Abstract
Introduction
Iron deficiency is associated with impaired dopaminergic signaling and externalizing behavior. However, whether iron stores in toddlerhood influence later response to psychostimulants is unknown.
Methods
Youths participating in a study monitoring the long-term safety of risperidone were included in this analysis if they had received psychostimulant monotherapy for at least 3 weeks and had a complete blood count obtained prior to psychostimulant treatment. Sensitivity to psychostimulants was defined based on the weight-adjusted dose during the first year of treatment. Regression analysis examined whether the hematological tests based on the characteristics of red blood cells were associated with sensitivity to psychostimulants.
Results
Twenty nine participants (93% males, 76% Caucasians), primarily with ADHD (93%), comprised the current sample. The hematological tests were obtained, on average, 3 years before the initiation of psychostimulants monotherapy which occurred at 5.8 years of age and continued for a median of 0.85 years, at an average daily dose of 0.98 mg/kg (SD=0.38) in methylphenidate-equivalent. Compared to those that were poorly sensitive to psychostimulants, after adjusting for age, mean corpuscular volume was significantly higher in the highly and moderately sensitive groups.
Conclusions
If replicated, our findings suggest that more attention should be paid to optimizing body iron in early childhood.
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most common neuropsychiatric disorders in children (APA, 2000). Psychosocial interventions effectively reduce symptom severity and impairment though psychotropics are often prescribed, especially in severe cases (Pliszka, 2007). Most of the proven pharmacological agents alter the dopaminergic system, consistent with research implicating dopamine in the various neuropsychological processes impaired in ADHD (Pliszka, 2007). In particular, psychostimulants, which represent the first-line treatment for ADHD (Pliszka, 2007), block the dopamine transporter and release catecholamines from storage granules in the presynaptic neurons (Ford et al., 2003). However, while many studies have established the efficacy of psychostimulants for ADHD, predictors of response remain somewhat elusive (Pliszka, 2007). Among the most consistent findings, brain abnormalities have been associated with decreased clinical efficacy.
Iron is a critical nutrient involved in brain development in general and that of the dopaminergic system in particular. In fact, it is a cofactor for tyrosine hydroxylase, the rate-limiting enzyme for catecholamine synthesis (Sachdev, 1993). In addition, iron deficiency (ID) in rats results in reduced density of the dopamine transporter as well as the D1 and D2 dopamine receptors in the basal ganglia (Beard et al., 1994; Burhans et al., 2005; Erikson et al., 2000; Erikson et al., 2001; Nelson et al., 1997). This effect appears more pronounced in males (Burhans et al., 2005; Erikson et al., 2001). Moreover, the response to cocaine, a potent inhibitor of the dopamine transporter, is attenuated in ID rats (Erikson et al., 2000; Nelson et al., 1997). Additional support comes from studies in children with ID showing reduced prolactin response to a clonidine challenge, again reflecting dysfunctional dopaminergic signaling (Felt et al., 2006a).
With iron playing a key role in neurotransmission, dendritogenesis, synaptogenesis, and myelination, concerns have been raised about the impact ID might have on cognitive, behavioral, and emotional development (Lozoff et al., 2006). This is particularly true since up to 7% of young children and 16% of adolescent females in the United States are affected by ID (CDC, 2002). This is an age when the brain is undergoing substantial growth and maturation. In fact, ID anemia has been linked to socio-emotional and academic impairment (Felt et al., 2006b; Lozoff et al., 2006; Lozoff et al., 2000; Wachs et al., 2005). In addition, studies in patients with ADHD have reported an inverse association between serum ferritin, a measure of body iron, and the severity of inattention, hyperactivity and impulsivity, or sleep disturbances (Cortese et al., 2009; Konofal et al., 2004; Oner et al., 2008).
Moreover, it is particularly disturbing that prospective studies have found persistent cognitive and neurochemical abnormalities, long after the resolution of ID identified postnatally (Burhans et al., 2005; Felt et al., 2006b; Lozoff et al., 2000). For example, more than ten years after their iron stores had been replenished, children with a history of ID were more likely to have repeated a grade and/or received educational services, compared to children with a normal iron status (Lozoff et al., 2000). They also exhibited increased behavior problems, including inattention (Lozoff et al., 2000). Furthermore, these children had altered prolactin response to a clonidine challenge (Felt et al., 2006b). These findings suggest that, at least to some extent, ID in infancy might have a long-lasting effect on brain development in general and on the dopaminergic system in particular. This would also be consistent with some studies in ID rats showing only partial recovery of neurotransmission following iron supplementation (Burhans et al., 2005). In fact, the severity of ID and the timing of iron repletion appear crucial in determining the extent to which recovery will be complete (Lozoff et al., 2006).
