolol for 2 weeks | |
| 8.20 | 97.24 | 0.4 | MTZ reduced to 0.25 mg/kg/d | |
| 8.25 | 2.67 | 1.1 | 310 | MTZ 0.25 mg/kg/d continued |
| 8.35 | 0.04 | 1.81 | MTZ increased to 0.7 mg/kg/d | |
| 8.45 | 110.41 | 0.6 | 94 | MTZ reduced to 0.25 mg/kg/d |
| 8.65 | 6.88 | 0.9 | 216 | MTZ reduced to 0.125 mg/kg/d |
| 8.75 | 0.05 | 1.9 | 318 | MTZ 0.125 mg/kg/d |
| 8.90 | 0.01 | 2.5 | MTZ went back to 0.7 mg/kg/d | |
| 9.00 | 35.29 | 0.2 | 47 | MTZ reduced to 0.25 mg/kg/d |
| 9.10 | 0.80 | 1.6 | 406 | MTX increased to 0.4 mg/kg/d. Recommended thyroidectomy |
Abbreviations: TSH, thyroid-stimulating hormone; MTZ, methimazole.
Repeat TFT 1 month after initiation of MTZ showed normal free T4 (1.0 ng/dL), but suppressed TSH (<0.01 μU/mL). By 2 months into treatment, while on the same dose of MTZ, her TSH became elevated (97.24 μU/mL) with low FT4 (0.4 ng/dL). MTZ dose was reduced to 0.25 mg/kg/d. Repeat TFT 2 weeks later while on reduced dose of MTZ showed slightly elevated T3, but normal FT4 and TSH (T3 = 310 ng/dL; FT4 = 1.1 ng/dL; TSH = 2.67 μU/mL). She was evaluated in the clinic 3 weeks later and was noticed to be fidgety and unable to sit still. She gained 2.16 kg in the interim since diagnosis. TFT revealed recurrence of thyrotoxicosis (FT4 = 1.81 ng/dL; TSH: 0.04 mcU/mL). MTZ dose was increased back up to 0.7 mg/kg/d (Figure 2). She has had few episodes of recurrence of hypothyroidism while on standard MTZ dose and resurgence of hyperthyroidism when the MTZ dose was reduced. During the period of hypothyroidism, she became more tired and goiter reappeared and when hyperthyroid she became very fidgety and hyperactive. She was demonstrated to have elevated thyrotropin receptor-binding inhibitory immunoglobulin (TBII) at 94% (normal = <16%), TSI at 247 % (normal: <125 %); and thyrotropin receptor antibody >40 IU/L (normal = <1.75 IU/L) confirming the presence of both thyroid stimulating and thyrotropin receptor blocking antibodies accounting for her swings in clinical presentation and thyroid function tests while on modest doses of MTZ. Because of her age, parental preference, and size of the thyroid goiter, she underwent total thyroidectomy, about 16 months after her initial diagnosis of Graves’s disease. Since surgery she has done well on levothyroxine supplementation with fewer blood tests, fewer clinic visits, and less worry and anguish to the patient and her parents alike.
Figure 2.
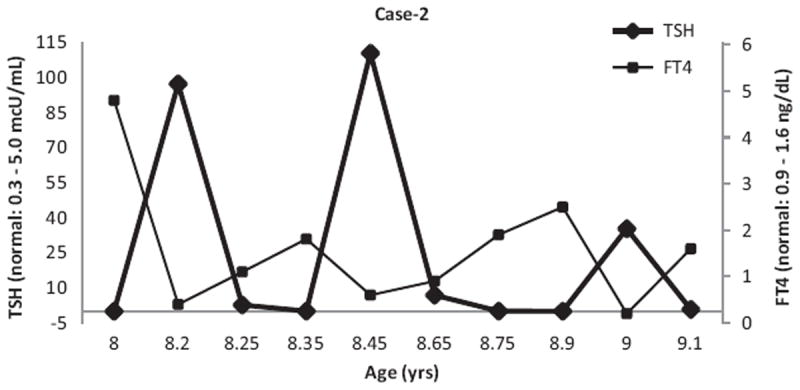
TSH and Free T4 values in Case 2
Discussion
One child each initially received a presumptive diagnosis of antibody-negative and antibody-positive Hashimoto’s thyroiditis leading to hypothyroidism and antibody-positive thyrotoxicosis, respectively. Neither child had evidence of ophthalmopathy. When they subsequently developed hyperthyroidism and hypothyroidism, respectively, those diagnoses were questioned as hashitoxicosis would more commonly precede the development of hypothyroidism and it is very unusual for those patients to go back to hyperthyroidism again. Overtreatment or lack of compliance with treatment was also possible, but their parents were reliable and made sure that both children complied with their treatment. Antibody testing revealed both stimulating and blocking antibodies in both children. Presumably, changes in the relative proportions/affinities of these antibodies over time gave rise to the transient, but recurrent, nature of their symptoms. Ultimately, thyroidectomy prevented further bouts of hyperthyroidism and hypothyroidism, simplified management for family and providers and reduced the overall cost while increasing the efficiency and convenience of care for the patients and their families.