In sum, preclinical and human research suggests that ID, inattention or ADHD, and impaired dopaminergic signaling may be interrelated, both acutely, during iron deficiency, and chronically, after it has been corrected. However, to our knowledge, only one published study has investigated the association between serum ferritin and response to treatment in ADHD (Millichap et al., 2006). In that study, there was no difference in clinical response to psychostimulants between children with low (<20 ng/ml) versus high (>60 ng/ml) serum ferritin concentration. This question is particularly pertinent in light of animal studies showing that ID dampens the inhibitory effect of cocaine on the dopamine transporter (Erikson et al., 2000; Nelson et al., 1997). Such evidence raises the possibility of a modulatory effect of iron status on the efficacy of psychostimulant treatment in patients with ADHD since, as noted earlier, they act by blocking the dopamine transporter (Ford et al., 2003).
Therefore, we here examine whether iron status in infancy and toddlerhood predicts response to psychostimulant treatment. We used hematological tests based on characteristics of red blood cells [i.e., hemoglobin concentration (Hb), hematocrit (Htc), mean cell volume (MCV), and red blood cell distribution width (RDW)] as proxies for iron status and tested the hypothesis that these parameters are associated with the dose of psychostimulants used to successfully treat ADHD symptoms. The dose of psychostimulants, we presumed, reflects the integrity of the central dopaminergic system.
Methods
Participants
This analysis uses data collected in an ongoing study evaluating the safety of risperidone during long-term treatment. The study aims, rationale, and initial findings have been described elsewhere (Calarge et al., 2009a; Calarge et al., 2009b). Briefly, 7 to 17 year-old males and females were enrolled if they had been taking risperidone, irrespective of indication, for a minimum of six months. Concomitant psychotropic treatment was allowed, except for other antipsychotic medications. Patients with intellectual disability, traumatic brain injury, other neurological disorders, or significant medical conditions were excluded as were pregnant females and those receiving hormonal contraception.
Procedures
The study was approved by the local Institutional Review Board. Written assent was obtained from children 11 years old or younger and written consent from adolescents and parents/guardians.
All medical and psychiatric records were obtained. In the majority of the cases, records were available since birth. We extracted all relevant clinical information including the psychiatric diagnoses and the medication history. We recorded the start and stop dates of each medication as well as changes in the dosage and formulation (Calarge et al., 2009a). This documentation, confirmed by a physician, also reflected any potential deviation from the prescribed treatments. All dosages of psychostimulants were expressed in methylphenidate (MPH) equivalents for amphetamines (x 2) (Calarge et al., 2009a; Swanson et al., 2007).
During the chart review, we also extracted all the results of hematological tests based on characteristics of red blood cells as well as the weight of the participants at each time point. The latter allowed us to adjust the dose of MPH for weight (i.e., computing a mg/kg/day dose). A time point for data entry was created in the data set every time a weight measurement was available in the medical record or a medication change took place.
Upon enrollment, the parent was asked to complete a questionnaire about the pregnancy, including the age of the parent at the time of the pregnancy as well as the gestational age upon birth, from the last menstrual period. We defined prematurity as birth before 37 weeks. These variables are relevant to ruling out the possibility of anemia resulting from gestational factors, including prematurity (Marks et al., 1998).
The psychiatric diagnoses were also obtained from the medical record. However, in order to evaluate the validity of these clinical diagnoses, a best-estimate diagnosis was generated in 96 subjects based on a review of all available clinical data, including a standardized interview of the parent and the youth (if 11 years old or older) using the NIMH Diagnostic Interview Schedule for Children (DISC-IV) (Shaffer et al., 2000). Agreement between the clinical diagnosis and the research-generated best-estimate diagnosis was compared, using κ statistic (Cohen, 1960), for five diagnostic categories: ADHD, disruptive behavior disorders, pervasive developmental disorders, tic disorders, and mood disorders. The κ values were 0.71 (95% confidence interval (CI): 0.50–0.93), 0.36 (95% CI: 0.17–0.56), 0.78 (95% CI: 0.60–0.97), 0.84 (95% CI: 0.72–0.96), and 0.39 (95% CI: 0.17–0.61), respectively, reflecting fair to substantial agreement. The lower agreement for mood disorders likely reflects the relapsing/remitting nature of this disorder, making it less likely that the symptoms would remain unaltered by the time subjects underwent the standardized assessment upon study enrollment.