Blocking TSH receptor antibodies (TSHRAb) are found in a significant number (18.5%) of patients with untreated hyperthyroid Graves disease in adults, and the presence of blocking antibodies is associated with clinically significant ophthalmopathy.4 Neither of our patients had significant ophthalmopathy. The association of blocking TSHRAb with ophthalmopathy may be an epiphenomenon reflecting the presence of a more severe degree of autoimmune response against the TSH receptor (TSHR) in patients with ophthalmopathy than in patients without ophthalmopathy.5 Thus patients with ophthalmopathy would have higher titers of both stimulating and blocking autoantibodies for TSHR, and in general, these patients would have severe immunologic perturbations targeting the TSHR. It is also possible that Graves’s patients with ophthalmopathy may have qualitatively different TSHRAb from those without ophthalmopathy. Blocking TSHRAb activity is preserved in this group relative to TBII activity and the 2 are associated with one another.6
Studies from multiple laboratories6,7 have demonstrated that stimulating TSHRAb differ from TSHRAb that inhibit TSH binding (TBIIs) or block TSH activity; and that Graves TBIIs do not inhibit stimulating TSHRAb activity.8 Some, but not all, TBIIs are functional and can activate the inositol phosphate signal transduction system.9 TBIIs associated with hypothyroidism were shown to be different from Graves TBIIs.10,11 and were shown to have their functional epitope on the C- rather than the N-terminus of TSHR extracellular domain.12 It is shown that there are 2 different TBII populations in Graves patients.13 One termed type 1 Graves TBII is unlikely to affect the stimulating TSHRAb activity to induce hyperthyroidism but could influence the course of disease during therapy. As the stimulating TSHRAb are decreased by MTZ and as TSH levels return to normal, this type of TBII could cause hypothyroidism by blocking TSH action.11 Type 2 Graves TBII target a different functional epitope from type 1 TBII, residues between 24 and 89 instead of residues between 90 and 165. These antibodies could have important functional consequences in increasing iodide efflux, increasing iodination of thyroglobulin by activating the peroxidase system, and increasing thyroid growth in concert with antibodies increasing cAMP levels.14 Graves’s and Hashimoto’s patients have a diversity of IgGs.15 Variations in the type of TBII might explain the variations in clinical presentations in our 2 patients. It has been suggested6 that the term blocking TSHRAb is best reserved for the TBII autoantibodies that inhibit both stimulating TSHRAb and TSH activities and that a Graves TBII should not be equated with it, because it has a different functional epitope and is not a blocker of stimulating TSHRAb activity.
Management of children with autoimmune alternating hypo- and hyperthyroidism is challenging both from a medical and familial perspective. We would have considered I-131 ablation of the thyroid rather than surgery if the children were older (>10 years). Moreover, as alternating hyper- and hypothyroidism are more commonly associated with opthalmopathy,4 radio-iodine therapy may involve some risk of appearance, progression or worsening of ophthalmopathy although this association remains controversial.16,17 One of the major advantages of surgical treatment that appeals to many children and their families is the rapid reversal of symptoms, especially thyrotoxic symptoms, whereas a 6- to 12-week delay in symptom resolution is not unusual for patients receiving radio-iodine therapy.18 Adding levothyroxine along with usual MTZ dose for their treatment would have been another option but was not considered because each of those 2 patients presented with typical primary hypothyroidism and Graves disease, respectively, initially and went on to develop the opposite phase during treatment. Titration of individual drugs to achieve euthyroid status would have required frequent office visits and blood draws with added worries, expense, and inconvenience to the individual child and her parents. The oscillating thyroid status also produced frustration in the parents, and they were reluctant to engage in further medical therapy when definitive therapy was available. Once both blocking and stimulating antibodies were confirmed as the reason for their alternating clinical presentation, thyroidectomy became the obvious choice to achieve a more permanent definitive solution, and this procedure subsequently reduced the frequency of hospital or clinic visits, blood tests, and the inconvenience and expense associated with those steps.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:DJM was supported by Ruth Kirschstein NRSA Fellowship 1F32DK083161.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Miyauchi A, Amino N, Tamaki H, Kuma K. Coexistence of thyroid-stimulating and thyroid blocking antibodies in a patient with Graves disease who had transient hypothyroidism. Am J Med. 1988;85:418–420. doi: 10.1016/0002-9343(88)90598-0. [DOI] [PubMed] [Google Scholar]