Statistical Analysis
Our overall hypothesis was that iron status, as reflected by the hematological indices, will be associated with “sensitivity to MPH” treatment. Due to the exploratory nature of this analysis, the absence of standardized measures for clinical response in this retrospective study, and the anticipated variability in the dose and duration of psychostimulant treatment in a clinical setting, we defined “sensitivity to MPH” as follows: First, we computed the average daily dose of MPH and adjusted it for weight (i.e., mg/kg/d). We then determined, during the first year of treatment, the percentage of time spent taking a low (i.e., ≤ 0.6mg/kg/d), a high (i.e., > 1.2 mg/kg/d), or a medium daily dose of MPH (Nigg et al., 1996). This variable captures the dose and duration components of response to psychostimulants, which are both important to consider when assessing responsiveness to treatment. Their distributions, however, were severely skewed, which prevented us from using these percentages as dependent variables (the regression diagnostics revealed severe violations of model assumptions). Therefore, we defined three groups reflecting declining sensitivity to MPH: The first group, referred to as “highly sensitive”, included those participants who took a low dose of MPH for at least 50% of the time but took a high dose for less than 20% of the time. The “low sensitive” group included those who took a high MPH dose for ≥50% of the time but a low dose for < 20% of the time. The “moderately sensitive” group included the rest of the participants. We have assumed that the treating clinicians modified the dose of psychostimulants based on clinical response and that maintenance treatment reflected patient and parental satisfaction.
We restricted the analysis to those participants whose hematological tests were obtained before a psychostimulant was initiated and who had received the medication for at least three weeks before it was stopped or another psychotropic was added (Figure 1). When multiple measurements for the same hematological parameter were present, we selected the one that was obtained closest to 1.5 years of age. This is because children are at the highest risk for ID between the ages of 9 and 18 months, coinciding with their rapid rate of growth (Marks et al., 1998). We also excluded measurements that were obtained before three months of age since these usually reflect iron stores accrued in utero (Marks et al., 1998).
Figure 1.
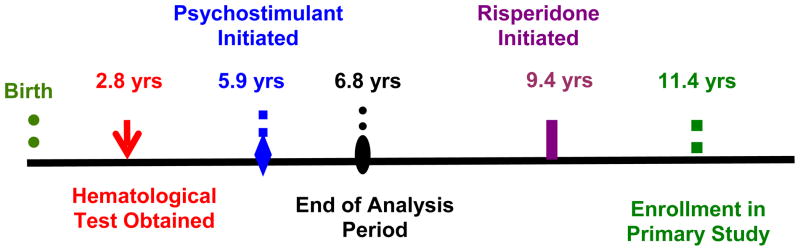
Time Course for a Prototypical Patient
As noted earlier, we used the participants’ weight at each time point to compute a daily, weight-adjusted dose of MPH. However, since clinicians do not measure or record weight at every clinic visit, weight information was missing in 27.5% of all the time points in the entire data set (n=8933). In those instances, we used the last weight available prior to the missing one and the first one available afterwards to estimate the missing weight. To confirm the accuracy of this linear interpolation method, we conducted a simulation analysis whereby we randomly excluded 30% of the non-missing weight values obtained after age 4 and interpolated them using the same linear method. The percentage difference between the interpolated and observed values was then computed (i.e., [observed weight − interpolated weight] × 100%/observed weight) (Bland et al., 1999). The mean (SD) of this percentage was −0.02 (3.56). The 95% confidence interval for the percent bias was [−6.96, 6.96] (Figure 2) (Bland et al., 1999). In other words, 95% of the interpolated weight values fell within ± 7% of the actual values. This suggests that our linear interpolation method is appropriate to estimate missing weight values in the dataset.
Figure 2.
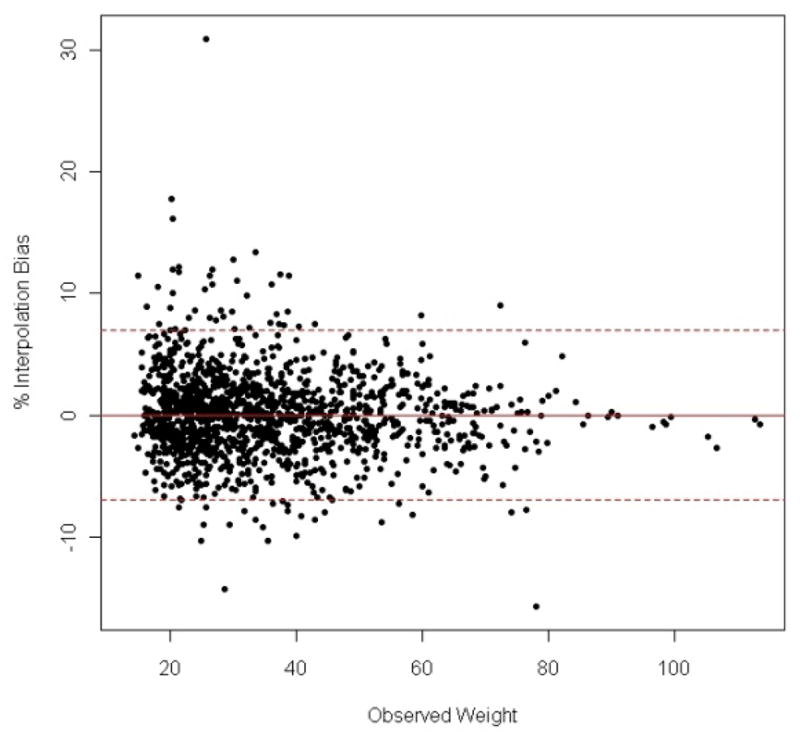
Percent difference between observed and interpolated weight measurements plotted against observed weight. The mean percent difference is 0 (full line) with a 95% confidence interval of ± 6.96 (dash-dotted lines).
Demographic and clinical variables across the three MPH “sensitivity” groups were compared using analysis of variance for continuous variables and the Fisher’s exact test for categorical variables. In order to test our primary hypotheses, we used analysis of covariance (ANCOVA) with each hematological index modeled as the dependent variable and the “MPH sensitivity” grouping serving as the predictor variables. Since the age at which the blood test was obtained and the age at which psychostimulants were initiated varied across the participants, though not across the “MPH sensitivity” groups, we controlled for these two variables in the analyses.
All the statistical tests performed were two-tailed. Given the exploratory nature of this analysis, we did not adjust statistical significance for the number of comparisons we conducted. We performed all the analyses using SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC).
Results
Of 144 participants with complete data recruited into the original study, 29 had at least one hematological test obtained before psychostimulants were started and received psychostimulants for at least three weeks during their first year of treatment. We found no differences between the 29 participants included in this analysis and those not (n=115) in sex (93% males vs. 89%, p>0.7), racial/ethnic composition (76% Caucasians vs. 84%, p>0.2), age (11.2 years ± 2.8 vs. 12.0 ± 2.8, p>0.1), or age when psychostimulants were started (5.8 years ± 1.7 vs. 6.2 ± 3.2, p>0.4). There were also no differences in the clinical diagnoses or in the indication for risperidone treatment across the two groups (all p>0.2).
Table 1 shows the demographic and clinical characteristics of the 29 participants included in this analysis divided in three groups based on the percentage of time, during the first year of psychostimulant treatment, they received a low, medium, or high weight-adjusted MPH dose. By study design, all participants were taking risperidone, upon recruitment, to target aggression and irritability in 93% (n=27) of the cases, impulsivity in one case (3%), and tics in another (3%). The most common clinical diagnosis, upon enrollment, was attention deficit hyperactivity disorder present in 27 (93%) of the children. However, comorbidity was the rule with 22 (76%) children having a disruptive behavior disorder, 13 (45%) an anxiety disorder, 4 (14%) a depressive disorder, 5 (17%) a tic disorder, and two children (7%) carrying a diagnosis of pervasive developmental disorder.
Table I.
Differences in Demographic, Treatment Characteristics, and Gestational Data Across the Three “MPH Sensitivity” Groups.
| Characteristic | High MPH Sensitivity n=8 | Medium MPH Sensitivity n=9 | Low MPH Sensitivity n=12 | Statistic | p value |
|---|---|---|---|---|---|
| Age at Enrollment, yrs | 10.8 ± 3.0 | 10.4 ± 2.8 | 12.0 ± 2.8 | F=0.90 | >.4 |
| Male, n (%) | 8 (100) | 8 (89) | 11 (92) | Fisher’s | >.99 |
| Age MPH Started, yrs | 5.3 ± 1.8 | 5.8 ± 1.5 | 6.2 ± 1.8 | F=0.58 | >.5 |
| Duration of MPH Treatment, yrs | 0.6 ± 0.4 | 0.7 ± 0.4 | 0.7 ± 0.4 | F=0.22 | >.8 |
| Mean MPH dose, mg/kg/d | 0.64 ± 0.22 | 0.91 ± 0.14 | 1.26 ± 0.38 | F=12.1 | <.0002 |
| Max MPH dose, mg/kg/d | 0.93 ± 0.46 | 1.05 ± 0.24 | 1.64 ± 0.34 | F=11.9 | <.0002 |
| MPH Start to Enrollment Interval, yrs | 5.4 ± 3.1 | 4.6 ± 2.2 | 5.8 ± 2.3 | F=0.58 | >.5 |
| Maternal Age at Birth, yrs § | 25.3 ± 3.9 | 26.4 ± 7.4 | 25.3 ± 4.7 | F=0.11 | >.8 |
| Gestational Age, wks § | 39.2 ± 1.8 | 40.0 ± 1.1 | 39.4 ± 2.1 | F=0.44 | >.6 |
| Prematurity, n (%) § | 1 (17%) | 0 | 1 (10%) | Fisher’s | >.7 |
High, medium, and low MPH sensitivity was based on the relative percent time spent, during the first year of treatment, receiving a high, medium, or low weight-adjusted dose of methylphenidate (MPH) (see Methods section for details).