- 2.Kasagi K, Takeda K, Goshi K, et al. Presence of both stimulating and blocking types of TSH-receptor antibodies in sera from three patients with primary hypothyroidism. Clin Endocrinol (Oxf) 1990;32:253–260. doi: 10.1111/j.1365-2265.1990.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 3.Takeda K, Takamatsu J, Kasagi K, et al. Development of hyperthyroidism following primary hypothyroidism: a case report with changes in thyroid-related antibodies. Clin Endocrinol (Oxf) 1988;28:341–344. doi: 10.1111/j.1365-2265.1988.tb03664.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim WB, Chung HK, Park YJ, et al. The prevalence and clinical significance of blocking thyrotropin receptor antibodies in untreated hyperthyroid Graves disease. Thyroid. 2000;10:579–586. doi: 10.1089/thy.2000.10.579. [DOI] [PubMed] [Google Scholar]
- 5.Beck K. Thyroid antibodies in endocrine ophthalmopahty. A review Acta Endocrinol (Copenh) 1989;121:117–122. [Google Scholar]
- 6.Kohn LD, Suzuki K, Hoffman WH, et al. Characterization of monoclonal thyroid-stimulating and thyrotropin binding-inhibiting autoantibodies from a Hashimoto’s patient whose children had intrauterine and neonatal thyroid disease. J Clin Endocrinol Metab. 1997;82:3998–4009. doi: 10.1210/jcem.82.12.4433. [DOI] [PubMed] [Google Scholar]
- 7.Yavin E, Yavin Z, Schneider MD, Kohn LD. Monoclonal antibodies to the thyrotropin receptor: implications for receptor strucuture and the action of autoantibodies in Graves disease. Proc Natl Acad Sci U S A. 1981;78:6680–6684. doi: 10.1073/pnas.78.5.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ealey PA, Kohn LD, Ekins RP, Marshall NJ. Characterization of monoclonal antibodies derived from lymphocytes from Graves disease patients in a cytochemical bioassay for thyroid stimulators. J Clin Endocrinol Metab. 1984;58:909–914. doi: 10.1210/jcem-58-5-909. [DOI] [PubMed] [Google Scholar]
- 9.Kohn LD, Alvarez F, Marcocci C, et al. Monoclonal antibody studies defining the origin and properties of autoantibodies in Graves disease. Ann N Y Acad Sci. 1986;475:157–173. doi: 10.1111/j.1749-6632.1986.tb20865.x. [DOI] [PubMed] [Google Scholar]
- 10.Tahara K, Ban T, Minegishi T, Kohn LD. Immunoglobulins from Graves disease patients interact with different sites on TSH receptor/LH-CG receptor chimera than either TSH or immunoglobulins from idiopathic myxedema patients. Biochem Biophys Res Commun. 1991;179:70–77. doi: 10.1016/0006-291x(91)91335-a. [DOI] [PubMed] [Google Scholar]
- 11.Kim WB, Cho BY, Park HY, et al. Epitopes for thyroid stimulating antibodies in Graves’ sera: a possible link of heterogeneity to differences in response to antithyroid drug treatment. J Clin Endocrinol Metab. 1996;81:1758–1767. doi: 10.1210/jcem.81.5.8626830. [DOI] [PubMed] [Google Scholar]
- 12.Kosugi S, Ban T, Kohn LD. Identification of thyroid stimulating antibody-specific interaction sites in the N-terminal region of the thyrotropin receptor. Mol Endocrinol. 1993;7:114–130. doi: 10.1210/mend.7.1.8095322. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe Y, Tahara K, Hirai A, Tada H, Kohn LD, Amino N. Subtypes of anti-TSH receptor antibodies classified by bio- and conversion assays using CHO cells expressing wild type or chimeric human TSH receptor. Thyroid. 1997;7:13–19. doi: 10.1089/thy.1997.7.13. [DOI] [PubMed] [Google Scholar]
- 14.Valente WA, Vitti P, Rotella CM, et al. Antibodies that promote thyroid growth: a distinct population of thyroid-stimulating autoantibodies. N Engl J Med. 1983;309:1028–1034. doi: 10.1056/NEJM198310273091705. [DOI] [PubMed] [Google Scholar]
- 15.Kim WB, Chung HK, Lee HK, Kohn LD, Tahara K, Cho BY. Changes in epitopes for thyroid stimulating antibodies in Graves’ disease sera during treatment of hyperthyroidism: therapeutic implications. J Clin Endocrinol Metab. 1997;82:1953–1959. doi: 10.1210/jcem.82.6.3999. [DOI] [PubMed] [Google Scholar]
- 16.Tallstedt L, Lundell G, Tørring O, et al. Occurance of ophthalmopathy after treatment for Graves’ hyperthyroidism. The Thyroid Study Group. N Engl J Med. 1992;326:1733–1738. doi: 10.1056/NEJM199206253262603. [DOI] [PubMed] [Google Scholar]
- 17.Bartalena L, Marcocci C, Bogazzi F, et al. Relationship between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73–78. doi: 10.1056/NEJM199801083380201. [DOI] [PubMed] [Google Scholar]
- 18.Mittendorf E, McHenry C. Thyroidectomy for selected patients with thyrotoxicosis. Arch Otolaryngol Head Neck Surg. 2001;127:61–65. doi: 10.1001/archotol.127.1.61. [DOI] [PubMed] [Google Scholar]