Birth information was available for 27 participants, the other two having been adopted.
Since the aim of the current investigation is to explore the association between iron status in toddlerhood and sensitivity to psychostimulants, we restricted the analysis to the initial psychiatric treatment period when the participants received only psychostimulants (Figure 1). On average, the participants were 5.8 years old (SD=1.7) when they started taking psychostimulants. Their average daily dose was 0.98 mg/kg (SD=0.38) and the median duration of treatment was 0.85 years (IQR=0.72). As noted earlier, the sample was divided in three groups based on the percentage of time each participant took MPH at a low, medium, and high dose. Using this definition, eight participants were considered to have high sensitivity to MPH, nine having medium sensitivity, and 12 having low sensitivity. No demographic or clinical differences were significant across the three groups, except those related to MPH dosage (Table I).
The hematological tests were obtained at a mean age of 2.8 years (SD=1.8), on average, 3.0 years (SD=1.5) before the onset of psychostimulant treatment. The mean Hb concentration was 11.9 g/dl (SD=1.1, range: 10–14.3), Htc 34.4% (SD=2.7, range: 26.5–38.4), MCV 76.3 femtoliters (SD=5.8, range: 63.4–84.4), and RDW 13.9 (SD=1.9, range: 10.8–17.3). Using the cutoffs for the different hematological tests, as defined by Marks et al. based on age and sex (Marks et al., 1998), low Hb was present in 18% (n=5) of the sample, low Htc in 22% (n=5), microcytosis in 48% (n=10), and large RDW in 42% (n=5). The indication to obtain the tests included a work up for a medical illness (n=16, 55%), screening during yearly physicals (n=8, 28%), a work up for anemia (n=3, 10%), and screening during a psychiatric evaluation (n=2, 7%). There were no significant differences across the three “MPH sensitivity” groups in the indications for the test (p>.7).
Table II presents the results of the hematological tests across the three “MPH sensitivity” groups. MCV was significantly different across the groups with the “high sensitivity” group having the largest value, followed by the “medium sensitivity” group. The “low sensitivity” group had the smallest MCV as would be expected if iron deficiency did, as hypothesized, reduce the susceptibility of the dopaminergic system to psychostimulants. Though none of the other hematological parameters was significantly different across the three groups, all the observed differences were in the expected directions. The rates of microcytocis and large RDW were numerically different across the three groups (Table II). However, these differences did not reach statistical significance, likely due to the small sample size.
Table II.
Differences in Hematological Tests Across the Three “MPH Sensitivity” Groups.
| Characteristic | High MPH Sensitivity n=8 | Medium MPH Sensitivity n=9 | Low MPH Sensitivity n=12 | Statistic | p value |
|---|---|---|---|---|---|
| Age Test Obtained | 3.0 ± 2.2 | 2.8 ± 1.8 | 2.7 ± 1.6 | F=0.07 | >.9 |
| Test to MPH Start Time Interval, yrs | 2.3 ± 1.2 | 2.9 ± 1.7 | 3.5 ± 1.6 | F=1.44 | >.2 |
| Hemoglobin, g/dl | 12.0 ± 1.1 | 12.0 ± 1.2 | 11.7 ± 1.0 | F=0.33 | >.7 |
| Low Hemoglobin, n (%)* | 2 (25) | 1 (11) | 2 (18) | Fisher’s | >.8 |
| Hematocrit, (%) | 34.3 ± 4.2 | 35.3 ± 1.6 | 33.8 ± 2.0 | F=0.59 | >.5 |
| Low Hematocrit, n (%)* | 2 (28) | 0 (0) | 3 (33) | Fisher’s | >.3 |
| MCV, fL | 79.2 ± 2.7 | 78.7 ± 1.0 | 71.9 ± 7.2 | F=5.3 | <.02 |
| Microcytosis, n (%)* | 3 (43) | 1 (17) | 6 (75) | Fisher’s | >.1 |
| RDW, (%) | 14.3 ± 1.5 | 13.0 ± 2.0 | 14.6 ± 1.9 | F=0.90 | >.4 |
| Large RDW, n (%)* | 1 (25) | 1 (17) | 3 (75) | Fisher’s | >.4 |
High, medium, and low MPH sensitivity was based on the relative percent time spent, during the first year of treatment, receiving a high, medium, or low weight-adjusted dose of methylphenidate (MPH) (see Methods section for details).
Cutoffs were based on Ref. (Marks et al., 1998), Tables 6 and 8. They varied by age and sex.
ANCOVA was then used to predict the four hematological parameters, after adjusting for the age when a psychostimulant was started as well as the age when the hematological test was obtained. Table III lists the beta estimates of the models predicting the four hematological parameters along with their standard errors. Compared to those that were poorly sensitive to psychostimulants, after adjusting for age, mean MCV was higher by 8.6 fL (p<.002) in the highly sensitive group and by 7.1 fL (p<.006) in the moderately sensitive one. An alternative analysis, with the three “MPH sensitivity” groups, rank ordered (low, moderate, high), also showed a significant association of MCV with MPH sensitivity (partial Spearman’s r=0.56, p<0.01).
Table III.
Multiple Linear Regression Models Predicting Individual Hematological Tests as a Function of “MPH Sensitivity”, Adjusting for the Age when Psychostimulants (MPH) were Started and when the Hematological Test was Obtained.
| Predictor Variables | β Estimates | SE | t | p Value | |
|---|---|---|---|---|---|
| Hemoglobin (Hb) | High MPH Sensitivity Group | 0.43 | 0.53 | 0.8 | >.4 |
| Medium MPH Sensitivity Group | 0.37 | 0.49 | 0.8 | >.4 | |
| Age MPH Initiated | 012 | 0.17 | 0.7 | >.4 | |
| Age Hb Measured | 0.13 | 0.15 | 0.9 | >.3 | |
|
| |||||
| Hematocrit (Hct) | High MPH Sensitivity Group | 0.62 | 1.55 | 0.4 | >.6 |
| Medium MPH Sensitivity Group | 1.56 | 1.47 | 1.1 | >.3 | |
| Age MPH Initiated | 0.15 | 0.47 | 0.3 | >.7 | |
| Age Hct Measured | −0.01 | 0.49 | −0.0 | >.9 | |
|
| |||||
| Mean Corpuscular Volume (MCV) | High MPH Sensitivity Group | 8.55 | 2.32 | 3.7 | .002 |
| Medium MPH Sensitivity Group | 7.07 | 2.25 | 3.1 | .006 | |
| Age MPH initiated | 1.20 | 0.73 | 1.6 | .12 | |
| Age MCV Measured | 0.31 | 0.73 | 0.4 | >.6 | |
|
| |||||
| Red Cell Distribution Width (RDW) | High MPH Sensitivity Group | −0.69 | 1.38 | −0.5 | >.6 |
| Medium MPH Sensitivity Group | −2.09 | 1.25 | −1.7 | .14 | |
| Age MPH initiated | −0.50 | 0.37 | −1.4 | >.2 | |
| Age RDW Measured | 0.06 | 0.39 | −0.1 | >.8 | |
High, medium, and low MPH sensitivity was based on the relative percent time spent, during the first year of treatment, receiving a high, medium, or low weight-adjusted dose of methylphenidate (MPH) (see Methods section for details). In this regression model, the low “MPH sensitivity” group represents the baseline to which the other two groups are compared.
Discussion
ID has been associated with altered dopaminergic signaling, cognitive impairment, inattention, and externalizing symptoms, including ADHD. In this analysis, we explored whether tests based on characteristics of red blood cells obtained, on average, 3 years prior to the onset of treatment with psychostimulants was correlated with response to stimulant treatment. MCV was significantly associated with sensitivity to psychostimulants as indexed by receiving a lower dose of psychostimulants during the first year of treatment.
Prior research has linked reduced serum ferritin with the severity of inattention, hyperactivity, and impulsivity (Konofal et al., 2004; Oner et al., 2008). However, serum ferritin was measured around the same time the clinical ratings were obtained. While such findings contribute to establishing an association between iron stores and ADHD, they fail to address the essential point raised by preclinical and human studies that ID, early during development, may lead to persistent changes in dopaminergic signaling and behavioral/cognitive sequelae (Burhans et al., 2005; Felt et al., 2006b; Lozoff et al., 2000). The alteration in dopaminergic neurotransmission affects various brain regions including the basal ganglia, a structure consistently implicated in ADHD (Dickstein et al., 2006). Due to the nature of our database, we were able to specifically test whether iron stores in the toddler years were associated with a differential sensitivity to psychostimulants in grade school. While MCV was strongly associated with “MPH sensitivity”, no other index reached significance, likely due to the small sample size. Nevertheless, the trends were in the expected duration with Hb and Hct being positively associated with “MPH sensitivity” and RDW negatively so.
In the one other published study, we are aware of, that investigated the association between serum ferritin and response to psychostimulant treatment, Millichap et al. found no difference in the response rate to psychostimulants based on ferritin concentration (Millichap et al., 2006). It was not clear, however, how response to treatment was defined. Moreover, patients with low serum ferritin were given iron supplements before treatment with psychostimulants was initiated (Millichap et al., 2006). Therefore, it is possible that iron repletion masked the potential link between serum ferritin and sensitivity to psychostimulants by attenuating ADHD symptoms as has been shown in a recent double-blind placebo-controlled trial (Konofal et al., 2008). By contrast, using a different sample in a multiphase clinical trial, our group has recently found that baseline ferritin concentration was negatively correlated with the weight-adjusted dose of amphetamine necessary to optimize clinical response in youths with ADHD (Calarge et al., Under Review).
This being a retrospective study, we could not evaluate how many of the participants had frank anemia upon the initiation of psychostimulants. These data were not available. However, a number of the participants did have abnormalities in their hematological parameters prior to treatment onset, as described in table II. Still, only one child with ID anemia appeared to have received iron supplementation. It is possible that the clinicians did not deem the abnormalities severe enough to treat, especially that the blood tests were obtained in order to specifically evaluate for anemia in only 3 of the children. Nevertheless, the absence of information regarding anemia at the start of psychostimulants should not detract from the significance of our findings since ID has been shown to affect cognitive and behavioral functioning even in the absence of frank anemia (Grantham-McGregor et al., 2001; Halterman et al., 2001; Lozoff et al., 2000). This is likely because, when intake is limited, iron is diverted towards hemoglobin synthesis, resulting in a reduction in brain iron concentration before anemia becomes manifested (Lozoff et al., 2006).
Though our findings contribute to the growing literature linking ID, ADHD, and dopaminergic signaling, a number of limitations are worth noting. First, the retrospective design of the study could have biased the results in one of several ways. It is possible that the indication for laboratory testing was itself the primary cause underlying the differences in “MPH sensitivity.” While we cannot rule out this possibility due to the small sample size, we believe it is unlikely since the indication for the blood test did not differ across the groups. Moreover, in several participants, the test was obtained as part of a general medical screen, in the absence of any particular medical condition. In addition, our findings would have been stronger had we had symptom ratings at the initiation of psychostimulants and later on during the course of the treatment. Furthermore, our sample is not representative of children with ADHD since comorbidity was prevalent and since, by study design, all the participants eventually received an antipsychotic medication. Therefore, it is necessary to replicate our findings in a larger sample of children whose ADHD was well-controlled with psychostimulants alone and where both males and females are equally represented. This would also minimize the risk for type 1 errors. Nevertheless, it is of interest that in the vast majority of our participants, risperidone was prescribed to treat irritability and aggression. In-utero iron deprivation in monkeys leads to impulsivity while, postnatally, it results in increased emotionality. Similarly, perinatal iron stores in humans are correlated with negative emotionality (Wachs et al., 2005). This temperamental trait is often observed in aggressive children with disruptive behavior (Briggs-Gowan et al., 2006). Finally, this being a convenience sample, we used hematological tests based on the characteristics of red blood cells as markers of iron stores. While the combination of low MCV and high RDW is an accepted index of ID, additional parameters could have included serum iron concentration, iron binding capacity, transferrin, and ferritin.
In sum, with food enrichment, ID has significantly declined in the developed countries, including the US. However, in at-risk populations, such as minorities and individuals from disadvantaged backgrounds, ID in infancy and the toddler years remains common (CDC, 2002; Marks et al., 1998). These groups are also at an elevated risk for externalizing disorders. This combination of risks not only makes them vulnerable to developing ADHD but, based on our findings reported here and elsewhere (Calarge et al., Under Review), it might attenuate their response to the gold standard treatment. Therefore, it is essential to optimize efforts to prevent ID early on in development while avoiding the adverse events associated with excessive iron supplementation (Iannotti et al., 2006).
Acknowledgments
Funding Support:
This study was funded by a 2005 Young Investigator Award, by the National Institute of Health (RR024979, R21MH080968, and K23MH085005), and through a Medical Student Summer Research Fellowship from American Academy of Child and Adolescent Psychiatry sponsored by the Campaign for America’s Kids. Dr. Bridget Zimmerman supervised the statistical analysis. We would like to thank the patients and their families for their commitment to this research, the staff of the University of Iowa child psychiatry division, the research coordinators, and the Clinical Research Unit Staff.
Biographies
Catharyn A. Turner, II received her A.B at Bryn Mawr College in 1991 and an M.Ed. from Towson University in 2003. She worked as a classroom teacher in Maryland Public Schools, before enrolling at The University of Iowa Carver College of Medicine where she is expected to graduate in May of 2010. Her research interests include general adolescent medicine and psychiatric health of adolescents.
Diquiong Xie, M.S.
Footnotes
Conflict of Interest
The authors report no competing interests.
Previous presentation
Aspects of this work have been presented at the annual meeting of the American Academy of Child and Adolescent Psychiatry, October, 2009, Honolulu, HI.
Contributor Information
Catharyn Turner, Medical Student, The University of Iowa Carver College of Medicine
Diqiong Xie, Graduate Student, The University of Iowa College of Public Health
Bridget Zimmerman, The University of Iowa College of Public Health, Director, Biostatistics Consultation Center
References
- APA. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Beard JL, Chen Q, Connor J, Jones BC. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacol Biochem Behav. 1994;48(3):621–624. doi: 10.1016/0091-3057(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Bosson-Heenan J, Guyer AE, Horwitz SM. Are infant-toddler social-emotional and behavioral problems transient? J Am Acad Child Adolesc Psychiatry. 2006;45(7):849–858. doi: 10.1097/01.chi.0000220849.48650.59. [DOI] [PubMed] [Google Scholar]
- Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, et al. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci. 2005;8(1):31–38. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA. Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol. 2009a;19(2):101–109. doi: 10.1089/cap.2008.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Farmer C, DiSilvestro R, Arnold LE. Serum Ferritin and Amphetamine Response in Youth with ADHD. (Under Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Zimmermann B, Xie D, Kuperman S, Schlechte JA. A Cross-sectional Evaluation of the Effect of Risperidone and Selective Serotonin Reuptake Inhibitors on Bone Mineral Density in Boys. J Clin Psychiatry, In Press. 2009b doi: 10.4088/JCP.08m04595gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Iron Deficiency — United States, 1999–2000. Atlanta, GA: 2002. [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- Cortese S, Konofal E, Bernardina BD, Mouren MC, Lecendreux M. Sleep disturbances and serum ferritin levels in children with attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2009;18(7):393–399. doi: 10.1007/s00787-009-0746-8. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130(11):2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69(3–4):409–418. doi: 10.1016/s0091-3057(01)00563-9. [DOI] [PubMed] [Google Scholar]
- Felt B, Jimenez E, Smith J, Calatroni A, Kaciroti N, et al. Iron deficiency in infancy predicts altered serum prolactin response 10 years later. Pediatr Res. 2006a;60(5):513–517. doi: 10.1203/01.PDR.0000242848.45999.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006b;171(2):261–270. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford RE, Greenhill LL, Posner K. Stimulants. In: Martin A, Scahill L, Charney DS, Leckman JF, editors. Pediatric Psychopharmacology, Principles and Practice. New York, NY: Oxford University Press; 2003. pp. 255–263. [Google Scholar]
- Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131(2S-2):649S–666S. doi: 10.1093/jn/131.2.649S. discussion 666S–668S. [DOI] [PubMed] [Google Scholar]
- Halterman JS, Kaczorowski JM, Aligne CA, Auinger P, Szilagyi PG. Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics. 2001;107(6):1381–1386. doi: 10.1542/peds.107.6.1381. [DOI] [PubMed] [Google Scholar]
- Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr. 2006;84(6):1261–1276. doi: 10.1093/ajcn/84.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konofal E, Lecendreux M, Arnulf I, Mouren MC. Iron deficiency in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2004;158(12):1113–1115. doi: 10.1001/archpedi.158.12.1113. [DOI] [PubMed] [Google Scholar]
- Konofal E, Lecendreux M, Deron J, Marchand M, Cortese S, et al. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatr Neurol. 2008;38(1):20–26. doi: 10.1016/j.pediatrneurol.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105(4):E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- Marks JS, Dietz WH, Holloway BR, Dean AG. Recommendations to Prevent and Control Iron Deficiency in the United States. Atlanta, GA: CDC; 1998. [Google Scholar]
- Millichap JG, Yee MM, Davidson SI. Serum ferritin in children with attention-deficit hyperactivity disorder. Pediatr Neurol. 2006;34(3):200–203. doi: 10.1016/j.pediatrneurol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Nelson C, Erikson K, Pinero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997;127(12):2282–2288. doi: 10.1093/jn/127.12.2282. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Hinshaw SP, Halperin JM. Continuous performance test in boys with attention deficit hyperactivity disorder: methylphenidate dose response and relations with observed behaviors. Journal of Clinical Child Psychology. 1996;25(3):330–340. [Google Scholar]
- Oner O, Alkar OY, Oner P. Relation of ferritin levels with symptom ratings and cognitive performance in children with attention deficit-hyperactivity disorder. Pediatr Int. 2008;50(1):40–44. doi: 10.1111/j.1442-200X.2007.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Sachdev P. The neuropsychiatry of brain iron. J Neuropsychiatry Clin Neurosci. 1993;5(1):18–29. doi: 10.1176/jnp.5.1.18. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol. 2005;46(2):141–153. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]


